Lignin from Residual Sawdust of Eucalyptus spp.—Isolation, Characterization, and Evaluation of the Antioxidant Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Biomass Composition
2.3. Sequential Acid-Alkaline Treatment
2.4. Crystallinity Index
2.5. Lignin Characterization
2.5.1. Thermal Analysis
2.5.2. Fourier-Transformed Infrared Spectroscopy
2.5.3. Nuclear Magnetic Resonance
2.6. Total Phenolic Content
2.7. Antioxidant Assays
2.8. Statistical Analysis
3. Results and Discussion
3.1. Lignocellulosic Composition
3.2. Acid Treatment
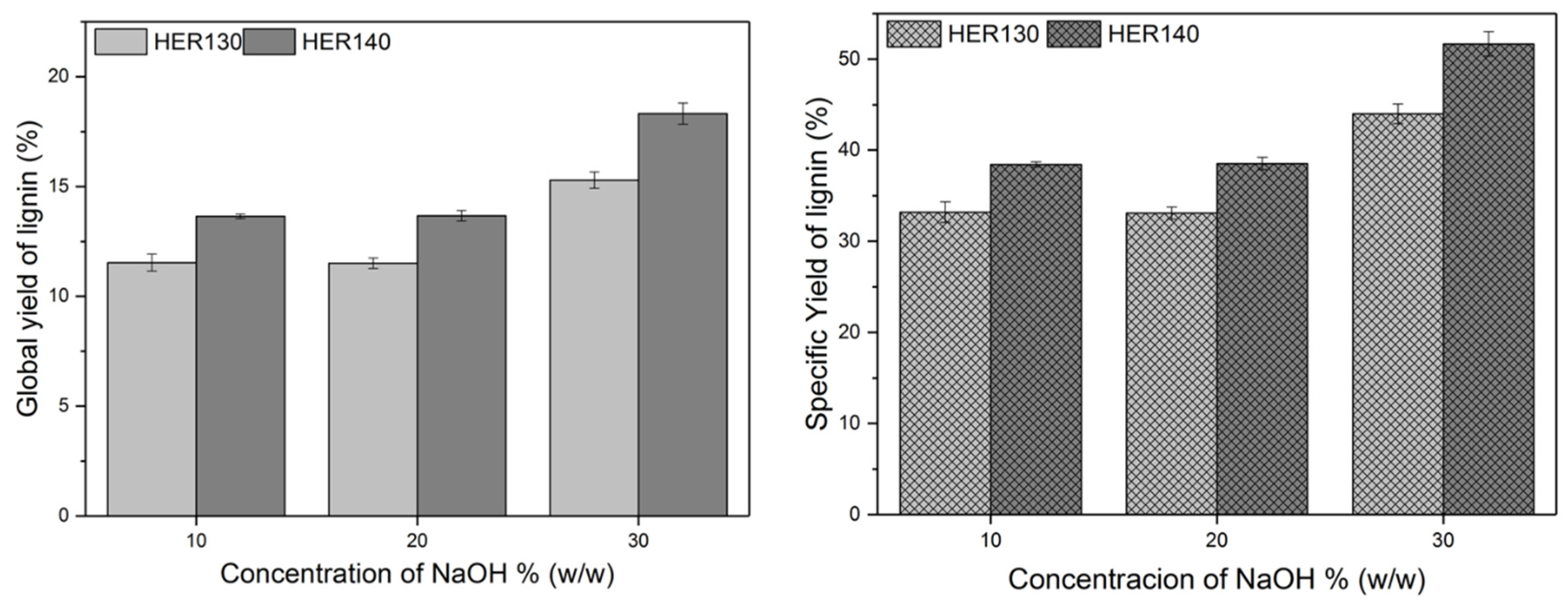
3.3. Alkaline Treatment and Lignin Recovery
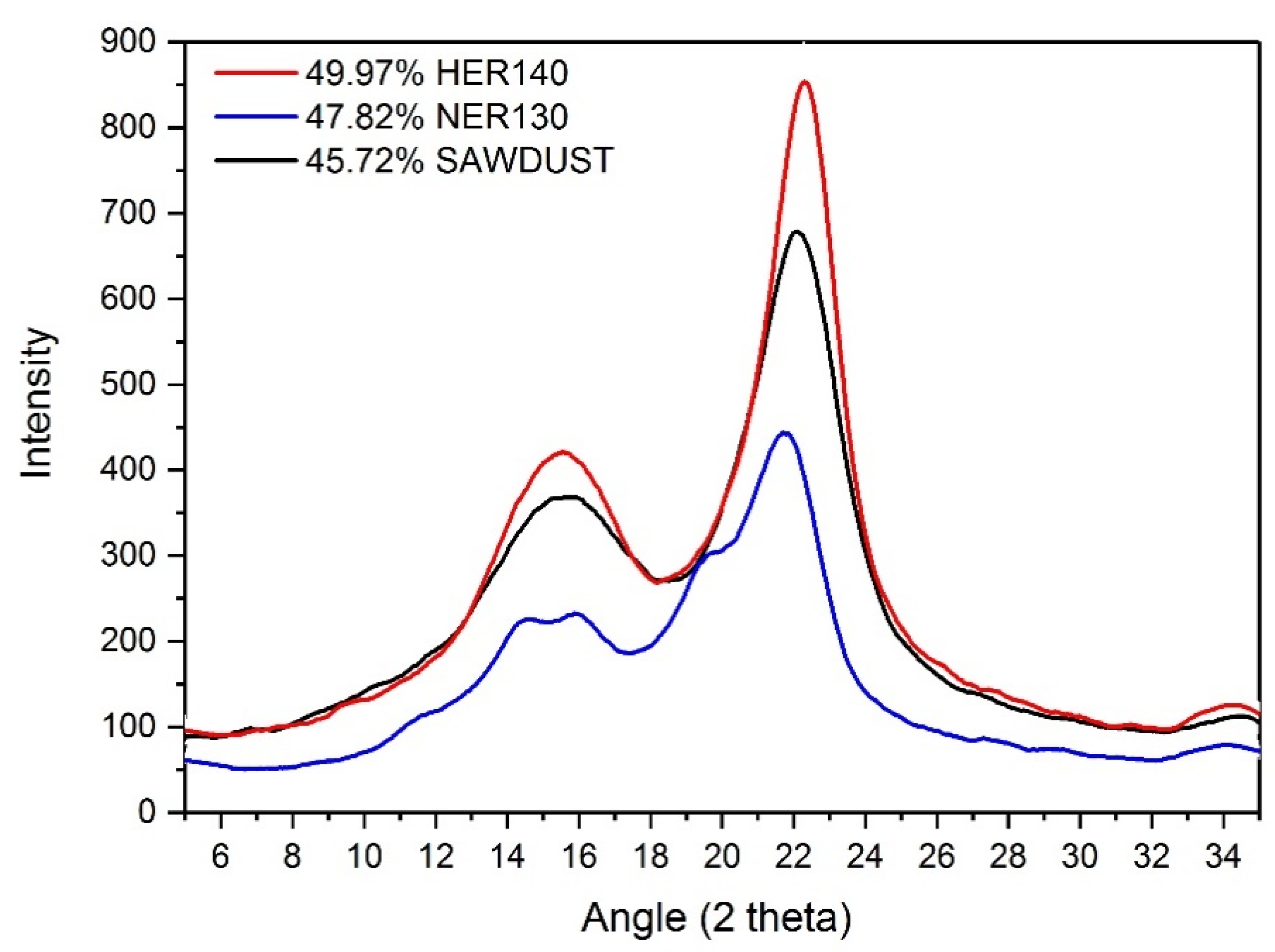
3.4. Crystallinity Index
3.5. Lignin Characterization
3.5.1. Thermogravimetric Analysis
3.5.2. Fourier-Transformed Infrared Spectroscopy
3.5.3. Nuclear Magnetic Resonance
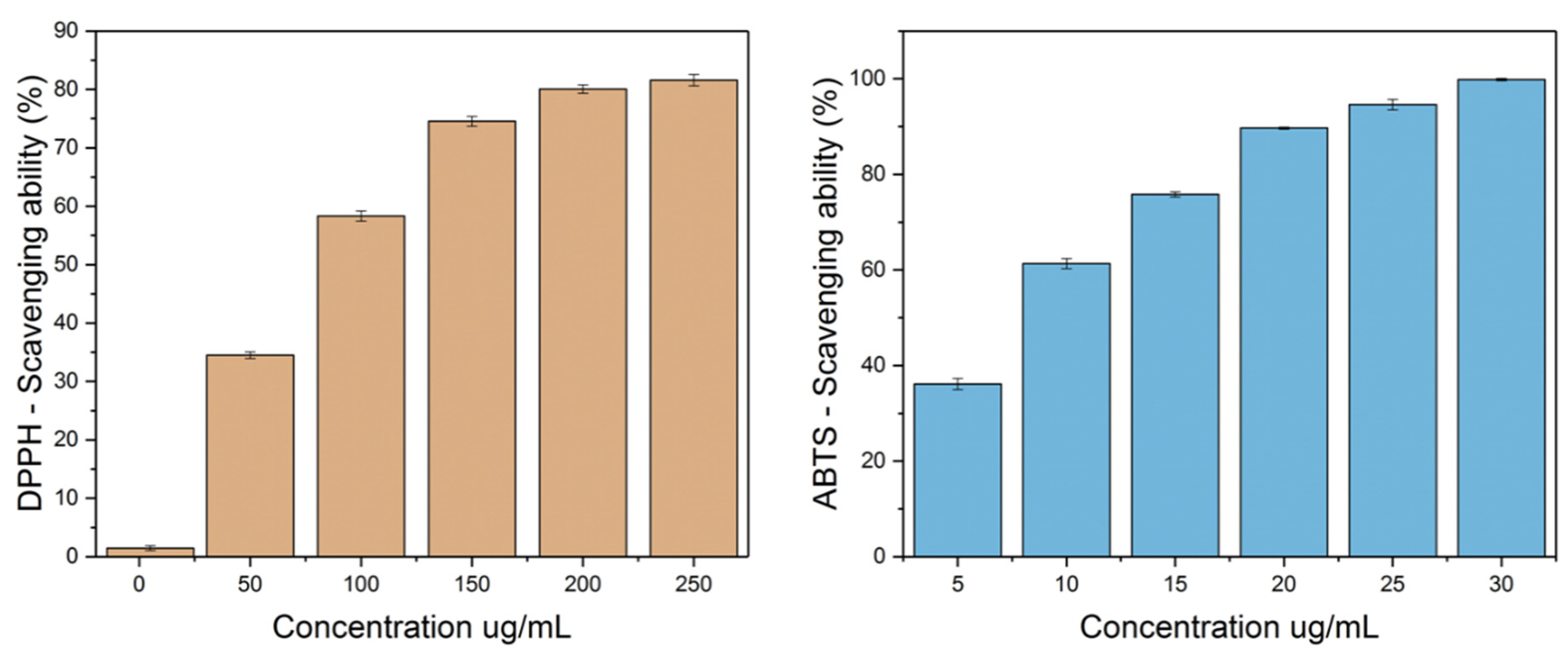
3.6. Total Phenolic Content and Antioxidant Assays
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arevalo-Gallegos, A.; Ahmad, Z.; Asgher, M.; Parra-Saldivar, R.; Iqbal, H.M. Lignocellulose: A sustainable material to produce value-added products with a zero waste approach—A review. Int. J. Biol. Macromol. 2017, 99, 308–318. [Google Scholar] [CrossRef]
- Medina, J.D.C.; Woiciechowski, A.; Filho, A.Z.; Noseda, M.D.; Kaur, B.S.; Soccol, C.R. Lignin preparation from oil palm empty fruit bunches by sequential acid/alkaline treatment—A biorefinery approach. Bioresour. Technol. 2015, 194, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Bohn, L.R.; Dresch, A.P.; Cavali, M.; Vargas, A.C.G.; Führ, J.F.; Tironi, S.P.; Fogolari, O.; Mibielli, G.M.; Alves, S.L., Jr.; Bender, J.P. Alkaline pretreatment and enzymatic hydrolysis of corn stover for bioethanol production. Res. Soc. Dev. 2021, 10, e149101118914. [Google Scholar] [CrossRef]
- Letti, L.A.J.; Woiciechowski, A.L.; Medeiros, A.B.P.; Rodrigues, C.; de Carvalho, J.C.; Vandenberghe, L.P.D.; Karp, S.G.; Torres, L.A.Z.; Guarnizo, A.F.C.; Colonia, B.S.O.; et al. Valorization of solid and liquid wastes from palm oil industry. In Waste Biorefinery: Value Addition through Resource Utilization; Bhaskar, T., Pandey, A., Varjani, S., Rene, E.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 235–265. [Google Scholar] [CrossRef]
