He–Ne Laser Priming Enhances Drought Tolerance in Wheat through Differential Modification of Photosynthetic Pigments and Antioxidative Enzymes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Material and Laser Irradiation Treatment
2.2. Growth Parameters
2.3. Photosynthetic Pigments
2.4. Anthocyanin
2.5. Enzymatic Antioxidants
2.6. Soluble Sugars
2.7. Soluble Proteins
2.8. Total Free Amino Acids
2.9. Ionic Contents of the Plant Sample
2.10. Statistical Analysis
3. Results
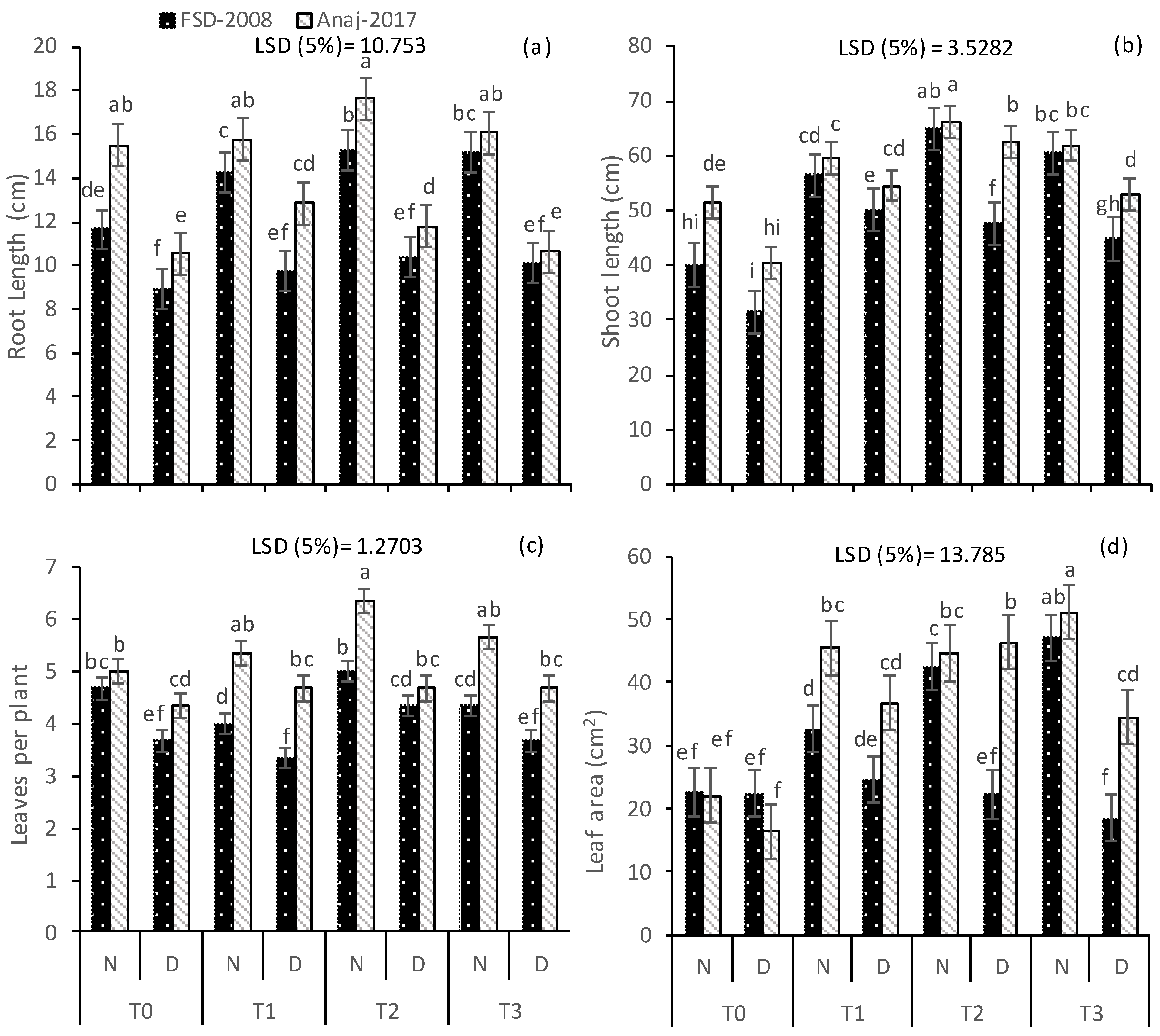
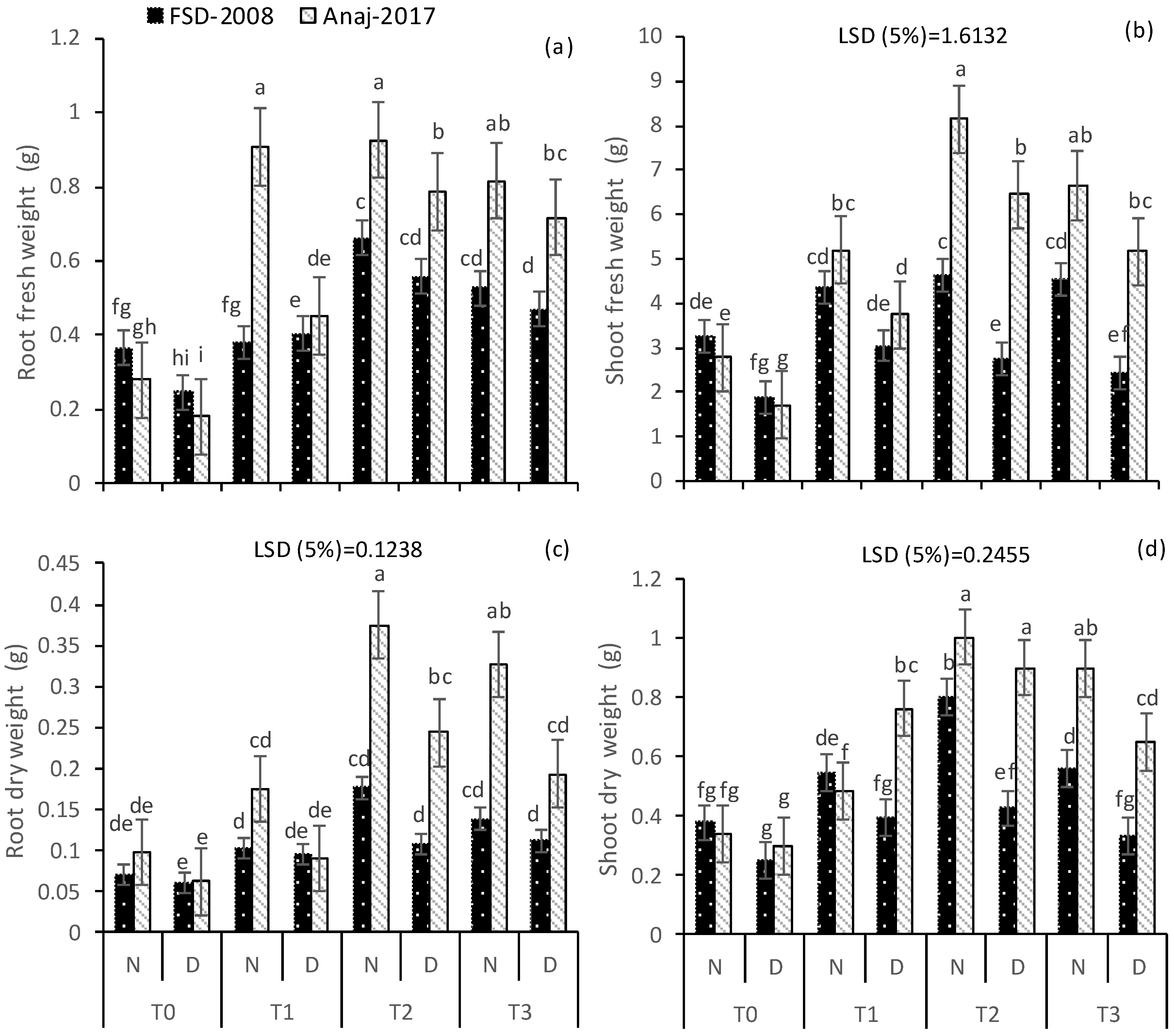
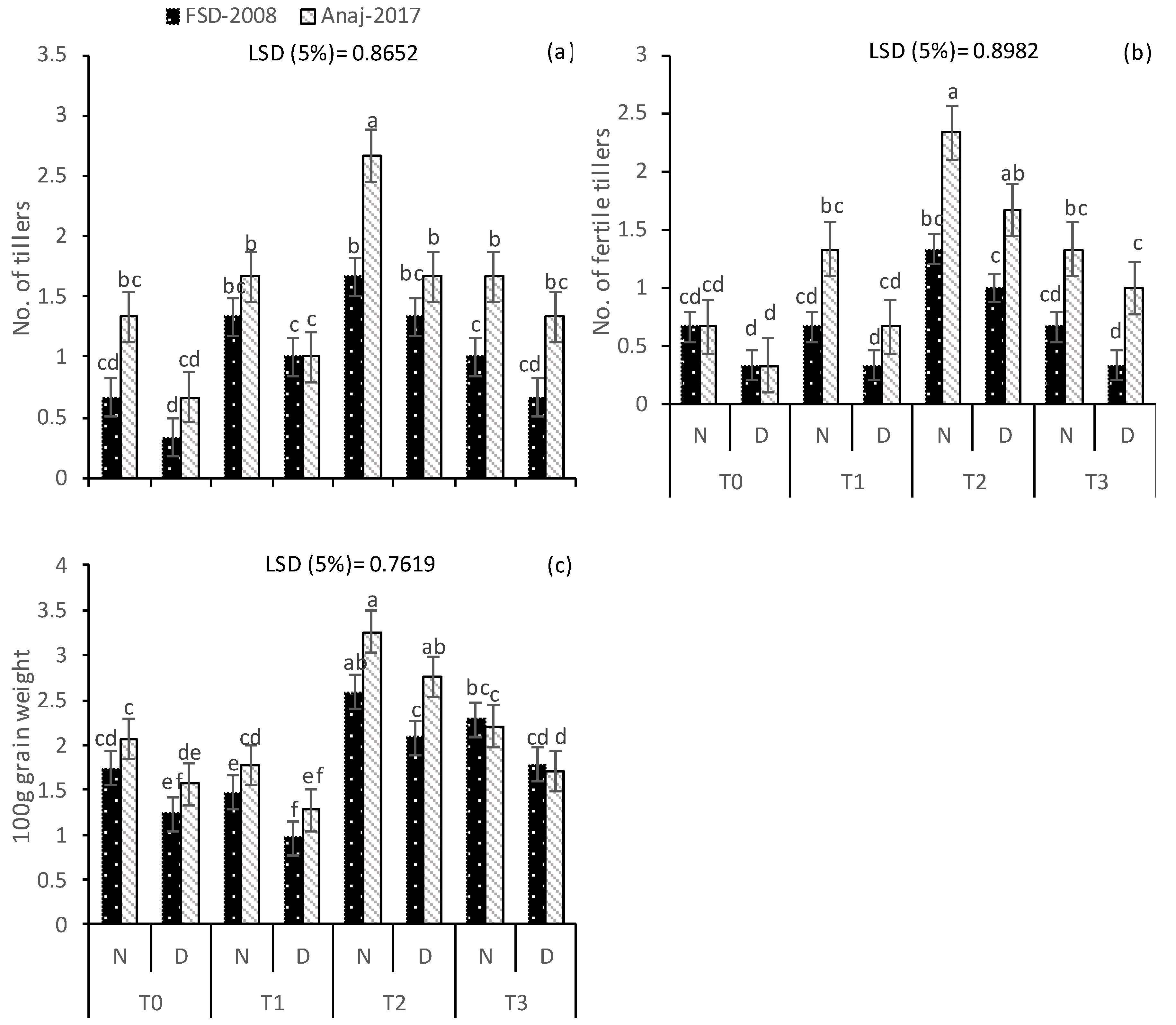
3.1. Growth and Yield Attributes
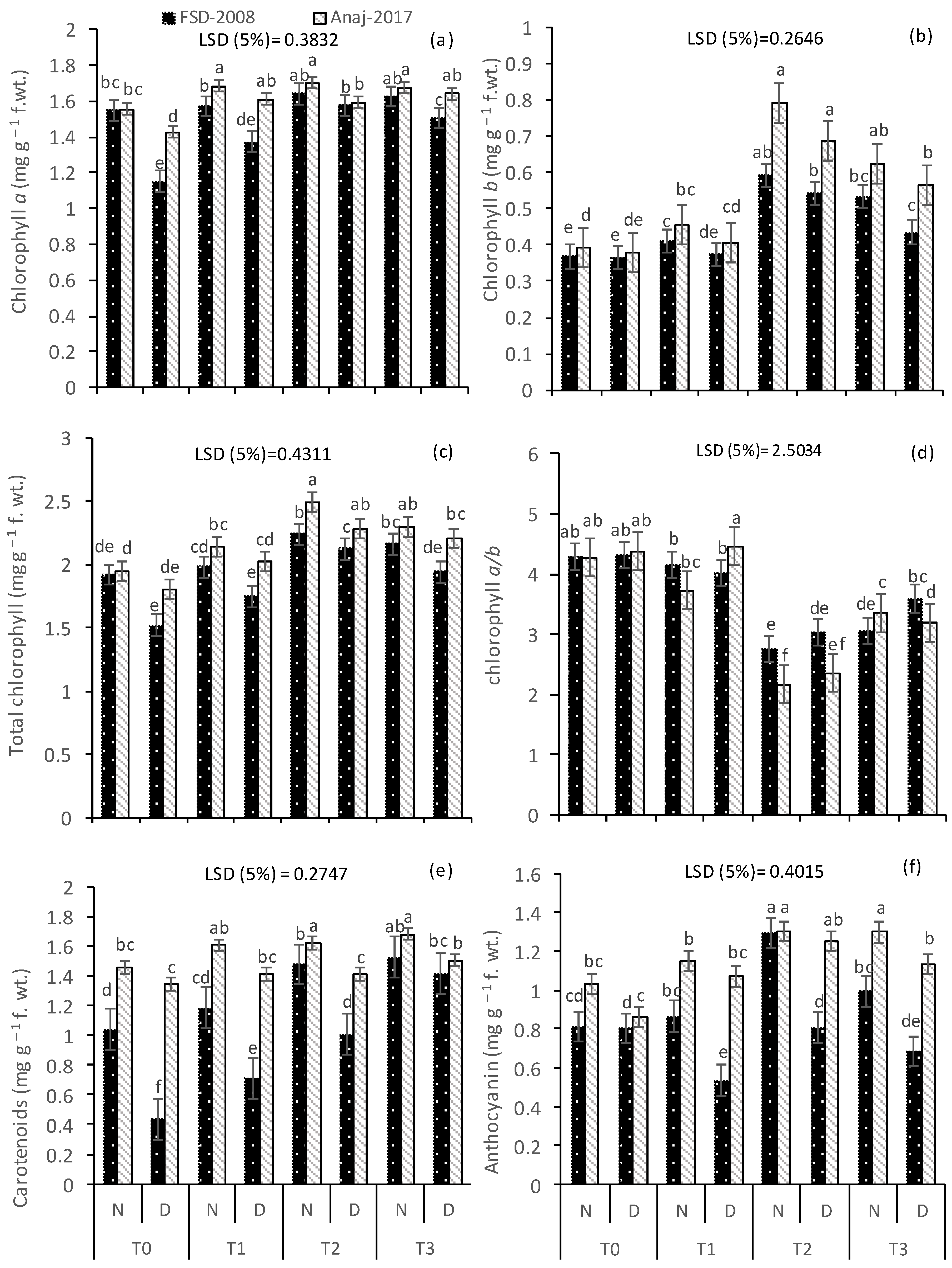
3.2. Photosynthetic Pigments
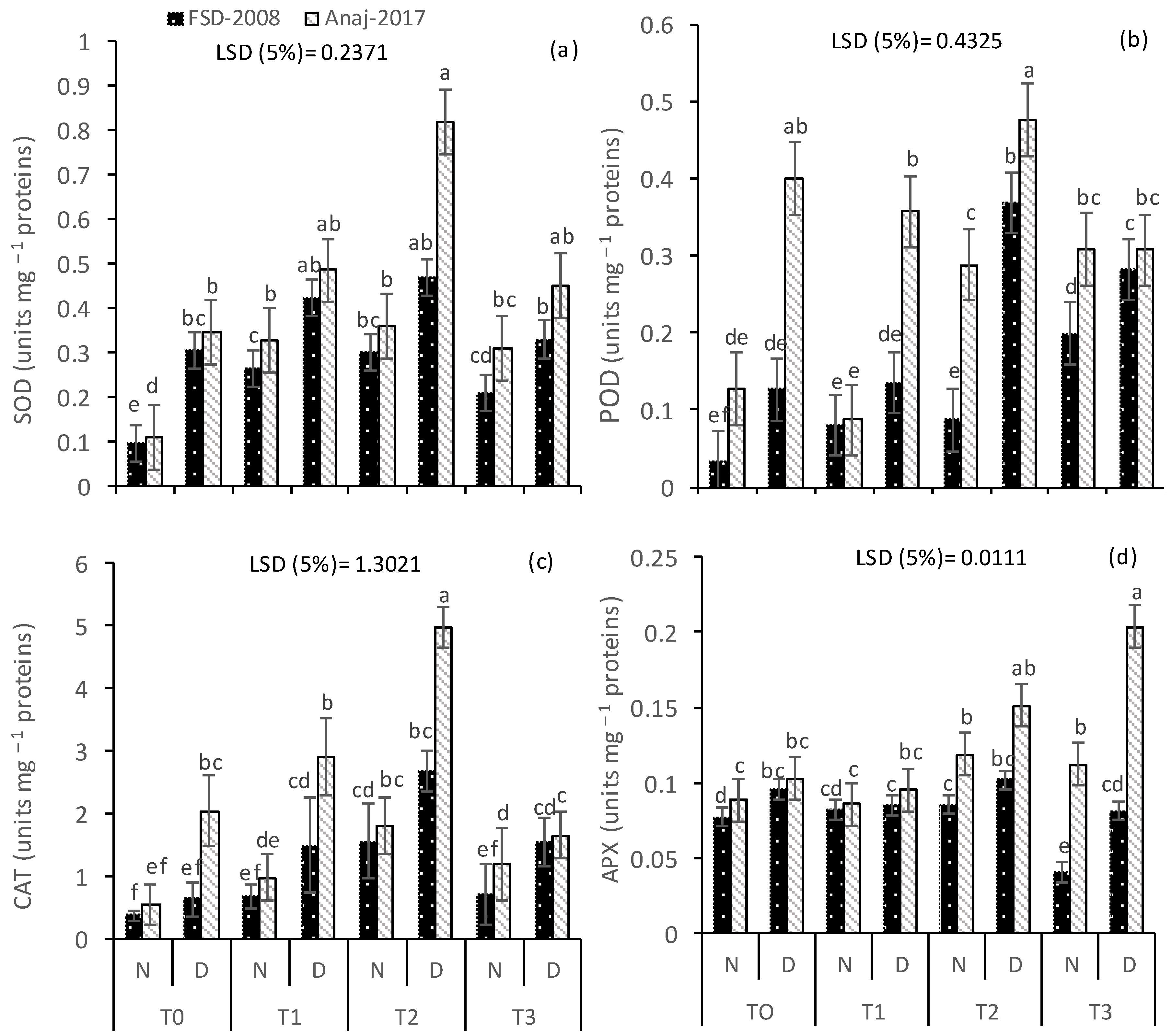
3.3. Antioxidative Enzymes
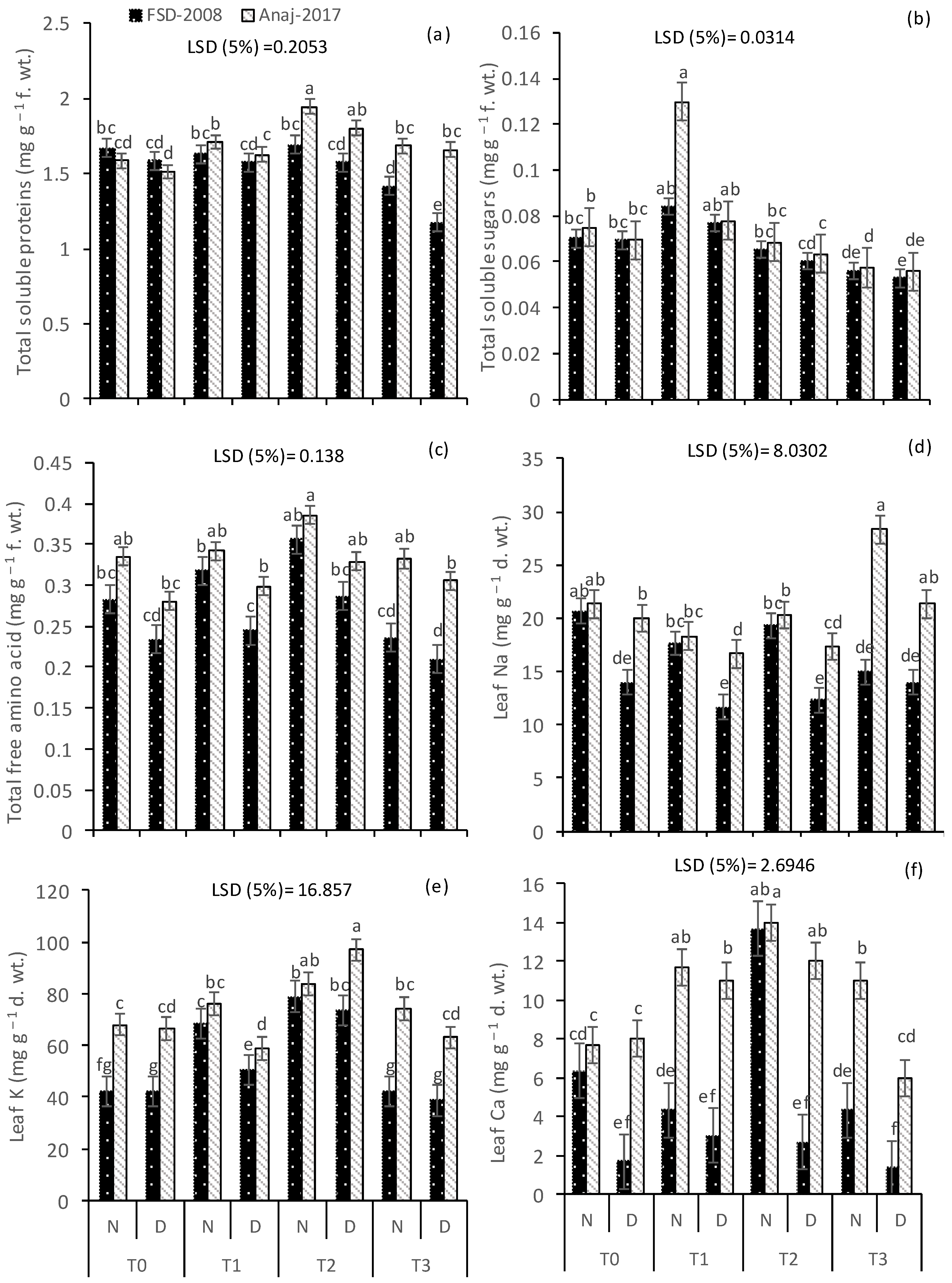
3.4. Soluble Proteins and Free Amino Acids
3.5. Leaf Ionic Contents
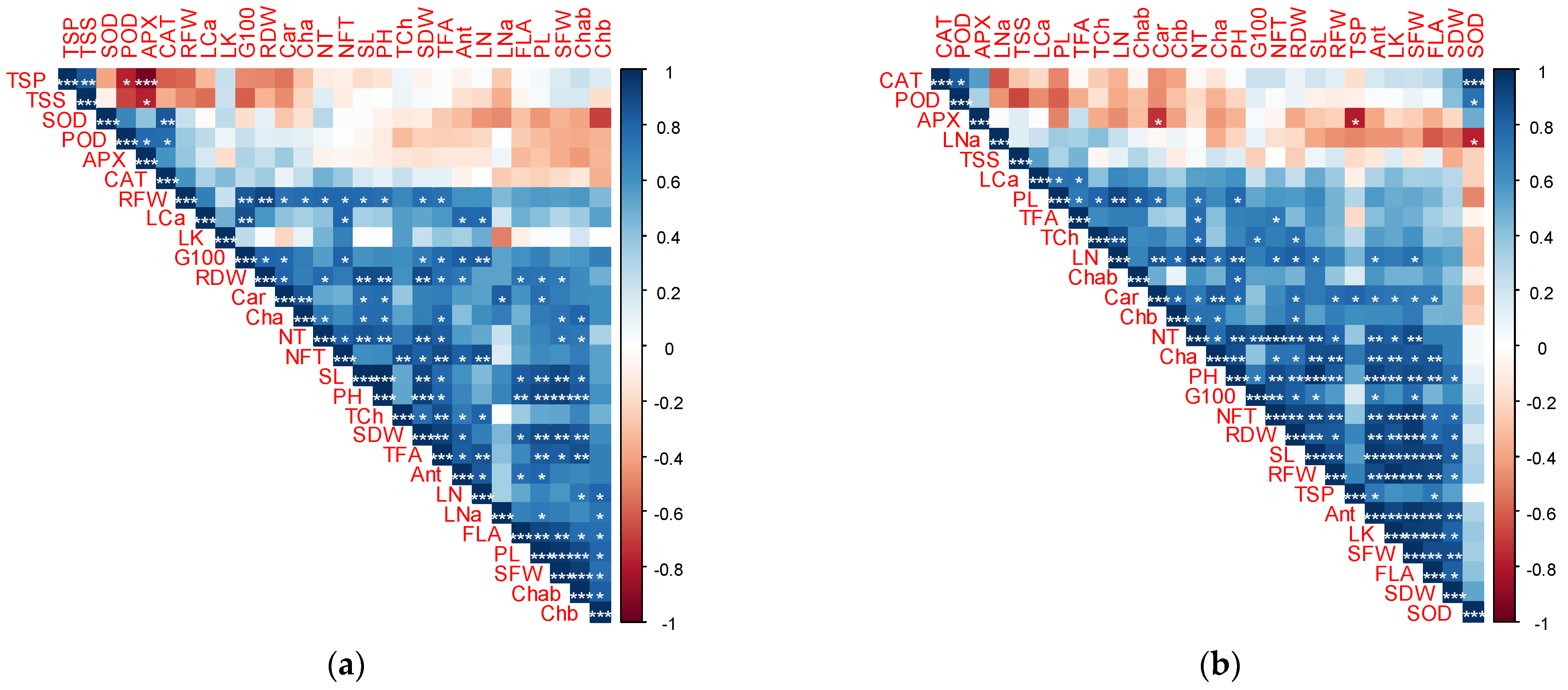
3.6. Heatmap Clustering
3.7. Pearson Correlation Coefficient
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, A. Characteristics and trends in various forms of the Palmer Drought Severity Index during 1900–2008. J. Geophys. Res. Atmos. 2011, 116, 115. [Google Scholar] [CrossRef] [Green Version]
- Penalba, O.C.; Rivera, J.A. Future changes in drought characteristics over Southern South America projected by a CMIP5 multi-model ensemble. Am. J. Clim. Change 2013, 2, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, W.; Ullah, N.; Xu, L.; El Sabagh, A. Global food and nutrition security under changing climates. Front Agron. 2022, 3, 799878. [Google Scholar] [CrossRef]
- United Nations. World population projected to reach 9.8 billion in 2050, and 11.2 billion in 2100. In World Population Prospects: The 2017 Revision; UN Department of Economics and Social Affairs: New York, NY, USA, 2017. [Google Scholar]
