CCN1/Integrin α5β1 Instigates Free Fatty Acid-Induced Hepatocyte Lipid Accumulation and Pyroptosis through NLRP3 Inflammasome Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Animal Procedures
2.3. Reagents
2.4. Transfection
2.5. RNA Extraction and Reverse Transcriptase qPCR (RT-qPCR)
2.6. Immunoblotting and Immunoprecipitation
2.7. ELISA
2.8. Oil Red O Staining
2.9. Statistical Analysis
3. Results
3.1. CCN1 Is Upregulated in the Livers of Obese Mice and in FFA-Treated Hepatocytes
3.2. Activation of the NLRP3 Inflammasome by FFA Is Reduced by CCN1 Silencing
3.3. FFA-Induced Lipid Accumulation and Pyroptosis Are Attenuated by CCN1 Silencing in Hepatocytes
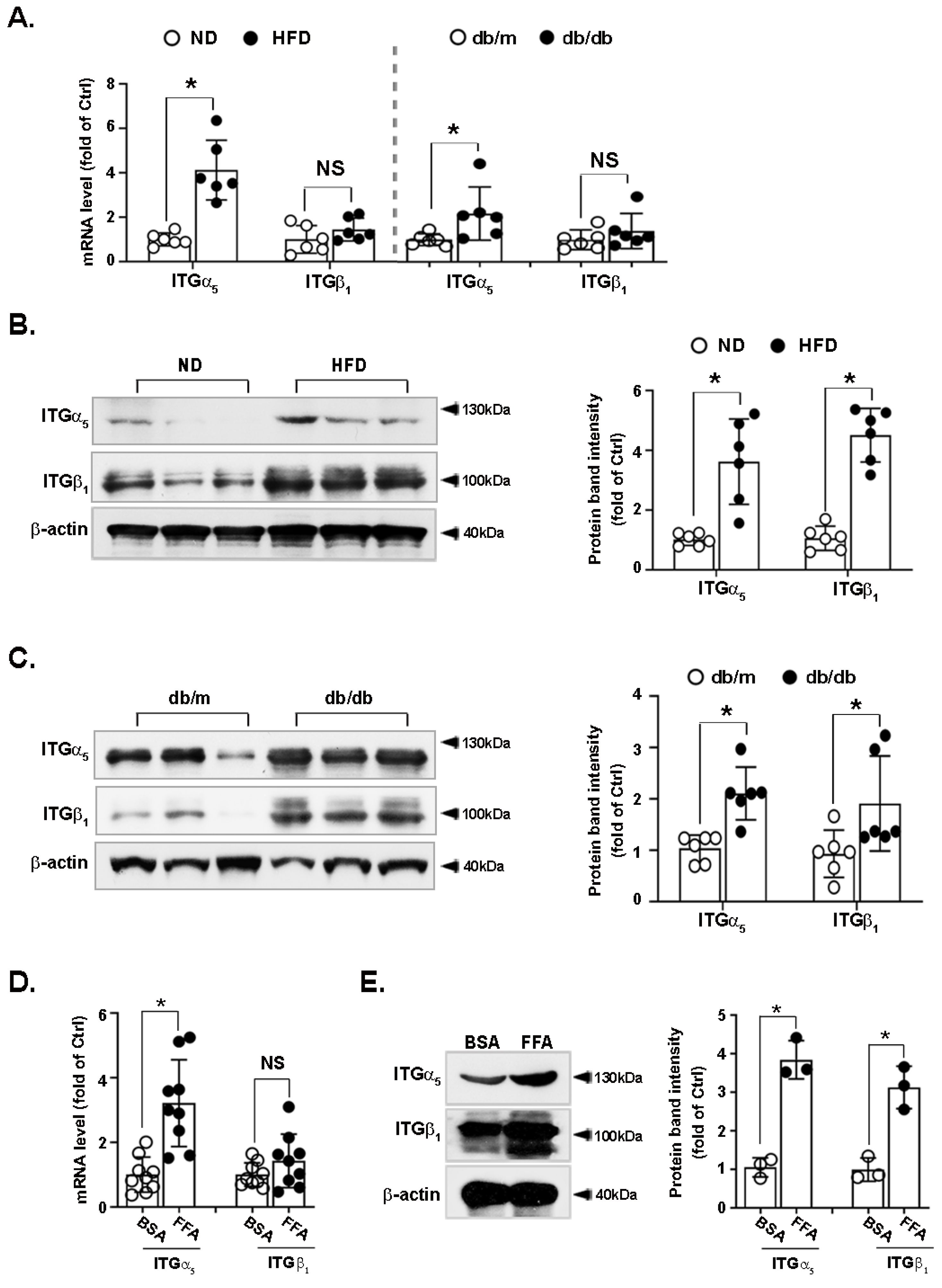
3.4. Integrin α5β1 Is Upregulated in the Liver of Obese Mice and FFA-Treated Hepatocytes
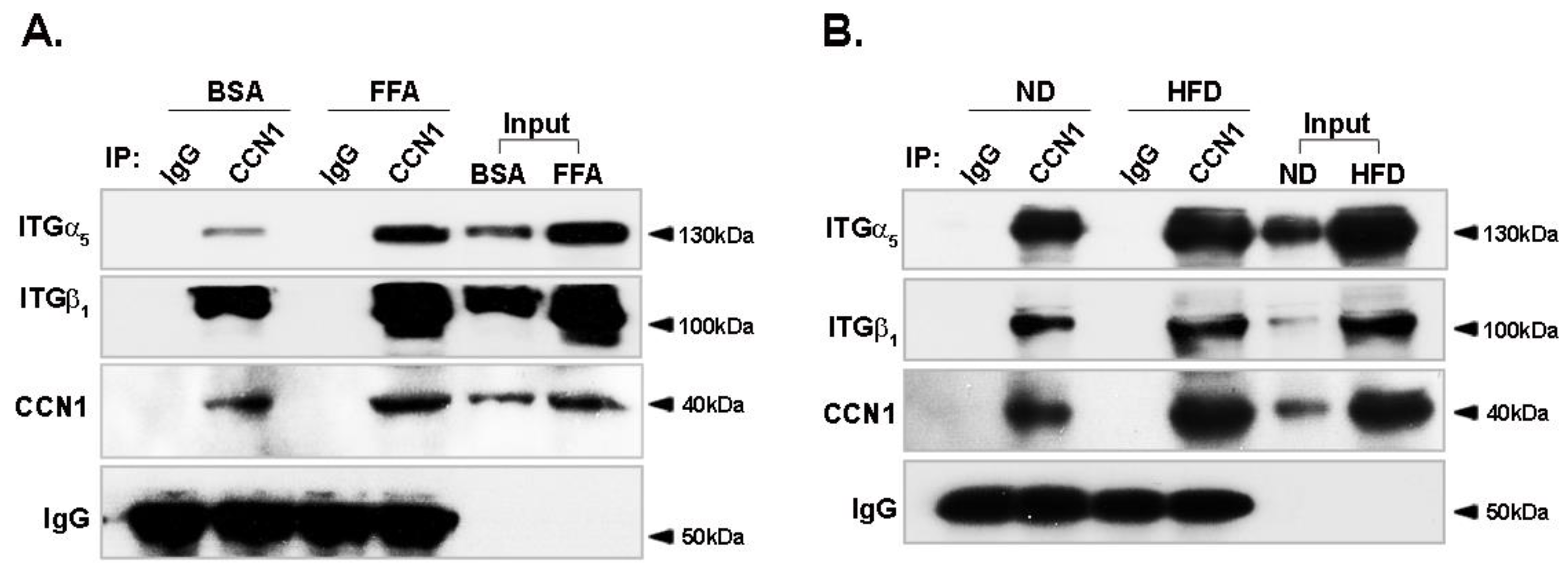
3.5. Binding of CCN1 to Integrin α5β1 Is Enhanced in Hepatocytes
3.6. FFA-Activated NLRP3 Inflammasome Is Reduced by Integrin α5β1 Silencing
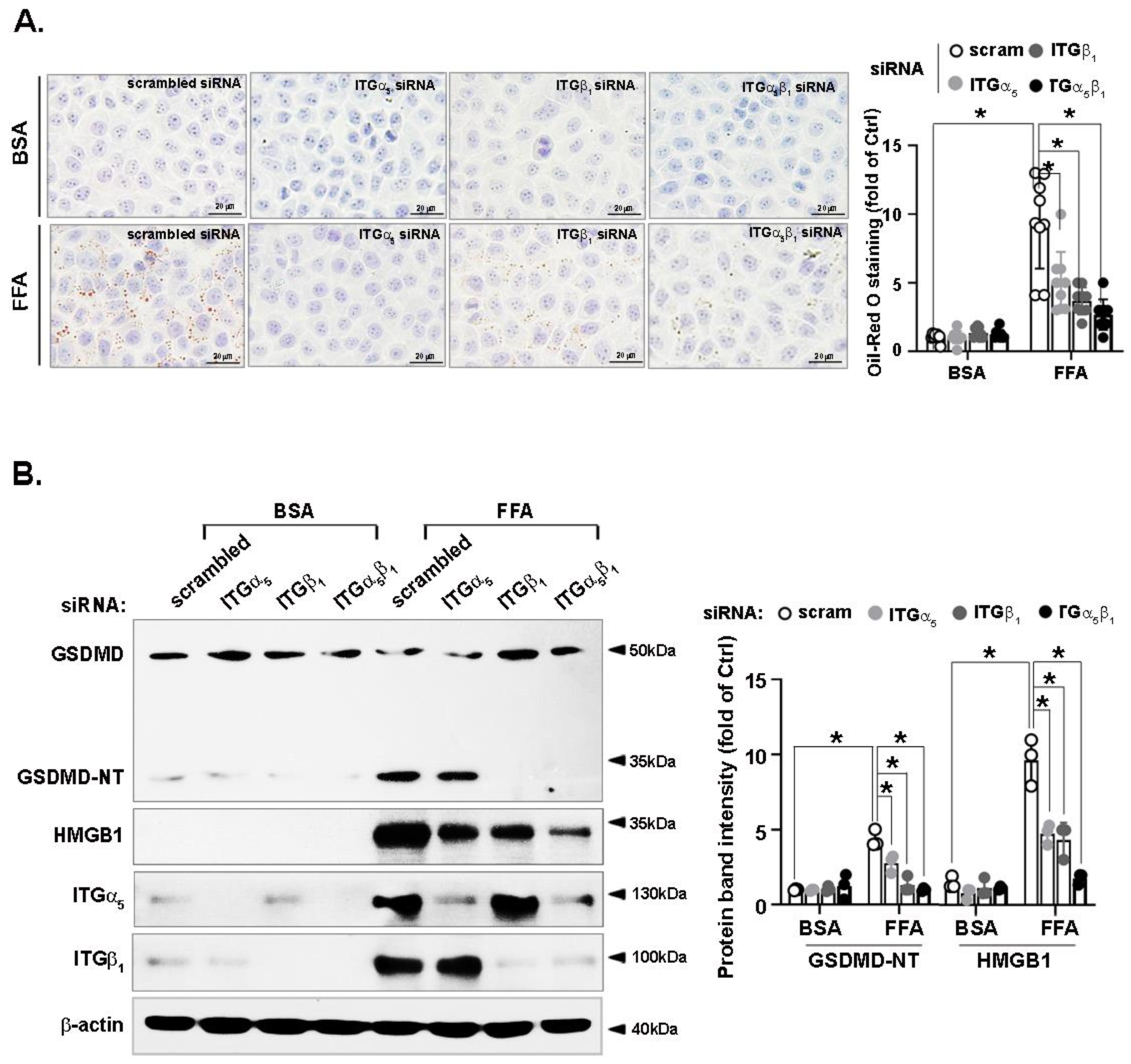
3.7. FFA-Induced Lipid Accumulation and Pyroptosis Are Attenuated by Integrin α5β1 Silencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finke, D.; Heckmann, M.B.; Frey, N.; Lehmann, L.H. Cancer-A Major Cardiac Comorbidity with Implications on Cardiovascular Metabolism. Front. Physiol. 2021, 12, 729713. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridi, A.; Chrysavgis, L.; Koutsilieris, M.; Chatzigeorgiou, A. The Role of Senescence in the Development of Nonalcoholic Fatty Liver Disease and Progression to Nonalcoholic Steatohepatitis. Hepatology 2020, 71, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Gurcel, L.; Abrami, L.; Girardin, S.; Tschopp, J.; van der Goot, F.G. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 2006, 126, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Gross, O.; Thomas, C.J.; Guarda, G.; Tschopp, J. The inflammasome: An integrated view. Immunol. Rev. 2011, 243, 136–151. [Google Scholar] [CrossRef]
