Human Mastadenovirus Infections in Children: A Review of the Current Status in the Arab World in the Middle East and North Africa
Abstract
:1. Introduction
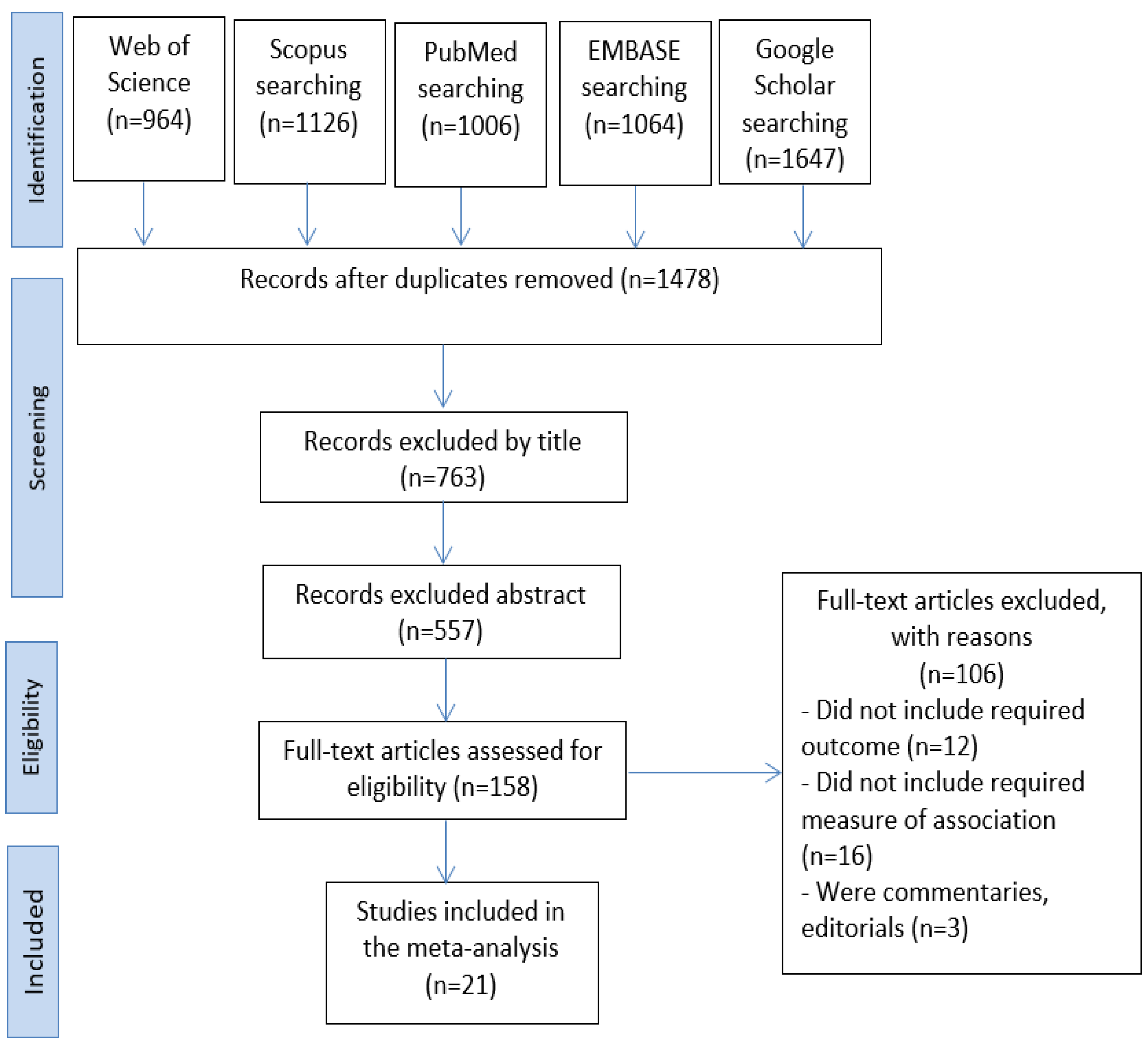
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samantaray, M.U.S.; Santra, M.P. Human Adenovirus Serotypes Efficiently Transducing HEK293 Cells: An In Vitro Propagation of HAdv. Int. J. Res. Appl. Sci. Biotechnol. 2021, 8, 17–21. [Google Scholar] [CrossRef]
- Rowe, W.P.; Huebner, R.J.; Gilmore, L.K.; Parrott, R.H.; Ward, T.G. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc. Soc. Exp. Biol. Med. 1953, 84, 570–573. [Google Scholar] [CrossRef]
- Moleirinho, M.G.; Feast, S.; Moreira, A.S.; Silva, R.J.; Alves, P.M.; Carrondo, M.J.; Huber, T.; Fee, C.; Peixoto, C. 3D-printed ordered bed structures for chromatographic purification of enveloped and non-enveloped viral particles. Sep. Purif. Technol. 2021, 254, 117681. [Google Scholar] [CrossRef]
- Takeuchi, A.; Hashimoto, K. Electron microscope study of experimental enteric adenovirus infection in mice. Infect. Immun. 1976, 13, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Krupovic, M.; Yutin, N. Evolution of double-stranded DNA viruses of eukaryotes: From bacteriophages to transposons to giant viruses. Ann. N. Y. Acad. Sci. 2015, 1341, 10–24. [Google Scholar] [CrossRef]
- Shaw, A.R.; Ziff, E.B. Transcripts from the adenovirus-2 major late promoter yield a single early family of 3′ coterminal mRNAs and five late families. Cell 1980, 22, 905–916. [Google Scholar] [CrossRef]
- Shek, L.P.-C.; Lee, B.-W. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatr. Respir. Rev. 2003, 4, 105–111. [Google Scholar] [CrossRef]
- Abbas, K.Z.; Lombos, E.; Duvvuri, V.R.; Olsha, R.; Higgins, R.R.; Gubbay, J.B. Temporal changes in respiratory adenovirus serotypes circulating in the greater Toronto area, Ontario, during December 2008 to April 2010. Virol. J. 2013, 10, 15. [Google Scholar] [CrossRef]
- Chow, W.Z.; Chan, Y.F.; Oong, X.Y.; Ng, L.J.; Nor’E, S.S.; Ng, K.T.; Chan, K.G.; Hanafi, N.S.; Pang, Y.K.; Kamarulzaman, A. Genetic diversity, seasonality and transmission network of human metapneumovirus: Identification of a unique sub-lineage of the fusion and attachment genes. Sci. Rep. 2016, 6, 27730. [Google Scholar] [CrossRef]
- La Rosa, G.; Fratini, M.; Libera, S.D.; Iaconelli, M.; Muscillo, M. Viral infections acquired indoors through airborne, droplet or contact transmission. Ann. Dell’istituto Super. Di Sanita 2013, 49, 124–132. [Google Scholar]
- Arnold, A.; MacMahon, E. Adenovirus infections. Medicine 2017, 45, 777–780. [Google Scholar] [CrossRef]