- Hunter, M.C.; Smith, R.G.; Schipanski, M.E.; Atwood, L.W.; Mortensen, D.A. Agriculture in 2050: Recalibrating targets for sustainable intensification. Bioscience 2017, 67, 386–391. [Google Scholar] [CrossRef] [Green Version]
- Castañares, J.L.; Bouzo, C.A. Effect of different priming treatments and priming durations on melon germination behavior under suboptimal conditions. Open Agri. 2018, 3, 386–392. [Google Scholar] [CrossRef]
- Dawood, M.G. Stimulating plant tolerance against abiotic stress through seed priming. In Advances in Seed Priming; Rakshit, A., Singh, H.B., Eds.; Springer: Singapore, 2018; pp. 147–183. [Google Scholar]
- Mohammadi, S.K.; Shekari, F.; Fotovat, R.; Darudi, A. Effect of laser priming on canola yield and its components under salt stress. Int. Agrophys. 2012, 26, 45–51. [Google Scholar] [CrossRef]
- Podleśny, J. Effect of laser irradiation on the biochemical changes in seeds and the accumulation of dry matter in the faba bean. Int. Agrophys. 2002, 16, 209–213. [Google Scholar]
- El Tayeb, T.A.; Habba, I.E. Studies on the effect of gamma, laser irradiation and progesterone treatments on gerbera leaves. Eur. J. Biophys. 2015, 3, 43–50. [Google Scholar]
- Klimek-Kopyra, A.; Dobrowolski, J.W.; Czech, T.; Neugschwandtner, R.W.; Gambuś, F.; Kot, D. The use of laser biotechnology in agri-environment as a significant agronomical advance increasing crop yield and quality. Adv. Agron. 2021, 170, 1–33. [Google Scholar]
- Singh, P.; Chatterjee, A.; Bhatia, V.; Prakash, S. Application of laser biospeckle analysis for assessment of seed priming treatments. Comput. Electron. Agric. 2020, 169, 105212. [Google Scholar] [CrossRef]
