A Collection of Molecular Fingerprints of Single Aerosol Particles in Air for Potential Identification and Detection Using Optical Trapping-Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. OT-RS Setup
2.2. Trapping Forces
2.3. Sample Materials
3. Results and Discussion
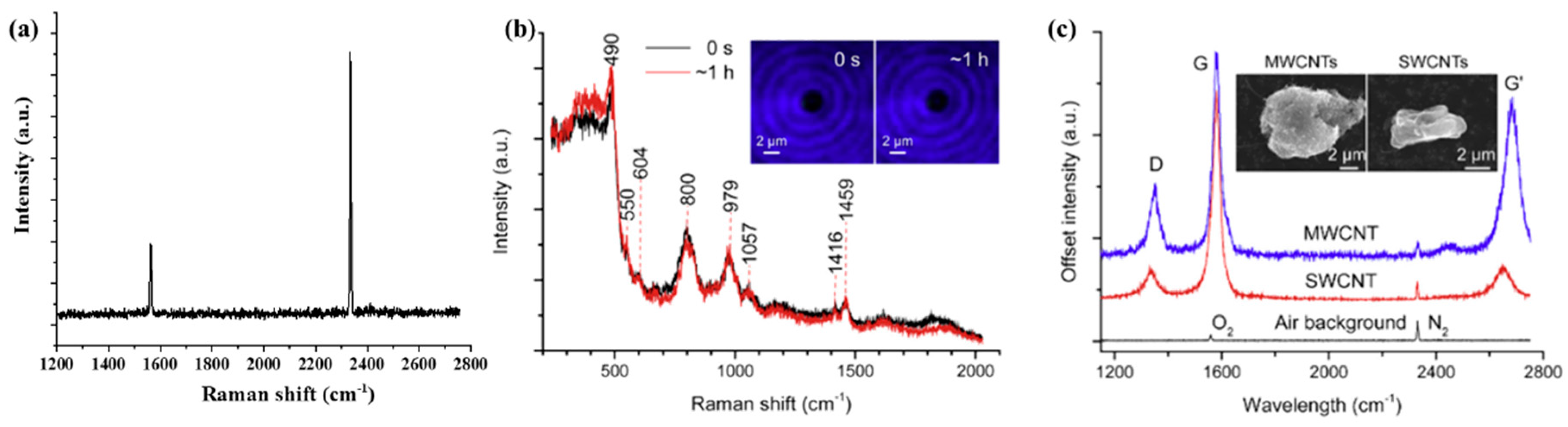
3.1. Single-Particle Raman Spectra of Standard Samples (Validation of the OT-RS System)
3.2. Raman Spectral Fingerprints of Single Bioaerosol Particles
3.2.1. Amino Acid
Glycine
L-glutamic
L-threonine
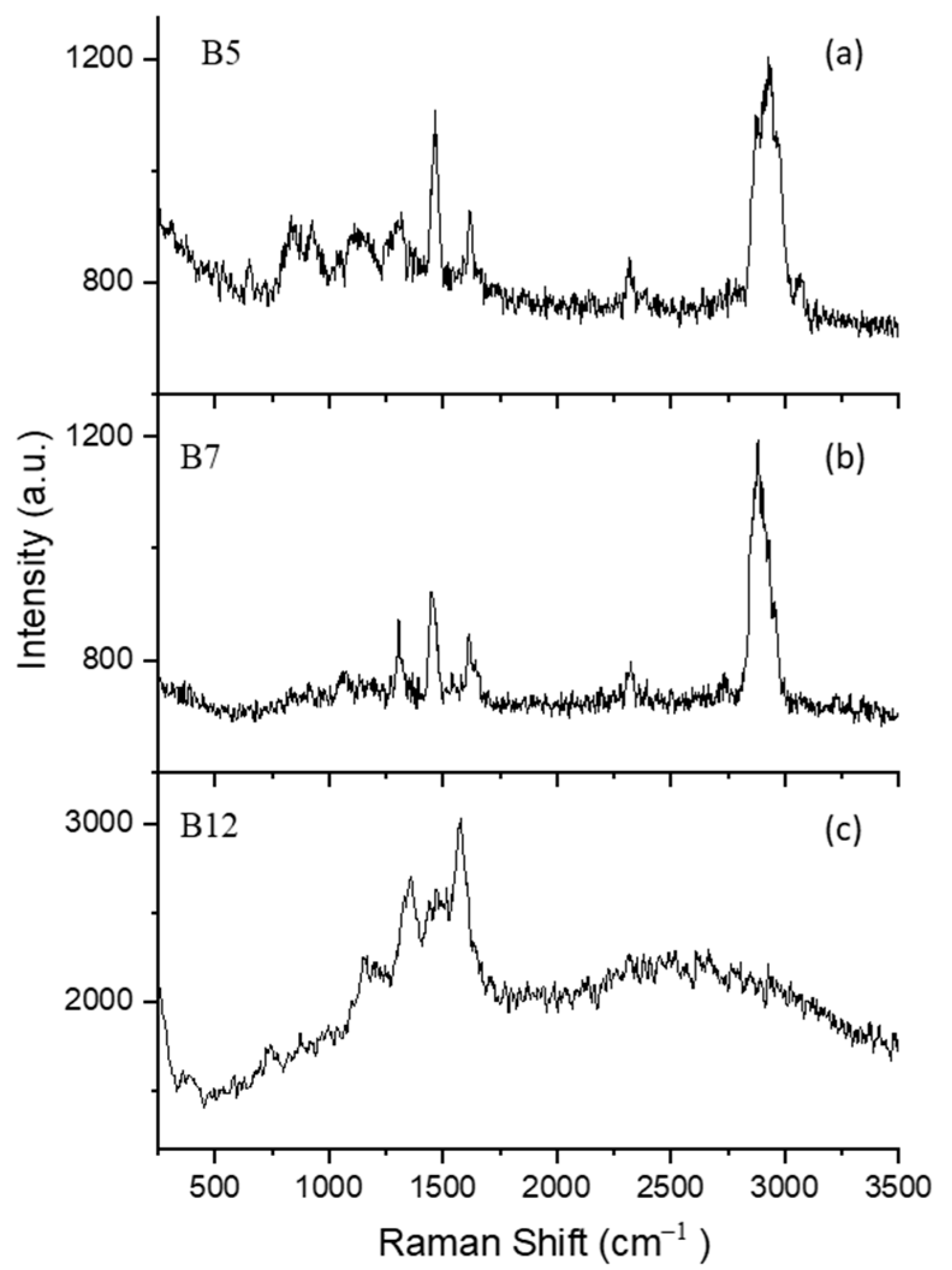
3.2.2. B Vitamins
B5 (D-Pantothenic Acid Hemicalcium Salt)
B7 (Biotin)
B12 (Cyanocobalamin)
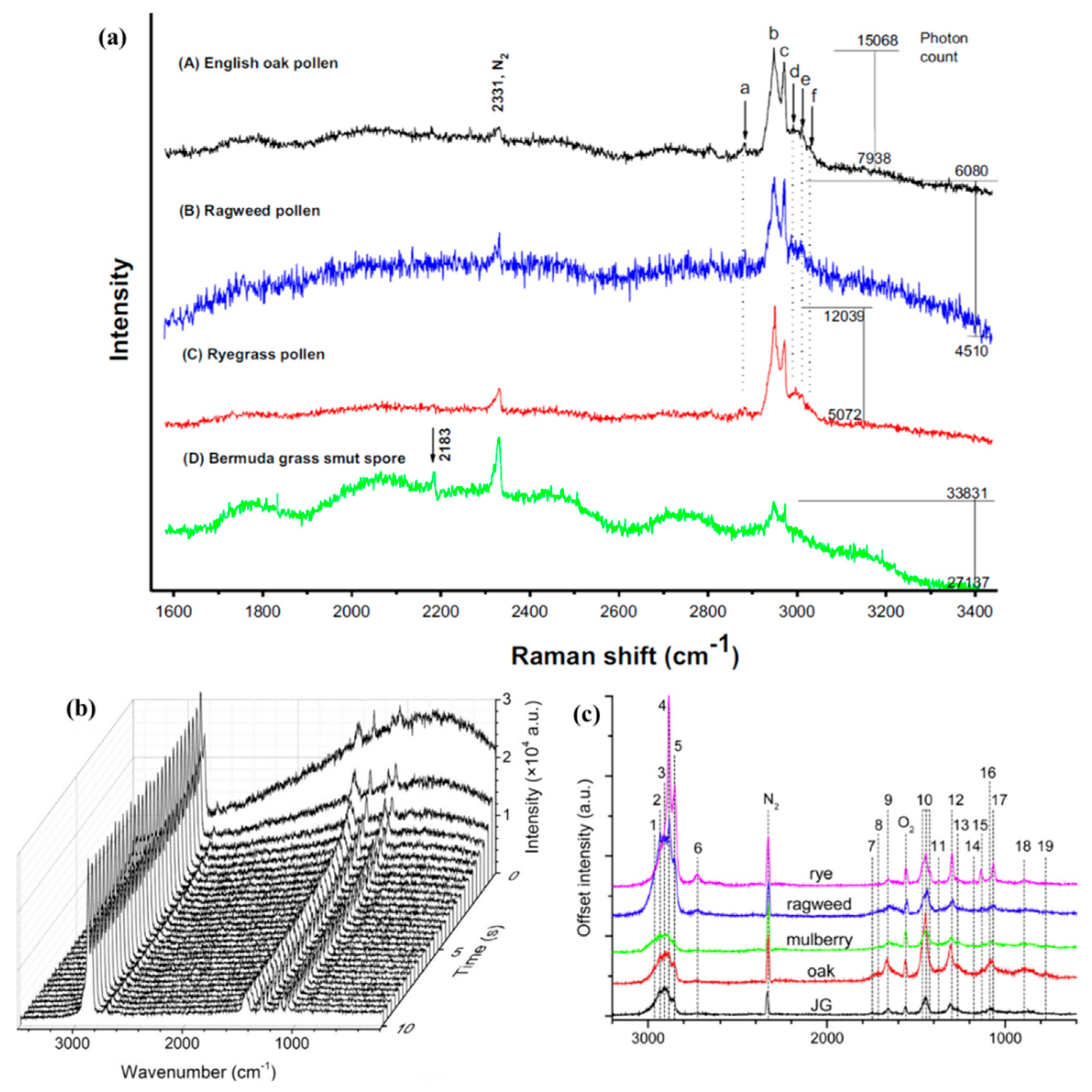
3.2.3. Pollens and Spores
3.2.4. Aerosolized Biowarfare Agent (BWA) Surrogates
3.2.5. Fungal Particles
3.3. Raman Spectral Fingerprints of Extraterrestrial and Terrestrial Dust Simulants
4. Future OT-RS for Potential Identification and Detection of Single Airborne Particles
- It helps minimize particle surface contamination, modification, and interaction with its surroundings.
- It helps mitigate background fluorescence interference via photo-bleaching and the WGM resonance effect [50].
- Measured data reveal temporal information about a particle in a simulated atmospheric state while the particle is being measured.
- It has high temporal and spatial resolutions (µs, and 300 nm × 300 nm), which facilitate the study of surface properties of a particle and its time-evolution.
- Integrated with an imaging system, it can concurrently monitor changes in physical and chemical properties of single particles in near-real time.
- It requires minimal volume of samples, essentially a single particle, which is critically important in detecting or sensing airborne particles in low concentration, e.g., life-threatening viruses and aerosolized BWA particles spread in air.