- O’Brien, B.; Goodridge, L.; Ronholm, J.; Nasheri, N. Exploring the potential of foodborne transmission of respiratory viruses. Food Microbiol. 2021, 95, 103709. [Google Scholar] [CrossRef]
- Smith, J.G.; Wiethoff, C.M.; Stewart, P.L.; Nemerow, G.R. Adenovirus. In Cell Entry by Non-Enveloped Viruses; Springer: Berlin/Heidelberg, Germany, 2010; pp. 195–224. [Google Scholar]
- Murtagh, P.; Giubergia, V.; Viale, D.; Bauer, G.; Pena, H.G. Lower respiratory infections by adenovirus in children. Clinical features and risk factors for bronchiolitis obliterans and mortality. Pediatric Pulmonol. 2009, 44, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, R.C.; Uil, T.G. Adenovirus DNA replication. Cold Spring Harb. Perspect. Biol. 2013, 5, a013003. [Google Scholar] [CrossRef]
- Lynch, J.P.; Fishbein, M.; Echavarria, M. Adenovirus. In Proceedings of the Seminars in Respiratory and Critical Care Medicine, Denver, Colorado, 13–18 May 2011; pp. 494–511. [Google Scholar]
- Gu, J.; Su, Q.-Q.; Zuo, T.-T.; Chen, Y.-B. Adenovirus diseases: A systematic review and meta-analysis of 228 case reports. Infection 2021, 49, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, G.L. The common cold. Prim. Care Clin. Off. Pract. 1996, 23, 657–675. [Google Scholar] [CrossRef]
- Shieh, W.-J. Human adenovirus infections in pediatric population-an update on clinic—Pathologic correlation. Biomed. J. 2021, 54, 38–49. [Google Scholar]
- Zhang, S.-Y.; Luo, Y.-P.; Huang, D.-D.; Fan, H.; Lu, Q.-B.; Wo, Y.; Chen, G.; Zhang, X.-A.; Li, Y.; Tong, Y.-G. Fatal pneumonia cases caused by human adenovirus 55 in immunocompetent adults. Infect. Dis. 2016, 48, 40–47. [Google Scholar] [CrossRef]
- Wang, H.-S. Updates in pediatrics. Biomed. J. 2022, 45, 9. [Google Scholar] [CrossRef]
- Ison, M.G.; Hayden, R.T. Adenovirus. In Diagnostic Microbiology of the Immunocompromised Host; Microbiology Spectrum, American Society for Microbiology Press: Washington, DC, USA, 2016; pp. 217–232. [Google Scholar]
- Matthes-Martin, S.; Boztug, H.; Lion, T. Diagnosis and treatment of adenovirus infection in immunocompromised patients. Expert Rev. Anti-Infect. Ther. 2013, 11, 1017–1028. [Google Scholar] [CrossRef]
- Bhatti, Z.; Dhamoon, A. Fatal adenovirus infection in an immunocompetent host. Am. J. Emerg. Med. 2017, 35, 1034.e1–1034.e2. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P., III; Kajon, A.E. Adenovirus: Epidemiology, global spread of novel serotypes, and advances in treatment and prevention. In Proceedings of the Seminars in Respiratory and Critical Care Medicine, San Francisco, CA, USA, 13–18 May 2016; pp. 586–602. [Google Scholar]