- Perveen, R.; Wang, X.; Jamil, Y.; Ali, Q.; Ali, S.; Zakaria, M.Q.; Afzaal, M.; Kasana, R.A.; Saleem, M.H.; Fiaz, S. Quantitative determination of the effects of He–Ne laser irradiation on seed thermodynamics, germination attributes and metabolites of safflower (Carthamus tinctorius L.) in relation with the activities of germination enzymes. Agronomy 2021, 1, 1411. [Google Scholar] [CrossRef]
- Hasan, M.; Hanafiah, M.M.; Taha, Z.A.; Said, M.N.M. Effect of low-intensity laser irradiation on field performance of maize (Zea mays L.) emergence, phenological and seed quality characteristics. Appl. Ecol. Environ. Res. 2020, 18, 6009–6023. [Google Scholar] [CrossRef]
- El Sabagh, A.; Hossain, A.; Barutcular, C.; Islam, M.S.; Awan, S.I.; Galal, A.; Iqbal, M.A.; Sytar, O.; Yildirim, M.; Meena, R.S.; et al. Wheat (Triticum aestivum L.) production under drought and heat stress–adverse effects, mechanisms and mitigation: A review. App. Ecol. Env. Res 2019, 8307–8332. [Google Scholar] [CrossRef]
- Peleg, Z.; Fahima, T.; Korol, A.B.; Abbo, S.; Saranga, Y. Genetic analysis of wheat domestication and evolution under domestication. J. Exp. Bot. 2011, 62, 5051–5061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed, A.; Raza, T.; Bhatti, T.T.; Eash, N.S. Climate impacts on the agricultural sector of Pakistan: Risks and solutions. Environ. Chall. 2022, 6, 100433. [Google Scholar] [CrossRef]
- Chen, X.; Min, D.; Yasir, T.A.; Hu, Y.G. Field crops research evaluation of 14 morphological, yield-related and physiological traits as indicators of drought tolerance in Chinese winter bread wheat revealed by analysis of the membership function value of drought tolerance (MFVD). Field Crop. Res. 2012, 137, 195–201. [Google Scholar] [CrossRef]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osorio, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field? Photosynthesis and growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef] [Green Version]