- A large, comprehensive database of Raman spectral features is needed. Although Raman spectra of most small molecules or chemical function groups have been established to date, the spectral data have been obtained predominantly using bulk samples and such samples are placed on a substrate. As a result, the specificity of Raman spectral features (relative peak intensity patterns, band structures, spectral overlap, etc.) for individual airborne particles is low and the fluorescence interference may hinder Raman-band assignments. For example, the formation and loss single airborne aerosol particles, which cannot be found by the averaged information from the Raman spectra from bulk measurements, can be characterized by the single-particle Raman spectra via observation of specific or signature Raman band(s) or the relative peak intensity patterns of the particle in each growth/loss phase [43]. On the other hand, in the atmosphere, the surface of each aerosol particle is chemically heterogeneous. Chemical reagents resulting from air pollution process (e.g., contamination) and the solar/UV radiation (e.g., photolytic reactions) can aggressively change the properties of the particle’s surface [51]. These changes cannot be characterized using measurements from bulk materials because the changes can be highly time and particle dependent and can be easily overwhelmed by the averaged Raman signal from the bulk materials. Finally, for some bioaerosol particles that usually contain some fluorescent compounds, the OT-RS technique can effectively mitigate the background fluorescence interference via the efficient photobleaching effect from the optical trap [50]. This point fundamentally differentiates the OT-RS from the bulk-sample RS. Therefore, the existing Raman spectra database cannot replace the single-particle Raman spectral database. Although the collection of single, airborne particle spectral features is not included in this work, only a limited number of particle types have been studied to date. Further collective efforts are needed to expand this data to include the vast number of aerosol types in existence.