- Vallejos, M.E.; Felissia, F.E.; Area, M.C.; Ehman, N.V.; Tarrés, Q.; Mutjé, P. Nanofibrillated cellulose (CNF) from eucalyptus sawdust as a dry strength agent of unrefined eucalyptus handsheets. Carbohydr. Polym. 2016, 139, 99–105. [Google Scholar] [CrossRef] [PubMed]
- European Organization of the Sawmill Industry. Annual Report of the European Sawmill Industry 2020–2021; European Organization of the Sawmill Industry: Brussels, Belgium, 2021. [Google Scholar]
- Schneider, V.E.; Peresin, D.; Trentin, A.C.; Bortolin, T.A.; Sambuichi, R.H.R. Diagnóstico dos Resíduos Orgânicos do Setor Agrossilvopastoril e Agroindústrias Associadas; Instituto de Pesquisa Econômica Aplicada (Ipea): Brasília, Brasil, 2012; 134p. [Google Scholar]
- Brazilian Tree Industry. Annual Report—2020; Brazilian Tree Industry: São Paulo, Brasil, 2020. [Google Scholar]
- Myburg, A.A.; Grattapaglia, D.; Tuskan, G.A.; Hellsten, U.; Hayes, R.D.; Grimwood, J.; Jenkins, J.; Lindquist, E.; Tice, H.; Bauer, D.; et al. The genome of Eucalyptus grandis. Nature 2014, 510, 356–362. [Google Scholar] [CrossRef]
- Rominiyi, O.L.; Adaramola, B.A.; Ikumapayi, O.M.; Oginni, O.T.; Akinola, S.A. Potential Utilization of Sawdust in Energy, Manufacturing and Agricultural Industry; Waste to Wealth. World J. Eng. Technol. 2017, 5, 526–539. [Google Scholar] [CrossRef]
- Gujjula, P.; Kumar, N.; Lynam, J.G. Pretreatment of Loblolly Pine Tree Needles Using Deep Eutectic Solvents. Biomass 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Celiktas, M.S.; Alptekin, F.M. Biorefinery Concept: Current Status and Future Prospects. Int. Conf. Eng. Technol. 2017, 7, 9. [Google Scholar]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Chemistry and Specialty Industrial Applications of Lignocellulosic Biomass. Waste Biomass-Valoriz. 2020, 12, 2145–2169. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, J.P. Sustainable bio-ethanol production from agro-residues: A review. Renew. Sustain. Energy Rev. 2015, 41, 550–567. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Xu, G.; Shi, Z.; Zhao, Y.; Deng, J.; Dong, M.; Liu, C.; Murugadoss, V.; Mai, X.; Guo, Z. Structural characterization of lignin and its carbohydrate complexes isolated from bamboo (Dendrocalamus sinicus). Int. J. Biol. Macromol. 