- Perveen, R.; Jamil, Y.; Ashraf, M.; Ali, Q.; Iqbal, M.; Ahmad, M.R. He–Ne laser induced improvement in biochemical, physiological, growth and yield characteristics in sunflower (Helianthus annuus L.). Photochem. Photobiol. 2011, 87, 1453–1463. [Google Scholar] [CrossRef]
- Chen, Z.; Li, P.; Jiang, S.; Chen, H.; Wang, J.; Cao, C. Evaluation of resource and energy utilization, environmental and economic benefits of rice water-saving irrigation technologies in a rice-wheat rotation system. Sci. Total Environ. 2021, 757, 143748. [Google Scholar] [CrossRef]
- Qiu, Z.; He, Y.; Zhang, Y.; Guo, J.; Wang, L. Characterization of miRNAs and their target genes in He–Ne laser pretreated wheat seedlings exposed to drought stress. Ecotoxicol Environ. Saf. 2018, 164, 611–617. [Google Scholar] [CrossRef]
- Qiu, Z.; Yuan, M.; He, Y.; Li, Y.; Zhang, L. Physiological and transcriptome analysis of He–Ne laser pretreated wheat seedlings in response to drought stress. Sci. Rep. 2017, 7, 6108. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.I.; Gaafar, A.A.; Metwally, S.A.; Habba, I.E. The reactive influences of pre-sowing He–Ne laser seed irradiation and drought stress on growth, fatty acids, phenolic ingredients, and antioxidant properties of Celosia argentea. Sci. Hort. 2020, 261, 108989. [Google Scholar] [CrossRef]