- Wree, A.; Eguchi, A.; McGeough, M.D.; Pena, C.A.; Johnson, C.D.; Canbay, A.; Hoffman, H.M.; Feldstein, A.E. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 2014, 59, 898–910. [Google Scholar] [CrossRef]
- Li, Z.Q.; Wu, W.R.; Zhao, C.; Zhang, X.L.; Yang, Z.; Pan, J.; Si, W.K. CCN1/Cyr61 enhances the function of hepatic stellate cells in promoting the progression of hepatocellular carcinoma. Int. J. Mol. Med. 2018, 41, 1518–1528. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Jiang, C.; Yuan, T.; Ma, H. Cellular communication network factor 1 (CCN1) knockdown exerts a protective effect for hepatic ischemia/reperfusion injury by deactivating the MEK/ERK pathway. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101737. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, Y.; Xu, W.; Wang, X.; Jin, H.; Bao, X.; Lu, C. Induction of Sestrin2 by pterostilbene suppresses ethanol-triggered hepatocyte senescence by degrading CCN1 via p62-dependent selective autophagy. Cell Biol. Toxicol. 2021, 1–21. [Google Scholar] [CrossRef]
- Bian, Z.; Peng, Y.; You, Z.; Wang, Q.; Miao, Q.; Liu, Y.; Han, X.; Qiu, D.; Li, Z.; Ma, X. CCN1 expression in hepatocytes contributes to macrophage infiltration in nonalcoholic fatty liver disease in mice. J. Lipid Res. 2013, 54, 44–54. [Google Scholar] [CrossRef]
- Kok, S.H.; Lin, L.D.; Hou, K.L.; Hong, C.Y.; Chang, C.C.; Hsiao, M.; Wang, J.H.; Lai, E.H.; Lin, S.K. Simvastatin inhibits cysteine-rich protein 61 expression in rheumatoid arthritis synovial fibroblasts through the regulation of sirtuin-1/FoxO3a signaling. Arthritis Rheum. 2013, 65, 639–649. [Google Scholar] [CrossRef]