2018, 126, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L.Q. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Roy, R.; Rahman, S.; Amit, T.A.; Jadhav, B. Recent Advances in Lignin Depolymerization Techniques: A Comparative Overview of Traditional and Greener Approaches. Biomass 2022, 2, 130–154. [Google Scholar] [CrossRef]
- Cavali, M.; Soccol, C.R.; Tavares, D.; Torres, L.A.Z.; Tanobe, V.O.D.A.; Filho, A.Z.; Woiciechowski, A.L. Valorization of lignin from pine (Pinus spp.) residual sawdust: Antioxidant activity and application in the green synthesis of silver nanoparticles for antibacterial purpose. Biomass Convers. Biorefinery 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Barapatre, A.; Meena, A.S.; Mekala, S.; Das, A.; Jha, H. In vitro evaluation of antioxidant and cytotoxic activities of lignin fractions extracted from Acacia nilotica. Int. J. Biol. Macromol. 2016, 86, 443–453. [Google Scholar] [CrossRef]
- de Carvalho, J.C.; Cardoso, L.C.; Ghiggi, V.; Woiciechowski, A.L.; de Souza Vandenberghe, L.P.; Soccol, C.R. Biotransformation of Waste Biomass into High Value Biochemicals; Springer: New York, NY, USA, 2014. [Google Scholar]
- Grossman, A.; Vermerris, W. Lignin-based polymers and nanomaterials. Curr. Opin. Biotechnol. 2019, 56, 112–120. [Google Scholar] [CrossRef]
- Li, T.; Lyu, G.; Liu, Y.; Lou, R.; Lucia, L.A.; Yang, G.; Chen, J.; Saeed, H.A.M. Deep Eutectic Solvents (DESs) for the Isolation of Willow Lignin (Salix matsudana cv. Zhuliu). Int. J. Mol. Sci. 2017, 18, 2266. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.-Y.; Patankar, S.; Chandra, R.P.; Sathitsuksanoh, N.; Saddler, J.; Renneckar, S. Valorization of Bark Using Ethanol–Water Organosolv Treatment: Isolation and Characterization of Crude Lignin. ACS Sustain. Chem. Eng. 2020, 8, 4745–4754. [Google Scholar] [CrossRef]
- Zikeli, F.; Vinciguerra, V.; Taddei, A.R.; D’Annibale, A.; Romagnoli, M.; Mugnozza, G.S. Isolation and characterization of lignin from beech wood and chestnut sawdust for the preparation of lignin nanoparticles (LNPs) from wood industry side-streams. Holzforschung 2018, 72, 961–972. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.; Sun, L.; Yuan, Q.; Cheng, G.; Argyropoulos, D.S. Extraction and characterization of lignin from corncob residue after acid-catalyzed steam explosion pretreatment. Ind. Crop. Prod. 2019, 133, 241–249. [Google Scholar] [CrossRef]