- Lopes, D.M.; Walford, N.; Viana, H.; Sette Junior, C.R. A proposed methodology for the correction of the leaf area index measured with a ceptometer for Pinus and Eucalyptus forests. Revista Arvore 2016, 40, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Arnon, D.L. Copper enzymes in isolation chloroplast. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Davis, B.H. Analysis of carotenoid pigments. In Chemistry and Biochemistry of Plant Pigments; Goodwin, T.W., Ed.; Academic Press: London, UK, 1965; pp. 489–532. [Google Scholar]
- Krizek, D.T.; Kramer, G.F.; Upadhyaya, A.; Mirecki, R.M. UV-B Response of cucumber seedling grown under metal halid and high pressure sodium/deluxe lamps. Physiol. Plant. 1993, 88, 350–358. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Biochem. Anal. 1955, 1, 357–424. [Google Scholar]
- Spitz, D.R.; Oberley, L.W. Measurement of Mn SOD and Cu Zn SOD activity in mammalian tissue homogenates. Curr. Protoc. Toxicol. 2001, 8, 7.5.1–7.5.11. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormod, D.P. Ultraviolet-B radiation and ozone-induced biochemical changes in the antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Dubois, M.; Gilles, K.; Hammiltron, J.K.; Robers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167–168. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Van Slyke, D.D. Amino acid determination with ninhydrin. J. Biol. Chem. 1943, 150, 231–233. [Google Scholar] [CrossRef]
- Wolf, B. An improved universal extracting solution and its use for diagnosing soil fertility. Commun. Soil Sci. Plant Anal. 1982, 13, 1005–1033. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods; Iowa State University Press: Ames, IA, USA, 1980. [Google Scholar]
- Krawiec, M.; Dziewulska-Hunek, A.; Kornarzynski, K. The use of physical factors for seed quality improvement of horticultural plants. J. Hort. Res. 2018, 26, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Metwally, S.A.; Mohamed, S.L.M.; Abou-Leila, B.H.; Aly, M.S. Effect of drought stress and helium neon (He–Ne) laser rays on growth, oil yield and fatty acids content in Caster bean (Ricinus communis L.). Agr. Forest. Fish. 2014, 3, 203–208. [Google Scholar]