- Maschmeyer, G.; Ljungman, P. Infections in hematopoietic stem cell transplant recipients. In Principles and Practice of cancer Infectious Diseases; Springer: Berlin/Heidelberg, Germany, 2011; pp. 17–25. [Google Scholar]
- Rabaan, A.A.; Bakhrebah, M.A.; Nassar, M.S.; Natto, Z.S.; Al Mutair, A.; Alhumaid, S.; Aljeldah, M.; Garout, M.; Alfouzan, W.A.; Alshahrani, F.S. Suspected Adenovirus Causing an Emerging HEPATITIS among Children below 10 Years: A Review. Pathogens 2022, 11, 712. [Google Scholar] [CrossRef]
- Lion, T. Adenovirus persistence, reactivation, and clinical management. FEBS Lett. 2019, 593, 3571–3582. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, W.; Yang, B.; Qian, R.; Wu, F.; He, X.; Zhu, Q.; Liu, J.; Ni, Y.; Wang, J. Epidemiological and virological characteristics of respiratory tract infections in children during COVID-19 outbreak. BMC Pediatrics 2021, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-C.; Guo, Y.-H.; Qiu, F.-Z.; Wang, L.; Yang, S.; Feng, Z.-S.; Li, G.-X. Molecular and clinical characterization of human adenovirus associated with acute respiratory tract infection in hospitalized children. J. Clin. Virol. 2020, 123, 104254. [Google Scholar] [CrossRef]
- Hess, M. Detection and differentiation of avian adenoviruses: A review. Avian Pathol. 2000, 29, 195–206. [Google Scholar] [CrossRef]
- Ko, G.; Cromeans, T.L.; Sobsey, M.D. Detection of infectious adenovirus in cell culture by mRNA reverse transcription-PCR. Appl. Environ. Microbiol. 2003, 69, 7377–7384. [Google Scholar] [CrossRef] [PubMed]
- Rohayem, J.; Berger, S.; Juretzek, T.; Herchenröder, O.; Mogel, M.; Poppe, M.; Henker, J.; Rethwilm, A. A simple and rapid single-step multiplex RT-PCR to detect Norovirus, Astrovirus and Adenovirus in clinical stool samples. J. Virol. Methods 2004, 118, 49–59. [Google Scholar] [CrossRef]
- Hiwarkar, P.; Kosulin, K.; Cesaro, S.; Mikulska, M.; Styczynski, J.; Wynn, R.; Lion, T. Management of adenovirus infection in patients after haematopoietic stem cell transplantation: State-of-the-art and real-life current approach: A position statement on behalf of the Infectious Diseases Working Party of the European Society of Blood and Marrow Transplantation. Rev. Med. Virol. 2018, 28, e1980. [Google Scholar]
- Jaff, D.O.; Aziz, T.A.; Smith, N.R. The incidence of rotavirus and adenovirus infections among children with diarrhea in Sulaimani Province, Iraq. J. Biosci. Med. 2015, 4, 124–131. [Google Scholar] [CrossRef]