- Torres, L.A.Z.; Woiciechowski, A.L.; Tanobe, V.O.D.A.; Filho, A.Z.; de Freitas, R.A.; Noseda, M.D.; Szameitat, E.S.; Faulds, C.; Coutinho, P.; Bertrand, E.; et al. Lignin from oil palm empty fruit bunches: Characterization, biological activities and application in green synthesis of silver nanoparticles. Int. J. Biol. Macromol. 2020, 167, 1499–1507. [Google Scholar] [CrossRef]
- Cavali, M.; Soccol, C.R.; Tavares, D.; Torres, L.A.Z.; Tanobe, V.O.D.A.; Filho, A.Z.; Woiciechowski, A.L. Effect of sequential acid-alkaline treatment on physical and chemical characteristics of lignin and cellulose from pine (Pinus spp.) residual sawdust. Bioresour. Technol. 2020, 316, 123884. [Google Scholar] [CrossRef] [PubMed]
- National Renewable Energy Laboratory. Biomass Compositional Analysis; National Renewable Energy Laboratory: Golden, CO, USA, 2020. [Google Scholar]
- Jaramillo, D.T.; Vélez, S.P.M.; Díaz, J.C.Q. Evaluación de pretratamientos químicos sobre materiales lignocelulósicos. Ingeniare Rev. Chil. Ing. 2017, 25, 733–743. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Y.; Gu, L.; Wu, W.; Zhao, H.; Jin, Y. Structural elucidation and antioxidant activity of lignin isolated from rice straw and alkali-oxygen black liquor. Int. J. Biol. Macromol. 2018, 116, 513–519. [Google Scholar] [CrossRef]
- Guigou, M.; Cabrera, M.N.; Vique, M.; Bariani, M.; Guarino, J.; Ferrari, M.D.; Lareo, C. Combined pretreatments of eucalyptus sawdust for ethanol production within a biorefinery approach. Biomass Convers. Biorefinery 2018, 9, 293–304. [Google Scholar] [CrossRef]
- Wang, H.-M.; Wang, B.; Wen, J.-L.; Yuan, T.-Q.; Sun, R.-C. Structural Characteristics of Lignin Macromolecules from Different Eucalyptus Species. ACS Sustain. Chem. Eng. 2017, 5, 11618–11627. [Google Scholar] [CrossRef]
- Gallina, G.; Cabeza, Á.; Biasi, P.; García-Serna, J. Optimal conditions for hemicelluloses extraction from Eucalyptus globulus wood: Hydrothermal treatment in a semi-continuous reactor. Fuel Process. Technol. 2016, 148, 350–360. [Google Scholar] [CrossRef] [Green Version]