- Given a sufficient database of single-particle Raman spectral features available for single-particle identification, it is necessary to develop data-processing algorithms to link measured spectral patterns to specific airborne particle or its chemical composition. Such studies are rare.
- Current studies of single particles using OT-RS are lab-based. In real-world applications, the characterization of single particles will rely on deployable, rugged, easy-to-use instruments. Development of such instrumentation is only beginning and must address several individual challenges such as automated, single-particle sample introduction, trapping efficiency and rigidness in the presence of field perturbations (airflow, vibration, etc.), as well as cost-effectiveness. Experience and knowledge derived from existing aerosol instrumentation can be helpful, yet new instrumentation schemes and innovative designs are expected in future studies.
- Like the evolution of many other technology developments, we should keep our minds open. This includes integrating OT-RS with other advanced optical and spectroscopic techniques (e.g., CRDS, LIF, ELS), as well as new data-processing algorithms (e.g., machine learning) to enhance detection specificity.
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, Y.L.; Kalume, A.; Wang, C.; Santarpia, J. Atmospheric aging processes of bioaerosols under laboratory-controlled conditions: A review. J. Aerosol Sci. 2021, 155, 105767. [Google Scholar] [CrossRef]
- Després, V.; Huffman, J.A.; Burrows, S.M.; Hoose, C.; Safatov, A.; Buryak, G.; Fröhlich-Nowoisky, J.; Elbert, W.; Andreae, M.; Pöschl, U.; et al. Primary biological aerosol particles in the atmosphere: A review. Tellus B Chem. Phys. Meteorol. 2012, 64, 15598. [Google Scholar] [CrossRef]
- Félix-Rivera, H.; Hernández-Rivera, S.P. Raman spectroscopy techniques for the detection of biological samples in suspensions and as aerosol particles: A review. Sens. Imaging 2012, 13, 1–25. [Google Scholar] [CrossRef]
- Mbareche, H.; Morawska, L.; Duchaine, C. On the interpretation of bioaerosol exposure measurements and impacts on health. J. Air Waste Manag. Assoc. 2019, 69, 789–804. [Google Scholar] [CrossRef]
- Suchorab, Z.; Frąc, Ł.M.; Guz, K.; Oszust, G.; Łagod, A.; Gryta, N.; Wielgus, B.; Czerwiński, J. A method for early detection and identification of fungal contamination of building materials using e-nose. PLoS ONE 2019, 14, e0215179. [Google Scholar] [CrossRef]
- Yoo, K.; Lee, T.K.; Choi, E.J.; Yang, J.; Shukla, S.K.; Hwang, S.; Park, J. Molecular approaches for the detection and monitoring of microbial communities in bioaerosols: A review. J. Environ. Sci. 2017, 51, 234–247. [Google Scholar] [CrossRef]
- Grewling, L.; Frątczak, A.; Kostecki, Ł.; Nowak, M.; Szymańska, A.; Bogawski, P. Biological and chemical air pollutants in an urban area of central Europe: Co-exposure assessment. Aerosol Air Qual. Res. 2019, 19, 1526–1537. [Google Scholar] [CrossRef]
- Ai, Y.; Alali, H.; Pan, Y.L.; Videen, G.; Wang, C. Single-particle optical-trapping Raman spectroscopy for the detection and identification of aerosolized airborne biological particles. Meas. Sci. Technol. 2021, 32, 055207. [Google Scholar] [CrossRef]
- Huffman, J.A.; Perring, A.E.; Savage, N.J.; Clot, B.; Crouzy, B.; Tummon, F.; Shoshanim, O.; Damit, B.; Schneider, J.; Sivaprakasam, V.; et al. Real-time sensing of bioaerosols: Review and current perspectives. Aerosol Sci. Technol. 2020, 54, 465–495. [Google Scholar] [CrossRef]
- Ault, A.P.; Axson, J.L. Atmospheric aerosol chemistry: Spectroscopic and microscopic advances. Anal. Chem. 2017, 89, 430–452. [Google Scholar] [CrossRef]
- Ziemann, P.J.; Atkinson, R. Kinetics, products, and mechanisms of secondary organic aerosol formation. Chem. Soc. Rev. 2012, 41, 6582–6605. [Google Scholar] [CrossRef] [PubMed]
- Pietrodangelo, A.; Pareti, S.; Perrino, C. Improved identification of transition metals in airborne aerosols by SEM-EDX combined backscattered and secondary electron microanalysis. Environ. Sci. Pollut. Res. 2014, 21, 4023–4031. [Google Scholar] [CrossRef] [PubMed]
- Wentzel, M.; Gorzawski, H.; Naumann, K.H.; Saathoff, H.; Weinbruch, S. Transmission electron microscopical and aerosol dynamical characterization of soot aerosols. J. Aerosol Sci. 2003, 34, 1347–1370. [Google Scholar] [CrossRef]
- Lebedev, A.T. Mass spectrometry in identification of ecotoxicants including chemical and biological warfare agents. Toxicol. Appl. Pharm. 2005, 207, 451–458. [Google Scholar] [CrossRef]