- Lau, L.F. Cell surface receptors for CCN proteins. J. Cell Commun. Signal. 2016, 10, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Sun, Y.; Xue, H.; Chen, L.; Gu, C.; Shao, J.; Lu, R.; Luo, X.; Wei, J.; Ma, X.; et al. CCN1 promotes hepatic steatosis and inflammation in non-alcoholic steatohepatitis. Sci. Rep. 2020, 10, 3201. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Twigg, S.M. Regulation and bioactivity of the CCN family of genes and proteins in obesity and diabetes. J. Cell Commun. Signal. 2018, 12, 359–368. [Google Scholar] [CrossRef]
- Guo, Q.; Furuta, K.; Lucien, F.; Sanchez, L.H.; Hirsova, P.; Krishnan, A.; Kabashima, A.; Pavelko, K.D.; Madden, B.; Alhuwaish, H.; et al. Integrin beta1-enriched extracellular vesicles mediate monocyte adhesion and promote liver inflammation in murine NASH. J. Hepatol. 2019, 71, 1193–1205. [Google Scholar] [CrossRef]
- Schuppan, D.; Surabattula, R.; Wang, X.Y. Determinants of fibrosis progression and regression in NASH. J. Hepatol. 2018, 68, 238–250. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, W.; Xiang, J.; Liu, P.; Ke, M.; Wang, B.; Wu, R.; Lv, Y. Collagen I promotes hepatocellular carcinoma cell proliferation by regulating integrin beta1/FAK signaling pathway in nonalcoholic fatty liver. Oncotarget 2017, 8, 95586–95595. [Google Scholar] [CrossRef]
- Rai, R.P.; Liu, Y.; Iyer, S.S.; Liu, S.; Gupta, B.; Desai, C.; Kumar, P.; Smith, T.; Singhi, A.D.; Nusrat, A.; et al. Blocking integrin alpha4beta7-mediated CD4 T cell recruitment to the intestine and liver protects mice from western diet-induced non-alcoholic steatohepatitis. J. Hepatol. 2020, 73, 1013–1022. [Google Scholar] [CrossRef]
- Bailey, W.P.; Cui, K.; Ardell, C.L.; Keever, K.R.; Singh, S.; Rodriguez-Gil, D.J.; Ozment, T.R.; Williams, D.L.; Yakubenko, V.P. Frontline Science: The expression of integrin alphaD beta2 (CD11d/CD18) on neutrophils orchestrates the defense mechanism against endotoxemia and sepsis. J. Leukoc. Biol. 2021, 109, 877–890. [Google Scholar] [CrossRef]