- Rangel, J.; Hornus, M.; Felissia, F.E.; Area, M.C. Hydrothermal treatment of eucalyptus sawdust for a forest biorefinery. Cellul. Chem. Technol. 2016, 50, 521–528. [Google Scholar]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef] [PubMed]
- Woiciechowski, A.L.; Neto, C.J.D.; de Souza Vandenberghe, L.P.; de Carvalho Neto, D.P.; Sydney, A.C.N.; Letti, L.A.J.; Karp, S.G.; Torres, L.A.Z.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance—Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Yáñez-S, M.; Matsuhiro, B.; Nuñez, C.; Pan, S.; Hubbell, C.A.; Sannigrahi, P.; Ragauskas, A.J. Physicochemical characterization of ethanol organosolv lignin (EOL) from Eucalyptus globulus: Effect of extraction conditions on the molecular structure. Polym. Degrad. Stab. 2014, 110, 184–194. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- dos Santos, P.S.B.; de Cademartori, P.H.G.; Prado, R.; Gatto, D.A.; Labidi, J. Composition and structure of organosolv lignins from four eucalypt species. Wood Sci. Technol. 2014, 48, 873–885. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, K.; Xiao, L.-P.; Sun, R.-C.; Song, G. Total utilization of lignin and carbohydrates in Eucalyptus grandis: An integrated biorefinery strategy towards phenolics, levulinic acid, and furfural. Biotechnol. Biofuels 2020, 13, 2. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Brienzo, M.; Fikizolo, S.; Benjamin, Y.; Tyhoda, L.; Görgens, J. Influence of pretreatment severity on structural changes, lignin content and enzymatic hydrolysis of sugarcane bagasse samples. Renew. Energy 2017, 104, 271–280. [Google Scholar] [CrossRef]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Torres, L.A.Z.; Woiciechowski, A.L.; Tanobe, V.O.D.A.; Karp, S.G.; Lorenci, L.C.G.; Faulds, C.; Soccol, C.R. Lignin as a potential source of high-added value compounds: A review. J. Clean. Prod. 2020, 263, 121499. [Google Scholar] [CrossRef]
- Canevarolo, S.V., Jr. Técnicas de Caracterização de Polímeros; Artliber: São Carlos, Brazil, 2017. [Google Scholar]
- García, A.; Toledano, A.; Andrés, M.; Labidi, J. Study of the antioxidant capacity of Miscanthus sinensis lignins. Process Biochem. 2010, 45, 935–940. [Google Scholar] [CrossRef]