- Brostrøm, A.; Kling, K.I.; Hougaard, K.S.; Mølhave, K. Complex aerosol characterization by scanning electron microscopy coupled with energy dispersive X-ray spectroscopy. Sci. Rep. 2020, 10, 9150. [Google Scholar] [CrossRef]
- Guicheteau, J.; Christesen, S.; Emge, D.; Tripathi, A. Bacterial mixture identification using Raman and surface-enhanced Raman chemical imaging. J. Raman Spectrosc. 2010, 41, 1632–1637. [Google Scholar] [CrossRef]
- Sivaprakasam, V.; Hart, M.B. Surface-enhanced Raman spectroscopy for environmental monitoring of aerosols. ACS Omega 2021, 6, 10150–10159. [Google Scholar] [CrossRef]
- Gabbarini, V.; Rossi, R.; Ciparisse, J.F.; Malizia, A.; Divizia, A.; Filippis, P.D.; Anselmi, M.; Carestia, M.; Palombi, L.; Divizia, M.; et al. Laser-induced fluorescence (LIF) as a smart method for fast environmental virological analyses: Validation on Picornaviruses. Sci. Rep. 2019, 9, 12598. [Google Scholar] [CrossRef]
- Gery, A.; Delanoe, A.; Heutte, N.; Chosson, E.; Bonhomme, J.; Garon, D. A novel qPCR based-method for detection and quantification of three recurrent species of Penicillium isolated from bioaerosols in mold-damaged homes. J. Microbiol. Methods 2021, 186, 106236. [Google Scholar] [CrossRef]
- Santarpia, J.L.; Shumate, S.R.; Haddrell, A. Laboratory study of bioaerosols: Traditional test systems, modern approaches, and environmental control. Aerosol Sci. Technol. 2020, 54, 585–600. [Google Scholar] [CrossRef] [Green Version]
- Kinahan, S.M.; Tezak, M.S.; Siegrist, C.M.; Lucero, G.; Servantes, B.L.; Santarpia, J.L.; Kalume, A.; Zhang, J.; Felton, M.; Williamson, C.C.; et al. Changes of fluorescence spectra and viability from aging aerosolized E. coli cells under various laboratory-controlled conditions in an advanced rotating drum. Aerosol Sci. Technol. 2019, 53, 1261–1276. [Google Scholar] [CrossRef]
- Ding, J.; Dai, Q.; Zhang, Y.; Xu, J.; Huangfu, Y.; Feng, Y. Air humidity affects secondary aerosol formation in different pathways. Sci. Total Environ. 2021, 759, 143540. [Google Scholar] [CrossRef] [PubMed]
- Bzdek, B.R.; Reid, J.P.; Cotterell, M.I. Open questions on the physical properties of aerosols. Commun. Chem. 2020, 3, 105. [Google Scholar] [CrossRef]
- Estillore, A.D.; Trueblood, J.V.; Grassian, V.H. Atmospheric chemistry of bioaerosols: Heterogeneous and multiphase reactions with atmospheric oxidants and other trace gases. Chem. Sci. 2016, 7, 6604–6616. [Google Scholar] [CrossRef]
- Gong, Z.; Pan, Y.L.; Videen, G.; Wang, C. Chemical reactions of single optically trapped bioaerosols in a controlled environment. Aerosol Sci. Technol. 2019, 53, 853–859. [Google Scholar] [CrossRef]
- Krieger, U.K.; Marcolli, C.; Reid, J.P. Exploring the complexity of aerosol particle properties and processes using single particle techniques. Chem. Soc. Rev. 2012, 41, 6631–6662. [Google Scholar] [CrossRef]
- Li, W.; Shao, L.; Zhang, D.; Ro, C.U.; Hu, M.; Bi, X.; Geng, H.; Matsuki, A.; Niu, H.; Chen, J. A review of single aerosol particle studies in the atmosphere of East Asia: Morphology, mixing state, source, and heterogeneous reactions. J. Clean. Prod. 2016, 112, 1330–1349. [Google Scholar] [CrossRef]
- Buajarern, J.; Mitchem, L.; Reid, J.P. Characterizing multiphase organic/inorganic/aqueous aerosol droplets. J. Phys. Chem. A 2007, 111, 9054–9061. [Google Scholar] [CrossRef]
- Reid, J.P. Particle levitation and laboratory scattering. J. Quant. Spectrosc. Radiat. Transf. 2009, 110, 1293–1306. [Google Scholar] [CrossRef]
- Jauffred, L.; Taheri, S.M.R.; Schmitt, R.; Linke, H.; Oddershede, L.B. Optical trapping of gold nanoparticles in air. Nano. Lett. 2015, 15, 4713–4719. [Google Scholar] [CrossRef] [Green Version]
- Bowman, R.W.; Padgett, M.J. Optical trapping and binding. Rep. Prog. Phys. 2013, 76, 026401. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Pan, Y.L.; Wang, C. Characterization of single airborne particle extinction using the tunable optical trap-cavity ringdown spectroscopy (OT-CRDS) in the UV. Opt. Express 2017, 25, 6732–6745. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.A.; Kalume, A.; Yu, H.; Wirth, C.L.; Videen, G.; Pan, Y.L. Measurements of elastic light-scattering patterns and images of single, oriented, optically trapped particles. J. Quant. Spectrosc. Radiat. Transf. 2022, 287, 108223. [Google Scholar] [CrossRef]
- Xie, C.; Goodman, C.; Dinno, M.A.; Li, Y.-Q. Real-time Raman spectroscopy of optically trapped living cells and organelles. Opt. Express 2004, 12, 6207–6214. [Google Scholar] [CrossRef] [PubMed]