- Shang, Y.; Jiang, M.; Chen, N.; Jiang, X.L.; Zhan, Z.Y.; Zhang, Z.H.; Zuo, R.M.; Wang, H.; Lan, X.Q.; Ren, J.; et al. Inhibition of HMGB1/TLR4 Signaling Pathway by Digitoflavone: A Potential Therapeutic Role in Alcohol-Associated Liver Disease. J. Agric. Food Chem. 2022, 70, 2968–2983. [Google Scholar] [CrossRef]
- Boden, G. Obesity and free fatty acids. Endocrinol. Metab. Clin. N. Am. 2008, 37, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.; Owens, D.; Collins, P.; Johnson, A.; Tomkin, G.H. The fatty acid distribution in low density lipoprotein in diabetes. Biochim. Biophys Acta 1999, 1439, 110–116. [Google Scholar] [CrossRef]
- Rinne, P.; Guillamat-Prats, R.; Rami, M.; Bindila, L.; Ring, L.; Lyytikäinen, L.-P.; Raitoharju, E.; Oksala, N.; Lehtimäki, T.; Weber, C.; et al. Palmitoylethanolamide Promotes a Proresolving Macrophage Phenotype and Attenuates Atherosclerotic Plaque Formation. Arter. Thromb. Vasc. Biol 2018, 38, 2562–2575. [Google Scholar] [CrossRef]
- Abdelmagid, S.A.; Clarke, S.E.; Nielsen, D.E.; Badawi, A.; El-Sohemy, A.; Mutch, D.M.; Ma, D.W.L. Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults. PLoS ONE 2015, 10, e0116195. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.A.; Carretta, M.D.; Burgos, R.A. Long Chain Fatty Acids as Modulators of Immune Cells Function: Contribution of FFA1 and FFA4 Receptors. Front. Physiol. 2021, 12, 668330. [Google Scholar] [CrossRef]
- Latz, E.; Xiao, T.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Yan, M.; Li, Y.; Luo, Q.; Zeng, W.; Shao, X.; Li, L.; Wang, Q.; Wang, D.; Zhang, Y.; Diao, H.; et al. Mitochondrial damage and activation of the cytosolic DNA sensor cGAS-STING pathway lead to cardiac pyroptosis and hypertrophy in diabetic cardiomyopathy mice. Cell Death Discov. 2022, 8, 258. [Google Scholar] [CrossRef]