- Zhao, J.; Xiuwen, W.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal degradation of softwood lignin and hardwood lignin by TG-FTIR and Py-GC/MS. Polym. Degrad. Stab. 2014, 108, 133–138. [Google Scholar] [CrossRef]
- Lopes, M.S. Obtenção e Caracterização de Ligninas e Nanopartículas de Lignina Klason e Kraft. Master’s Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 2018. [Google Scholar]
- Romaní, A.; Ruiz, H.A.; Teixeira, J.; Domingues, L. Valorization of Eucalyptus wood by glycerol-organosolv pretreatment within the biorefinery concept: An integrated and intensified approach. Renew. Energy 2016, 95, 1–9. [Google Scholar] [CrossRef]
- Sunthornvarabhas, J.; Liengprayoon, S.; Suwonsichon, T. Antimicrobial kinetic activities of lignin from sugarcane bagasse for textile product. Ind. Crop. Prod. 2017, 109, 857–861. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Yuan, T.-Q.; Sun, R.-C. Structural elucidation of whole lignin from Eucalyptus based on preswelling and enzymatic hydrolysis. Green Chem. 2015, 17, 1589–1596. [Google Scholar] [CrossRef]
- Klamrassamee, T.; Tana, T.; Laosiripojana, N.; Moghaddam, L.; Zhang, Z.; Rencoret, J.; Gutierrez, A.; del Rio, J.C.; Doherty, W.O.S. Effects of an alkali-acid purification process on the characteristics of eucalyptus lignin fractionated from a MIBK-based organosolv process. RSC Adv. 2016, 6, 92638–92647. [Google Scholar] [CrossRef]
- Fernández-Costas, C.; Gouveia, S.; Sanromán, M.A.; Moldes, D. Structural characterization of Kraft lignins from different spent cooking liquors by 1D and 2D Nuclear Magnetic Resonance spectroscopy. Biomass Bioenergy 2014, 63, 156–166. [Google Scholar] [CrossRef]
- Faustino, H.; Gil, N.; Baptista, C.; Duarte, A.P. Antioxidant Activity of Lignin Phenolic Compounds Extracted from Kraft and Sulphite Black Liquors. Molecules 2010, 15, 9308–9322. [Google Scholar] [CrossRef]
- Medina, J.D.C.; Woiciechowski, A.L.; Filho, A.Z.; Bissoqui, L.; Noseda, M.; Vandenberghe, L.P.D.S.; Zawadzki, S.F.; Soccol, C.R. Biological activities and thermal behavior of lignin from oil palm empty fruit bunches as potential source of chemicals of added value. Ind. Crop. Prod. 2016, 94, 630–637. [Google Scholar] [CrossRef]