- Harb, A.; Abraham, S.; Rusdi, B.; Laird, T.; O’Dea, M.; Habib, I. Molecular detection and epidemiological features of selected bacterial, viral, and parasitic enteropathogens in stool specimens from children with acute diarrhea in Thi-Qar Governorate, Iraq. Int. J. Environ. Res. Public Health 2019, 16, 1573. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, N.M.; Dove, W.; Abd-Eldayem, S.A.; Abu-Zeid, A.F.; Shamoon, H.E.; Hart, C.A. Molecular epidemiology and disease severity of respiratory syncytial virus in relation to other potential pathogens in children hospitalized with acute respiratory infection in Jordan. J. Med. Virol. 2008, 80, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Meqdam, M.M.; Nasrallah, G.; Al-Shurman, A. Detection of adenovirus infection in children in Jordan. Ann. Trop. Paediatr. 2001, 21, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, H.A.; Madi, N.M.; Al-Nakib, W. Analysis of viral diversity in stool samples from infants and children with acute gastroenteritis in Kuwait using Metagenomics approach. Virol. J. 2020, 17, 1–12. [Google Scholar] [CrossRef]
- Chehadeh, W.; Al-Adwani, A.; John, S.E.; Al-Dhufairi, S.; Al-Dousari, H.; Alkhaledi, M.; Al-Nakib, W. Adenovirus types associated with severe respiratory diseases: A retrospective 4-year study in Kuwait. J. Med. Virol. 2018, 90, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Al-Moyed, K.A.; Al-Jamrah, K.M.; Al-Robasi, A.B.A.; Nabhan, A.S.B.; Al-Haddad, A.M. Prevalence of enteric adenovirus among infants and young children suffering from acute gastroenteritis in Sana’a city, Yemen. Andal. J. Appl. Sci. 2015, 4, 79–81. [Google Scholar]
- Al Amad, M.A.; Al Mahaqri, A.A.; Al Serouri, A.A.; Khader, Y.S. Severe acute respiratory infections with influenza and noninfluenza respiratory viruses: Yemen, 2011–2016. INQUIRY J. Health Care Organ. Provis. Financ. 2019, 56, 1–7. [Google Scholar] [CrossRef]
- Aysha Waheed, A.; Muneera Abdulla, A.N.; Ghada Al, B.A. Adenovirus isolated from an outbreak of acute hemorrhagic conjunctivitis. Bahrain Med. Bull. 2016, 38, 224–226. [Google Scholar]
- Zaraket, R.; Salami, A.; Bahmad, M.; El Roz, A.; Khalaf, B.; Ghssein, G.; Bahmad, H.F. Prevalence, risk factors, and clinical characteristics of rotavirus and adenovirus among Lebanese hospitalized children with acute gastroenteritis. Heliyon 2020, 6, e04248. [Google Scholar] [CrossRef]
- Alsuwaidi, A.R.; Al Dhaheri, K.; Al Hamad, S.; George, J.; Ibrahim, J.; Ghatasheh, G.; Issa, M.; Al-Hammadi, S.; Narchi, H. Etiology of diarrhea by multiplex polymerase chain reaction among young children in the United Arab Emirates: A case-control study. BMC Infect. Dis. 2021, 21, 1–9. [Google Scholar] [CrossRef]