- Kalume, A.; Wang, C.; Santarpia, J.; Pan, Y.L. Liquid-liquid phase separation and evaporation of a laser-trapped organic-organic airborne droplet using temporal spatial-resolved Raman spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 19151. [Google Scholar] [CrossRef]
- Gong, Z.; Pan, Y.L.; Videen, G.; Wang, C. Optical trapping-Raman spectroscopy (OT-RS) with embedded microscopy imaging for concurrent characterization and monitoring of physical and chemical properties of single particles. Anal. Chim. Acta 2018, 10120, 86–94. [Google Scholar] [CrossRef]
- Ashkin, A. Acceleration and trapping of particles by radiation pressure. Phys. Rev. Lett. 1970, 24, 156–159. [Google Scholar] [CrossRef]
- Daly, M.; Sergides, M.; Chormaic, S. Optical trapping and manipulation of micrometer and submicrometer particles. Laser Photonics Rev. 2015, 9, 3309–3329. [Google Scholar] [CrossRef]
- Gong, Z.; Pan, Y.L.; Videen, G.; Wang, C. Optical trapping and manipulation of single particles in air: Principles, technical details, and applications. J. Quant. Spectrosc. Radiat. Transf. 2018, 214, 94–119. [Google Scholar] [CrossRef]
- Kalume, A.; Wang, C.; Pan, Y.L. Optical-trapping laser techniques for characterizing airborne aerosol particles and its application in chemical aerosol study. Micromachines 2021, 12, 466. [Google Scholar] [CrossRef]
- Corsetti, S.; Dholakia, K. Optical manipulation: Advances for biophotonics in the 21st century. J. Biomed. Opt. 2021, 26, 070602. [Google Scholar] [CrossRef] [PubMed]
- Redding, B.; Pan, Y.L. Optical trap for both transparent and absorbing particles in air using a single shaped laser beam. Opt. Lett. 2015, 40, 2798–2801. [Google Scholar] [CrossRef]
- Gallimore, P.J.; Davidson, N.M.; Kalberer, M.; Pope, F.D.; Ward, A.D. 1064 nm dispersive Raman microspectroscopy and optical trapping of pharmaceutical aerosols. Anal. Chem. 2018, 90, 8838–8844. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Chen, S.; Liu, Q. Review of fluorescence suppression techniques in Raman spectroscopy. Appl. Spectrosc. Rev. 2015, 50, 387–406. [Google Scholar] [CrossRef]
- Thurn, R.; Kiefer, W. Raman-microsampling technique applying optical levitation by radiation pressure. Appl. Spectrosc. 1984, 38, 78–83. [Google Scholar] [CrossRef]
- Hopkins, R.J.; Mitchem, L.; Ward, A.D.; Reid, J.P. Control and characterisation of a single aerosol droplet in a single-beam gradient-force optical trap. Phys. Chem. Chem. Phys. 2004, 6, 4924–4927. [Google Scholar] [CrossRef]
- Pan, Y.L.; Hill, S.C.; Coleman, M. photophoretic trapping of absorbing particles in air and measurement of their single-particle Raman spectra. Opt. Express 2012, 20, 5325–5334. [Google Scholar] [CrossRef]
- Ling, L.; Li, Y.-Q. Measurement of Raman spectra of single airborne absorbing particles trapped by a single laser beam. Opt. Lett. 2013, 38, 416–418. [Google Scholar] [CrossRef]
- Wang, C.; Pan, Y.L.; Hill, S.C.; Redding, B. Photophoretic trapping-Raman spectroscopy for single pollens and fungal spores trapped in air. J. Quant. Spectrosc. Radiat. Transf. 2015, 153, 4–12. [Google Scholar] [CrossRef]
- Gong, Z.; Pan, Y.L.; Videen, G.; Wang, C. The temporal evolution process of fluorescence bleaching and Raman spectra from single solid particles optically trapped in air. Chem. Phys. Lett. 2017, 689, 100–104. [Google Scholar] [CrossRef]
- Ai, Y.; Wang, C.; Pan, Y.L.; Videen, G. Characterization of single fungal aerosol particles in a reactive atmospheric environment using time-resolved optical trapping-Raman spectroscopy (OT-RS). Environ. Sci. Atmos. 2022, 2, 591–600. [Google Scholar] [CrossRef]
- Redding, B.; Schwab, M.J.; Pan, Y.L. Raman spectroscopy of optically trapped single biological micro-particles. Sensors 2015, 15, 19021–19046. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pan, Y.L.; Videen, G. Optical trapping and laser-spectroscopy measurements of single particles in air: A review. Meas. Sci. Technol. 2021, 32, 102005. [Google Scholar] [CrossRef]
- Liang, Z.; Chu, Y.; Gen, M.; Chan, C.K. Single-particle Raman spectroscopy for studying physical and chemical processes of atmospheric particles. Atmos. Chem. Phys. 2022, 22, 3017–3044. [Google Scholar] [CrossRef]
- Alali, H.; Gong, Z.; Videen, G.; Pan, Y.L.; Muñoz, O.; Wang, C. Laser spectroscopic characterization of single extraterrestrial dust particles using optical trapping-cavity ringdown and Raman spectroscopy. J. Quant. Spectrosc. Radiat. Transf. 2020, 255, 107249. [Google Scholar] [CrossRef]
- Gong, Z.; Pan, Y.L.; Wang, C. Optical configurations for photophoretic trap of single particles in air. Rev. Sci. Instrum. 2016, 87, 103104. [Google Scholar] [CrossRef]