- Aadil, K.R.; Barapatre, A.; Sahu, S.; Jha, H.; Tiwary, B.N. Free radical scavenging activity and reducing power of Acacia nilotica wood lignin. Int. J. Biol. Macromol. 2014, 67, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Alzagameem, A.; El Khaldi-Hansen, B.; Büchner, D.; Larkins, M.; Kamm, B.; Witzleben, S.; Schulze, M. Lignocellulosic biomass as source for lignin-based Environmentally Benign Lignin-based Antioxidants isolated from Lignocellulose Feedstock. Molecules 2018, 23, 2664. [Google Scholar] [CrossRef] [PubMed]
- Dizhbite, T.; Telysheva, G.; Jurkjane, V.; Viesturs, U. Characterization of the radical scavenging activity of lignins—Natural antioxidants. Bioresour. Technol. 2004, 95, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Qiu, X.; Zhu, S. Lignin: A nature-inspired sun blocker for broad-spectrum sunscreens. Green Chem. 2015, 17, 320–324. [Google Scholar] [CrossRef]
- Ponomarenko, J.; Tatiana, D.; Lauberts, M.; Viksna, A.; Dobele, G.; Bikovens, O.; Telysheva, G. Characterization of Softwood and Hardwood LignoBoost Kraft Lignins with Emphasis on their Antioxidant Activity. Bioresources 2014, 9, 2051–2068. [Google Scholar] [CrossRef]
- Harahap, A.F.P.; Gozan, M. Utilization of lignin as natural antioxidant for biodiesel Utilization of lignin as natural antioxidant for biodiesel. J. Clin. Immunol. 2018, 2024, 020024. [Google Scholar] [CrossRef]



| Test | Independent Variables | Total Recovered Carbohydrates (g/L) | ||
|---|---|---|---|---|
| Time (min) | Temperature (°C) | H2SO4 (% w/w) | ||
| 1 | 20 (−) | 100 (−) | 1.5 (−) | 0.40 |
| 2 | 60 (+) | 100 (−) | 1.5 (−) | 0.96 |
| 3 | 20 (−) | 130 (+) | 1.5 (−) | 11.93 |
| 4 | 60 (+) | 130 (+) | 1.5 (−) | 14.90 |
| 5 | 20 (−) | 100 (−) | 4.5 (+) | 0.95 |
| 6 | 60 (+) | 100 (−) | 4.5 (+) | 5.98 |
| 7 | 20 (−) | 130 (+) | 4.5 (+) | 15.09 |
| 8 | 60 (+) | 130 (+) | 4.5 (+) | 14.60 |
| 9 | 40 (0) | 115 (0) | 3 (0) | 9.97 |
| 10 | 40 (0) | 115 (0) | 3 (0) | 11.69 |
| 11 | 40 (0) | 115 (0) | 3 (0) | 10.40 |
| SAWDUST | HER140 | NER130 | LIRES * | |
|---|---|---|---|---|
| ASL (%) | 1.74 ± 0.05 | 0.71 ± 0.06 | 0.98 ± 0.04 | 3.83 ± 0.24 |
| AIL (%) | 21.99 ± 0.05 | 33.13 ± 0.57 | 10.60 ± 0.79 | 89.64 ± 1.31 |
| Hemicellulose (%) | 16.05 ± 0.24 | 6.54 ± 0.07 | 7.23 ± 0.01 | 1.61 ± 0.02 |
| Cellulose (%) | 42.67 ± 0.80 | 47.58 ± 0.80 | 60.45 ± 2.32 | 1.96 ± 0.02 |