- Korrani, M.F.; Amooaghaie, R.; Ahadi, A. He–Ne Laser enhances seed germination and salt acclimation in Salvia officinalis seedlings in a manner dependent on phytochrome and H2O2. Protoplasma 2022, 1–14. [Google Scholar]
- Rybinski, W.; Garczynski, S. Influence of laser light on leaf area and parameters of photosynthetic activity in DH lines of spring barley [Hordeum vulgare L.]. Int. Agrophys. 2004, 18, 261–267. [Google Scholar]
- Al-Sherbini, A.; El-Gawad, H.A.; Kamal, M. Potential of He–Ne laser irradiation and iron nanoparticles to increase growth and yield of pea. Nanotechnology 2015, 3, 4. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Tashish, W.; Al-Osaif, S.S.; Atif, M. Effects of He–Ne laser and argon laser irradiation on growth, germination, and physico-biochemical characteristics of wheat seeds (Triticum aestivum L.). Laser Phy. 2018, 29, 015602. [Google Scholar] [CrossRef]
- Zhu, M.; Duan, X.; Zeng, Q.; Liu, Y.; Qiu, Z. He–Ne laser irradiation ameliorates cadmium toxicity in wheat by modulating cadmium accumulation, nutrient uptake and antioxidant defense system. Ecotoxicol. Environ. Safet. 2022, 236, 113477. [Google Scholar] [CrossRef]
- Kataria, S.; Guruprasad, K.N.; Ahuja, S.; Singh, B. Enhancement of growth, photosynthetic performance and yield by exclusion of ambient UV components in C3 and C4 plants. J. Photochem. Photobiol. B Biol. 2013, 127, 140–152. [Google Scholar] [CrossRef]