- Rampanelli, E.; Orsó, E.; Ochodnicky, P.; Liebisch, G.; Bakker, P.J.; Claessen, N.; Butter, L.M.; Weerman, M.A.V.D.B.; Florquin, S.; Schmitz, G.; et al. Metabolic injury-induced NLRP3 inflammasome activation dampens phospholipid degradation. Sci. Rep. 2017, 7, 2861. [Google Scholar] [CrossRef]
- Zeng, X.; Zhu, M.; Liu, X.; Chen, X.; Yuan, Y.; Li, L.; Liu, J.; Lu, Y.; Cheng, J.; Chen, Y. Oleic acid ameliorates palmitic acid induced hepatocellular lipotoxicity by inhibition of ER stress and pyroptosis. Nutr. Metab. 2020, 17, 11. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.-H.; Chen, X.-Y.; Jian-Hua, Z.; Wang, M.-X.; Jin-Sheng, L.; Zhang, Q.-Y.; Wang, W.; Wang, R.; Kang, L.-L.; et al. Reactive oxygen species-induced TXNIP drives fructose-mediated hepatic inflammation and lipid accumulation through NLRP3 inflammasome activation. Antioxid Redox Signal. 2015, 22, 848–870. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Hao, M.; Liu, Y.; Ma, X.; Lin, W.; Xu, Q.; Zhou, H.; Shao, N.; Kuang, H. Liraglutide ameliorates non-alcoholic steatohepatitis by inhibiting NLRP3 inflammasome and pyroptosis activation via mitophagy. Eur. J. Pharmacol. 2019, 864, 172715. [Google Scholar] [CrossRef]
- Zhang, N.-P.; Liu, X.-J.; Xie, L.; Shen, X.-Z.; Wu, J. Impaired mitophagy triggers NLRP3 inflammasome activation during the progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis. Lab. Investig. 2019, 99, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Chen, C.-C.; Monzon, R.I.; Lau, L.F. Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts. Mol. Cell. Biol. 2013, 33, 2078–2090. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, H.; Lu, J.; Lin, K.; Ni, J.; Wu, G.; Tang, H. Caspase-11-Mediated Hepatocytic Pyroptosis Promotes the Progression of Nonalcoholic Steatohepatitis. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Kang, R.; Tang, D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef]