- Zaki, M.E.S.; El Kheir, N.A. Molecular study of astrovirus, adenovirus and norovirus in community acquired diarrhea in children: One Egyptian center study. Asian Pac. J. Trop. Biomed. 2017, 7, 987–990. [Google Scholar] [CrossRef]
- Elmahdy, E.M.; Ahmed, N.I.; Shaheen, M.N.; Mohamed, E.-C.B.; Loutfy, S.A. Molecular detection of human adenovirus in urban wastewater in Egypt and among children suffering from acute gastroenteritis. J. Water Health 2019, 17, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Allayeh, A.K.; El Baz, R.M.; Saeed, N.M.; Osman, M.E.S. Detection and genotyping of viral gastroenteritis in hospitalized children below five years old in Cairo, Egypt. Arch. Pediatric Infect. Dis. 2018, 6, e60288. [Google Scholar]
- Jroundi, I.; Mahraoui, C.; Benmessaoud, R.; Moraleda, C.; Tligui, H.; Seffar, M.; Kettani, S.C.; Benjelloun, B.S.; Chaacho, S.; Maaroufi, A. The epidemiology and aetiology of infections in children admitted with clinical severe pneumonia to a university hospital in Rabat, Morocco. J. Trop. Pediatrics 2014, 60, 270–278. [Google Scholar] [CrossRef]
- Bimouhen, A.; Regragui, Z.; El Falaki, F.; Ihazmade, H.; Benkerroum, S.; Cherkaoui, I.; Rguig, A.; Ezzine, H.; Benamar, T.; Triki, S.; et al. Viral aetiology of influenza-like illnesses and severe acute respiratory illnesses in Morocco, September 2014 to December 2016. J. Glob. Health. 2022, 12, 04062. [Google Scholar] [CrossRef]
- Elhag, W.I.; Saeed, H.A.; Omer, E.F.E.; Ali, A.S. Prevalence of rotavirus and adenovirus associated with diarrhea among displaced communities in Khartoum, Sudan. BMC Infect. Dis. 2013, 13, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Adam, M.A.; Wang, J.; Enan, K.-A.; Shen, H.; Wang, H.; El Hussein, A.R.; Musa, A.B.; Khidir, I.M.; Ma, X. Molecular survey of viral and bacterial causes of childhood diarrhea in Khartoum state, Sudan. Front. Microbiol. 2018, 9, 112. [Google Scholar] [CrossRef]
- Derrar, F.; Izri, K.; Kaddache, C.; Boukari, R.; Hannoun, D. Virologic study of acute lower respiratory tract infections in children admitted to the paediatric department of Blida University Hospital, Algeria. New Microbes New Infect. 2019, 30, 100536. [Google Scholar] [CrossRef]
- Rahouma, A.; Klena, J.D.; Krema, Z.; Abobker, A.A.; Treesh, K.; Franka, E.; Abusnena, O.; Shaheen, H.I.; El Mohammady, H.; Abudher, A. Enteric pathogens associated with childhood diarrhea in Tripoli-Libya. Am. J. Trop. Med. Hyg. 2011, 84, 886. [Google Scholar] [CrossRef]