- Abou-Dahabe, A.D.M.; Mohammed, T.A.; Heikal, A.A.; Taha, L.S.; Gabr, A.M.; Metwally, S.A.; Ali, A.I. In vitro laser radiation induces mutation and growth in Eustoma grandiflorum plant. Bull. Natl. Res. Cent. 2019, 43, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Rosema, A.; Snel, J.F.H.; Zahn, H.; Buurmeijer, W.F.; Van Hove, L.W.A. The relation between laser-induced chlorophyll fluorescence and photosynthesis. Remote Sens. Environ. 1998, 65, 143–154. [Google Scholar] [CrossRef]
- Sarwar, M.; Saleem, M.F.; Najeeb, U.; Shakeel, A.; Ali, S.; Bilal, M.F. Hydrogen peroxide reduces heat-induced yield losses in cotton (Gossypium hirsutum L.) by protecting cellular membrane damage. J. Agron. Crop Sci. 2017, 203, 429–441. [Google Scholar] [CrossRef]
- Chen, Y.P.; Liu, Q. Effect of laser irradiation and ethylene on chilling tolerance of wheat seedlings. Russ. J. Plant Physiol. 2015, 62, 299–306. [Google Scholar] [CrossRef]
- Sacala, E.; Demczuk, A.; Grzys, E.; Prosba-Bialczyk, U.; Szajsner, H. Effect of laser-and hydropriming of seeds on some physiological parameters in sugar beet. J. Elementol. 2016, 21, 527–538. [Google Scholar] [CrossRef]
- An, J.; Hu, P.; Li, F.; Wu, H.; Shen, Y.; White, J.C.; Tian, X.; Li, Z.; Giraldo, J.P. Emerging investigator series: Molecular mechanisms of plant salinity stress tolerance improvement by seed priming with cerium oxide nanoparticles. Environ. Sci. Nano 2020, 7, 2214–2228. [Google Scholar] [CrossRef]
- Podleśny, J.; Stochmal, A.; Podleśna, A.; Misiak, L.E. Effect of laser light treatment on some biochemical and physiological processes in seeds and seedlings of white lupine and faba bean. Plant Growth Regul. 2012, 67, 227–233. [Google Scholar] [CrossRef]
- Jamil, Y.; Perveen, R.; Ashraf, M.; Ali, Q.; Iqbal, M.; Ahmad, M.R. He–Ne laser-induced changes in germination, thermodynamic parameters, internal energy, enzyme activities and physiological attributes of wheat during germination and early growth. Laser Phys. Lett. 2013, 10, 045606. [Google Scholar] [CrossRef]