- Walsh, C.T.; Radeff-Huang, J.; Matteo, R.; Hsiao, A.; Subramaniam, S.; Stupack, D.; Brown, J.H. Thrombin receptor and RhoA mediate cell proliferation through integrins and cysteine-rich protein 61. FASEB J. 2008, 22, 4011–4021. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Li, Y.; Tai, R.; Sun, Z.; Wu, Q.; Liu, Y.; Sun, C. Collagen XV Promotes ER Stress-Induced Inflammation through Activating Integrin beta1/FAK Signaling Pathway and M1 Macrophage Polarization in Adipose Tissue. Int. J. Mol. Sci. 2021, 22, 9997. [Google Scholar] [CrossRef]
- Takano, M.; Hirose, N.; Sumi, C.; Yanoshita, M.; Nishiyama, S.; Onishi, A.; Asakawa, Y.; Tanimoto, K. ANGPTL2 Promotes Inflammation via Integrin alpha5beta1 in Chondrocytes. Cartilage 2021, 13 (Suppl. 2), 885S–897S. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, X.; Ding, J.; Pan, Q.; Zheng, M.-H.; Liu, W.-Y.; Luo, G.; Qu, J.; Li, M.; Li, L.; et al. SEMA7AR148W mutation promotes lipid accumulation and NAFLD progression via increased localization on the hepatocyte surface. JCI Insight 2022, 7, e154113. [Google Scholar] [CrossRef]
- Campden, R.I.; Warren, A.L.; Greene, C.J.; Chiriboga, J.A.; Arnold, C.R.; Aggarwal, D.; McKenna, N.; Sandall, C.F.; MacDonald, J.A.; Yates, R.M. Extracellular cathepsin Z signals through the alpha5 integrin and augments NLRP3 inflammasome activation. J. Biol. Chem. 2022, 298, 101459. [Google Scholar] [CrossRef]
- Rohn, F.; Kordes, C.; Buschmann, T.; Reichert, D.; Wammers, M.; Poschmann, G.; Stühler, K.; Benk, A.S.; Geiger, F.; Spatz, J.S.; et al. Impaired integrin alpha5/beta1-mediated hepatocyte growth factor release by stellate cells of the aged liver. Aging Cell 2020, 19, e13131. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Lv, X.P.; Li, S.; Bai, B.; Zhan, L.L. Synergy of urokinasetype plasminogen activator receptor isomer (D1D2) and integrin alpha5beta1 causes malignant transformation of hepatic cells and the occurrence of liver cancer. Mol. Med. Rep. 2014, 10, 2568–2574. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Xin, X.; Ma, J.; Tan, C.; Osna, N.; Mahato, R.I. Therapeutic targets, novel drugs, and delivery systems for diabetes associated NAFLD and liver fibrosis. Adv. Drug Deliv. Rev. 2021, 176, 113888. [Google Scholar] [CrossRef] [PubMed]
- Kazankov, K.; Jørgensen, S.M.D.; Thomsen, K.L.; Møller, H.J.; Vilstrup, H.; George, J.; Schuppan, D.; Grønbæk, H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol 2019, 16, 145–159. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Q.; Liu, J.; Cui, Q.; Jiang, T.; Xie, X.; Du, X.; Zhao, Z.; Lai, B.; Xiao, L.; Wang, N. CCN1/Integrin α5β1 Instigates Free Fatty Acid-Induced Hepatocyte Lipid Accumulation and Pyroptosis through NLRP3 Inflammasome Activation. Nutrients 2022, 14, 3871. https://doi.org/10.3390/nu14183871
Yao Q, Liu J, Cui Q, Jiang T, Xie X, Du X, Zhao Z, Lai B, Xiao L, Wang N. CCN1/Integrin α5β1 Instigates Free Fatty Acid-Induced Hepatocyte Lipid Accumulation and Pyroptosis through NLRP3 Inflammasome Activation. Nutrients. 2022; 14(18):3871. https://doi.org/10.3390/nu14183871
Chicago/Turabian StyleYao, Qinyu, Jia Liu, Qi Cui, Tingting Jiang, Xinya Xie, Xiong Du, Ziwei Zhao, Baochang Lai, Lei Xiao, and Nanping Wang. 2022. "CCN1/Integrin α5β1 Instigates Free Fatty Acid-Induced Hepatocyte Lipid Accumulation and Pyroptosis through NLRP3 Inflammasome Activation" Nutrients 14, no. 18: 3871. https://doi.org/10.3390/nu14183871
APA StyleYao, Q., Liu, J., Cui, Q., Jiang, T., Xie, X., Du, X., Zhao, Z., Lai, B., Xiao, L., & Wang, N. (2022). CCN1/Integrin α5β1 Instigates Free Fatty Acid-Induced Hepatocyte Lipid Accumulation and Pyroptosis through NLRP3 Inflammasome Activation. Nutrients, 14(18), 3871. https://doi.org/10.3390/nu14183871