- Kalume, A.; Zhu, E.; Wang, C.; Santarpia, J.; Pan, Y.L. Position-resolved Raman spectra from a laser-trapped single airborne chemical droplet. Opt. Lett. 2017, 42, 5113–5116. [Google Scholar] [CrossRef]
- Parameswari, A.; Premkumar, S.; Premkumar, R.; Milton Franklin Benial, A. Surface enhanced Raman spectroscopy and quantum chemical studies on glycine single crystal. J. Mol. Struct. 2016, 1116, 180–187. [Google Scholar] [CrossRef]
- Tortonda, F.R.; Pascual-Ahuir, J.L.; Silla, E.; Tuñón, I.; Ramírez, F.J. Aminoacid zwitterions in solution: Geometric, energetic, and vibrational analysis using density functional theory-continuum model calculations. J. Chem. Phys. 1998, 109, 592–603. [Google Scholar] [CrossRef]
- Stewart, S.; Fredericks, P.M. Surface-enhanced Raman spectroscopy of amino acids adsorbed on an electrochemically prepared silver surface. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1998, 55, 1641–1660. [Google Scholar] [CrossRef]
- Podstawka, E.; Ozaki, Y.; Proniewicz, L.M. Part II: Surface-enhanced Raman spectroscopy investigation of methionine containing heterodipeptides adsorbed on colloidal silver. Appl. Spectrosc. 2004, 58, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Manogaran, S. Vibrational analysis of glycine zwitterion—An ab initio study. Chem. Phys. Lett. 1998, 294, 56–64. [Google Scholar] [CrossRef]
- Guicheteau, J.; Argue, L.; Hyre, A.; Jacobson, M.; Christesen, S.D. Raman and surface-enhanced Raman spectroscopy of amino acids and nucleotide bases for target bacterial vibrational mode identification. Chem. Bio. Sens. VII 2006, 6218, 174–184. [Google Scholar]
- Culka, A.; Jehlička, J.; Edwards, H.G.M. Acquisition of Raman spectra of amino acids using portable instruments: Outdoor measurements and comparison. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 77, 978–983. [Google Scholar] [CrossRef]
- Freire, P.T.C.; Barboza, F.M.; Lima, J.A.; Melo, F.E.A.; Filho, J.M. Raman spectroscopy of amino acid crystals. In Raman Spectroscopy and Applications; Khan, M., Ed.; Intech Open: London, UK, 2017; pp. 201–223. ISBN 978-953-51-2908-0. [Google Scholar]
- Zhu, G.; Zhu, X.; Fan, Q.; Wan, X. Raman spectra of amino acids and their aqueous solutions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 78, 1187–1195. [Google Scholar] [CrossRef]
- Pawlukoj, A.; Leciejewicz, J.; Tomkinson, J.; Parker, S.F. Neutron Scattering, infra red, Raman spectroscopy and ab initio study of L-threonine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2001, 57, 2513–2523. [Google Scholar] [CrossRef]
- Liu, Y.-K.; Kuo, H.-C.; Lai, C.-H.; Chou, C.-C. Single amino acid utilization for bacterial categorization. Sci. Rep. 2020, 10, 12686. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Singh, N.P.; Yadav, R.A. Experimental Raman and IR spectral and theoretical studies of vibrational spectrum and molecular structure of pantothenic acid (vitamin B5). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 129, 131–142. [Google Scholar] [CrossRef]
- Srivastava, M.; Singh, P.; Yadav, A.; Yadav, R.A. Experimental and quantum chemical investigation of vibrational and molecular structural experimental and quantum chemical investigations on vibrational and molecular structural characteristics of vitamin B7: Biotin Molecule. Asian J. Phys. 2015, 24, 635–656. [Google Scholar]
- Kim, N.H.; Lee, S.J.; Kim, K. Isocyanide and biotin-derivatized ag nanoparticles: An efficient molecular sensing mediator via surface-enhanced Raman spectroscopy. Chem. Commun. 2003, 3, 724–725. [Google Scholar] [CrossRef]
- Andruniow, T.; Zgierski, M.Z.; Kozlowski, P.M. Vibrational analysis of methylcobalamin. J. Phys. Chem. A 2002, 106, 1365–1373. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.; Yin, Y.; Mo, Y. Surface-enhanced Raman spectroscopy of vitamin B12 on silver particles in colloid and in atmosphere. J. Mol. Struct. 2009, 927, 88–90. [Google Scholar] [CrossRef]
- Ibáñez, D.; Pérez-Junquera, A.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Spectroelectrochemical elucidation of B vitamins present in multivitamin complexes by EC-SERS. Talanta 2020, 206, 120190. [Google Scholar] [CrossRef]
- Piedra, P.; Kalume, A.; Zubko, E.; Mackowski, D.; Pan, Y.L.; Videen, G. Particle-shape classification using light scattering: An exercise in deep learning. J. Quant. Spectrosc. Radiat. Transf. 2019, 231, 140–156. [Google Scholar] [CrossRef]
- A website for “An open database for molecular fingerprints of single aerosol particles in air using optical trapping—Raman spectroscopy” is being constructed and will be open to public. 2022; in press.