| Legend | δC/δH ppm | Assignment | Reference |
|---|---|---|---|
| Cβ | 53.5/3.07 | Cβ-Hβ in β-β resinol substructures | [55] |
| OCH3 | 54.5/3.7 and 56.8/3.75 | C-H in methoxyl groups | [56] |
| Bα | 59.3/3.23 | Cα-Hα in β–O–4 linked to a syringyl units | [57] |
| X5 | 62.7/3.20 | C5-H5 in xylan | [57] |
| Cγ | 70.7/3.78 and 71.2/4.17 | Cγ-Hγ in β-β resinol substructures | [55] |
| Bγ | 71.2/4.86 | Cγ-Hγ in β–O–4 substructures | [57] |
| X2 | 72.4/3.06 | C2-H2 in xylan | [57] |
| X3 | 73.7/3.27 | C3-H3 in xylan | [57] |
| X4 | 74.7/3.53 | C4-H4 in xylan | [57] |
| Cα | 84.9/4.62 | Cα-Hα in β-β resinol substructures | [55] |
| X1 | 101.4/4.29 | C1-H1 in xylan | [57] |
| S2,6 | 103.8/6.68 | C2,6-H2,6 in syringyl units | [56] |
| S’2,6 | 106.9/7.21 | C2,6-H2,6 in oxidized syringyl units | [56] |
| G5/G6 | 115.1/6.71 | C2,6-H2,6 in guaiacyl units | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, D.; Cavali, M.; Tanobe, V.d.O.A.; Torres, L.A.Z.; Rozendo, A.S.; Zandoná Filho, A.; Soccol, C.R.; Woiciechowski, A.L. Lignin from Residual Sawdust of Eucalyptus spp.—Isolation, Characterization, and Evaluation of the Antioxidant Properties. Biomass 2022, 2, 195-208. https://doi.org/10.3390/biomass2030013
Tavares D, Cavali M, Tanobe VdOA, Torres LAZ, Rozendo AS, Zandoná Filho A, Soccol CR, Woiciechowski AL. Lignin from Residual Sawdust of Eucalyptus spp.—Isolation, Characterization, and Evaluation of the Antioxidant Properties. Biomass. 2022; 2(3):195-208. https://doi.org/10.3390/biomass2030013
Chicago/Turabian StyleTavares, Débora, Matheus Cavali, Valcineide de Oliveira Andrade Tanobe, Luis Alberto Zevallos Torres, Anderson Steyner Rozendo, Arion Zandoná Filho, Carlos Ricardo Soccol, and Adenise Lorenci Woiciechowski. 2022. "Lignin from Residual Sawdust of Eucalyptus spp.—Isolation, Characterization, and Evaluation of the Antioxidant Properties" Biomass 2, no. 3: 195-208. https://doi.org/10.3390/biomass2030013
APA StyleTavares, D., Cavali, M., Tanobe, V. d. O. A., Torres, L. A. Z., Rozendo, A. S., Zandoná Filho, A., Soccol, C. R., & Woiciechowski, A. L. (2022). Lignin from Residual Sawdust of Eucalyptus spp.—Isolation, Characterization, and Evaluation of the Antioxidant Properties. Biomass, 2(3), 195-208. https://doi.org/10.3390/biomass2030013