- Nadimi, M.; Sun, D.W.; Paliwal, J. Recent applications of novel laser techniques for enhancing agricultural production. Laser Phys. 2021, 31, 053001. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslam, H.; Ahmad, M.S.A.; Alvi, A.K.; Rani, W.; Athar, H.-u.-R.; Al-Ashkar, I.; Almutairi, K.F.; Ullah, N.; Ayman, E.-S. He–Ne Laser Priming Enhances Drought Tolerance in Wheat through Differential Modification of Photosynthetic Pigments and Antioxidative Enzymes. Agronomy 2022, 12, 2376. https://doi.org/10.3390/agronomy12102376
Aslam H, Ahmad MSA, Alvi AK, Rani W, Athar H-u-R, Al-Ashkar I, Almutairi KF, Ullah N, Ayman E-S. He–Ne Laser Priming Enhances Drought Tolerance in Wheat through Differential Modification of Photosynthetic Pigments and Antioxidative Enzymes. Agronomy. 2022; 12(10):2376. https://doi.org/10.3390/agronomy12102376
Chicago/Turabian StyleAslam, Hamza, Muhammad Sajid Aqeel Ahmad, Ambreen Khadija Alvi, Wasifa Rani, Habib-ur-Rehman Athar, Ibrahim Al-Ashkar, Khalid F. Almutairi, Najeeb Ullah, and El-Sabagh Ayman. 2022. "He–Ne Laser Priming Enhances Drought Tolerance in Wheat through Differential Modification of Photosynthetic Pigments and Antioxidative Enzymes" Agronomy 12, no. 10: 2376. https://doi.org/10.3390/agronomy12102376
APA StyleAslam, H., Ahmad, M. S. A., Alvi, A. K., Rani, W., Athar, H.-u.-R., Al-Ashkar, I., Almutairi, K. F., Ullah, N., & Ayman, E.-S. (2022). He–Ne Laser Priming Enhances Drought Tolerance in Wheat through Differential Modification of Photosynthetic Pigments and Antioxidative Enzymes. Agronomy, 12(10), 2376. https://doi.org/10.3390/agronomy12102376