| Sample Class | Name (a) | Supplier | Descriptions (Size, Shape, Color, etc.) | Trapping Efficiency (b) | Stability | Ref. |
|---|---|---|---|---|---|---|
| Standard samples | SWCNT | US nano-Materials (Houston, TX, USA) | Cylinderical shape, black | H | H | [36] |
| MWCNT | H | H | ||||
| SM | White House Scientific (Chester, PB, USA) | 2 µm, silica shpere, white | L | H | ||
| Amino Acid | Glycine L-glutamic L-threonine | Sigma-Aldrich (St. Louis, MO, USA) | Submicron to several microns, | H | H | |
| irregular shape, white to off-white | M | H | ||||
| H | H | |||||
| Vitamin | B5 | Sigma-Aldrich | Submicron to several microns, irregular shape, white to off-white, except for B12 is glitter dark red | L | M | |
| B7 | L | M | ||||
| B12 | M | H | ||||
| BWA | YR | Edgewood Chemical Biological center (Seattle, WA, USA) | ~10 µm, irregular shape, light brow | M | M | [8] |
| MS2 | M | M | ||||
| BS | M | M | ||||
| BG | H | M | ||||
| Pollens | rye | Greer lab (Lenoir, NC, USA) | ~35 μm, irregular shape, light brown | M | M | [36,49] |
| ragweed | ~24 μm, irregular shape, yellow | L | M | |||
| mulberry | ~14 μm, irregular shape, light brown | L | M | |||
| oak | ~34 μm, irregular shape, yellow | M | M | |||
| spores | JG | Greer lab | ~8 μm, irregular shape, dark brown | L | L | [36,49] |
| BGS | 5.6–8.2 μm, irregular shape, dark black | M | L | |||
| Fungi | AF | Greer lab | Actual size is in millimeters, the | M | M | [51] |
| AV | samples were further ground | M | L | |||
| CH | to submicron to tens of | M | M | |||
| PV | microns for experimental | M | M | |||
| PCa | purpose. Irregular shape, | M | M | |||
| PCh | black and dark gray | L | L | |||
| PD | L | L | ||||
| Extraterrestrial dust | LA | Johnson sapce center (Houston, TX, USA) | ~4 µm, irregular shape with clear edge | L | M | [55] |
| MA | Irregular shape, more | L | M | |||
| stickie, brown | ||||||
| Terrestrial dust | VA | volcanic eruption | Irregular shape, dark gray | M | H | [55] |
| CS | Sigma-Aldrich | 2–4 μm, spherical, black | H | H | ||
| Fluorescent particle | RhB-PEMS | Cospheric (Santa Barbara, CA, USA) | 10–30 μm, sphere RhB% = ~1% | H | H | [50] |
| Class | Name | RS Bands (cm−1) | Assignments | Ref. |
|---|---|---|---|---|
| Amino Acid | Glycine | 369 | CH2 torsional mode | [58] |
| 513 | COO− coupled with C-N deformation mode | [58,59,60] | ||
| 616 | COO− wagging | [58] | ||
| 709 | COO− coupled with C-C stretching | [58] | ||
| 905 | CH2 rocking coupled with COO− deformation | [58] | ||
| 1048 | C-NH2 stretching coupled with CH2 twisting | [58,61] | ||
| 1122 | NH3+ rocking coupled with CH2 twisting | [58,61] | ||
| 1338 | CH2 wagging vibration | [58] | ||
| 1416 | CH2 scissoring coupled with COO− symmetric stretching vibration | [58] | ||
| 1452 | CH2 scissoring coupled with COO− asymmetric stretching vibration | [58] | ||
| 1580 | NH3+ symmetric deformation mode | [58,62] | ||
| 1677 | NH3+ asymmetric deformation mode | [58] | ||
| 2331 | N2 | [8] | ||
| 2976 | CH2 symmetric starching vibration | [58] | ||
| 3013 | CH2 symmetric starching vibration | [58] | ||
| 3162 | NH3+ asymmetric stretching vibration | [58] | ||
| L-glutamic | 1064 | C-C stretching vibration | [63] | |
| 1122 | Deformation of NH3+ | [60] | ||
| 1301 | CH2 wagging band | [63] | ||
| 1443 | COO− symmetric stretching vibration | [64] | ||
| 2848 | CH2 stretching | [65] | ||
| 2880 | CH2 stretching | [65] | ||
| L-threonine | 876 | CCN stretching | [66] | |
| 1076 | OH deformation motion | [66] | ||
| 1299 | CH2 wagging vibration | [67] | ||
| 1443 | COO− symmetric | [60,67] | ||
| 2878 | CH2 stretching | [67] | ||
| Vitamin | B5 | 658 | C=C=C angle deformation mode | [69] |
| 842 | C=C stretching | [69] | ||
| 933 | CH2 rocking | [69] | ||
| 1041 | C-O(H) stretching | [69] | ||
| 1139 | C-O(H) stretching | [69] | ||
| 1306 | CH deformation | [69] | ||
| 1477 | CH3 antisymmetric deformation | [69] | ||
| 1628 | C=O stretching | [69] | ||
| 3000 | CH stretching | [69] | ||
| B7 | 1071 | Ureido ring | [70] | |
| 1318 | CH deformation | [70] | ||
| 1458 | CH2 deformation | [70] | ||
| 1654 | C=O stretching vibration | [70] | ||
| 2892 | CH stretching vibration | [71] | ||
| B12 | 733 | CH3 rocking vibration | [72] | |
| 1149 | Corrin ring vibration | [73,74] | ||
| 1359 | Corrin ring vibration | [73,74] | ||
| 1459 | Corrin ring vibration | [73,74] | ||
| 1574 | Corrin ring vibration | [73,74] | ||
| BWA surrogates | YR | 732 | C-C adenosine ring stretching | [8] |
| MS2 | 840 | Tyrosine | [8] | |
| BS | 955 | C-C backbone stretching, protein | [8] | |
| BG | 1032 | Phenylalanine | [8] | |
| 1080 | C-C stretching, C-O-C glycosidic link | [8] | ||
| 1136 | C-N or C-C stretching | [8] | ||
| 1187 | Cytosine, nucleotides: base ν(CN), guanine | [8] | ||
| 1350 | Amide III | [8] | ||
| 1451 | Lipids, protein, or the C-H2 deformation | [8] | ||
| 1618 | Amide I | [8] | ||
| 2720 | CH stretching | [8] | ||
| 3100 | CH stretching | [8] | ||
| Pollens and spores | rye | 771 | ν(O-P-O) diester, phosphatidylinositol | [36,49] |
| regweed | 892 | C-C skeletal stretching, C-O-C backbone | [36,49] | |
| mulberry | 1065 | C-C or C-N stretching | [36,49] | |
| oak | 1080 | C-C skeletal stretching mode, C-O-C glycosidic link | [36,49] | |
| JG | 1136 | C-C or C-N starching | [36,49] | |
| BGS | 1177 | Cytosine, nucleotides: base ν(CN), guanine | [36,49] | |
| 1265 | Proteins, Amide III | [36,49] | ||
| 1298 | CH2 twisting, fatty acid, lipids | [36,49] | ||
| 1420–1480 | Lipids, protein, CH2 deformation | [36,49] | ||
| 1660 | Amide I, lipids, v(C=C) | [36,49] | ||
| 1713 | C=O | [36,49] | ||
| 1750 | Lipids, C=O | [36,49] | ||
| 2720–3000 | CH stretching, sporopollenin | [36,49] | ||
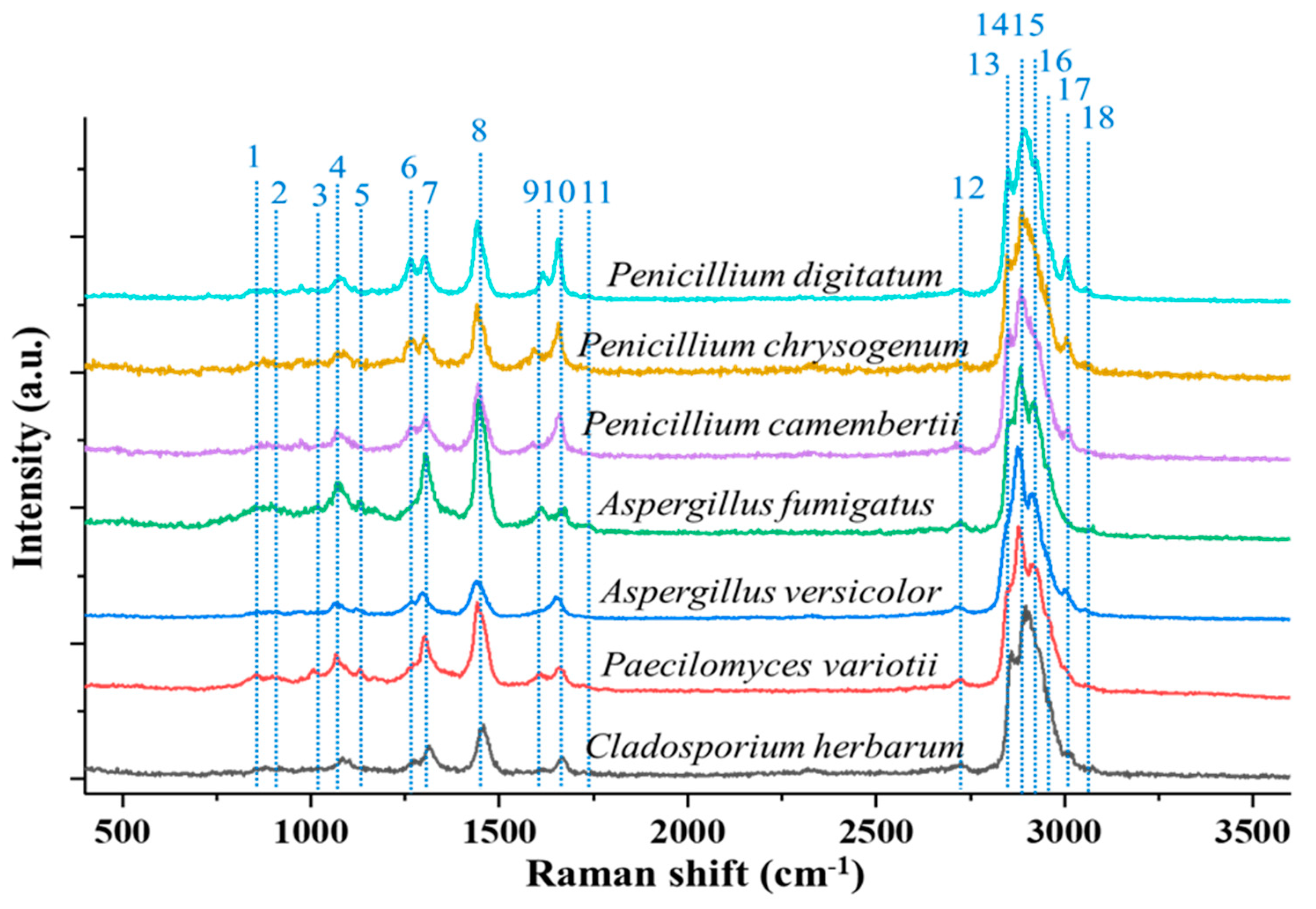
| Fungal aerosols | AF | 840 | Tyrosine | [51] |
| AV | 892 | C-O-C backbone, C-C skeletal stretches | [51] | |
| CH | 1032 | Phenylalanine | [51] | |
| PV | 1080 | C-O-C glycosidic link, C–C skeletal stretches | [51] | |
| PCa | 1131 | Palmitic acid | [51] | |
| PCh | 1267 | Triacylglycerol, CH (lipid in normal tissue) | [51] | |
| PD | 1298 | CH2 deformation | [51] | |
| 1450 | Lipid/protein, CH deformation | [51] | ||
| 1610 | Cytosine | [51] | ||
| 1660 | Amide I | [51] | ||
| 1750 | Lipids, C=O | [51] | ||
| 2739 | Stretching vibrations of CH | [51] | ||
| 2855 | CH2 symmetric stretch of lipids | [51] | ||
| 2878 | CH2 symmetric stretch of lipids | [51] | ||
| 2915 | CH2 stretch lipids and proteins | [51] | ||
| 2960 | Out-of-plane chain end antisymmetric CH3 stretch band | [51] | ||
| 3008 | νas(=CH), lipids, fatty acids | [51] | ||
| 3059 | (C=CH) aromatic stretching | [51] | ||
| Extraterrestrial dust | LA | 500 | Symmetric T-O-T stretching mode (T = Al or Si) | [55] |
| MA | 371 | Mg-O stretch | [55] | |
| 503 | O-Si-O band | [55] | ||
| 627 | Si-O stretching vibrations | [55] | ||
| 1078 | Band of the ion | [55] | ||
| 1565 | G-band of graphite | [55] | ||
| 3382 | O-H | [55] | ||
| 3614 | O-H | [55] | ||
| Terrestrial dust | VA | 361 | Ca-O stretching vibration | [55] |
| 468 | Quartz SiO2 | [55] | ||
| 665 | Si-O-Si symmetric band | [55] | ||
| 1006 | Sulfates | [55] | ||
| CS | 1367 | D-band of graphite | [55] | |
| 1585 | G-band of graphite | [55] | ||
| 2700 | G’-band | [55] | ||
| 2900 | 2LO-band | [55] | ||
| SWCNT | 490 | Breathing modes of 4- and membered rings | [36] | |
| MWCNT | 604 | Breathing modes of 4- and membered rings | [36] | |
| 800 | Optical mode of the SiO2 network | [36] | ||
| 979 | Vibration of (OH)-Si | [36] | ||
| 1349 | D-band | [36] | ||
| 1518 | G-band | [36] | ||
| 2684 | G’-band | [36] | ||
| RhB-PEMS | 1080 | C-C stretching band | [50] | |
| 1305 | CH2 twisting | [50] | ||
| 1450 | CH2 bending | [50] | ||
| 2800–3000 | CH stretching vibrational modes | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alali, H.; Ai, Y.; Pan, Y.-L.; Videen, G.; Wang, C. A Collection of Molecular Fingerprints of Single Aerosol Particles in Air for Potential Identification and Detection Using Optical Trapping-Raman Spectroscopy. Molecules 2022, 27, 5966. https://doi.org/10.3390/molecules27185966
Alali H, Ai Y, Pan Y-L, Videen G, Wang C. A Collection of Molecular Fingerprints of Single Aerosol Particles in Air for Potential Identification and Detection Using Optical Trapping-Raman Spectroscopy. Molecules. 2022; 27(18):5966. https://doi.org/10.3390/molecules27185966
Chicago/Turabian StyleAlali, Haifa, Yukai Ai, Yong-Le Pan, Gorden Videen, and Chuji Wang. 2022. "A Collection of Molecular Fingerprints of Single Aerosol Particles in Air for Potential Identification and Detection Using Optical Trapping-Raman Spectroscopy" Molecules 27, no. 18: 5966. https://doi.org/10.3390/molecules27185966
APA StyleAlali, H., Ai, Y., Pan, Y.-L., Videen, G., & Wang, C. (2022). A Collection of Molecular Fingerprints of Single Aerosol Particles in Air for Potential Identification and Detection Using Optical Trapping-Raman Spectroscopy. Molecules, 27(18), 5966. https://doi.org/10.3390/molecules27185966