- Brini, I.; Guerrero, A.; Ezzine, I.K.; Orth-Höller, D.; Hetzer, B.; Würzner, R.; Hazgui, O.; Handous, I.; Nouri-Merchaoui, S.; Bouguila, J. Human adenoviruses associated with respiratory illness in neonates, infants, and children in the Sousse area of Tunisia. J. Med. Virol. 2020, 92, 3081–3092. [Google Scholar] [CrossRef]
- Svensson, L.; Wadell, G.; Uhnoo, I.; Johansson, M.; Von Bonsdorff, C.-H. Cross-reactivity between enteric adenoviruses and adenovirus type 4: Analysis of epitopes by solid-phase immune electron microscopy. J. Gen. Virol. 1983, 64, 2517–2520. [Google Scholar] [CrossRef] [PubMed]
- Grimwood, K.; Carzino, R.; Barnes, G.L.; Bishop, R.F. Patients with enteric adenovirus gastroenteritis admitted to an Australian pediatric teaching hospital from 1981 to 1992. J. Clin. Microbiol. 1995, 33, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Saderi, H.; Roustai, M.; Sabahi, F.; Sadeghizadeh, M.; Owlia, P.; De Jong, J. Incidence of enteric adenovirus gastroenteritis in Iranian children. J. Clin. Virol. 2002, 24, 1–5. [Google Scholar] [CrossRef]
- Subekti, D.; Lesmana, M.; Tjaniadi, P.; Safari, N.; Frazier, E.; Simanjuntak, C.; Komalarini, S.; Taslim, J.; Campbell, J.; Oyofo, B. Incidence of Norwalk-like viruses, rotavirus and adenovirus infection in patients with acute gastroenteritis in Jakarta, Indonesia. FEMS Immunol. Med. Microbiol. 2002, 33, 27–33. [Google Scholar] [CrossRef]
- Koh, H.; Baek, S.Y.; Shin, J.I.; Chung, K.S.; Jee, Y.M. Coinfection of viral agents in Korean children with acute watery diarrhea. J. Korean Med. Sci. 2008, 23, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Bányai, K.; Esona, M.; Liu, A.; Wang, Y.; Tu, X.; Jiang, B. Molecular detection of novel adenoviruses in fecal specimens of captive monkeys with diarrhea in China. Vet. Microbiol. 2010, 142, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Tekin, A. Mardin\′deki akut gastroenteritli çocuklarda Rotavirüs ve Enterik Adenovirüs sıklığı. J. Clin. Exp. Investig. 2010, 1, 41–45. [Google Scholar]
- Costa, L.C.P.d.N.; Siqueira, J.A.M.; Portal, T.M.; Sousa, E.C.; Linhares, A.d.C.; Gabbay, Y.B.; Resque, H.R. Detection and genotyping of human adenovirus and sapovirus in children with acute gastroenteritis in Belém, Pará, between 1990 and 1992: First detection of GI. 7 and GV. 2 sapoviruses in Brazil. Rev. Da Soc. Bras. De Med. Trop. 2017, 50, 621–628. [Google Scholar] [CrossRef]
- CDC. Adenovirus VIS: Vaccine Information Statements (VISs); Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020. [Google Scholar]
- Kujawski, S.A.; Lu, X.; Schneider, E.; Blythe, D.; Boktor, S.; Farrehi, J.; Haupt, T.; McBride, D.; Stephens, E.; Sakthivel, S.K. Outbreaks of adenovirus-associated respiratory illness on 5 college campuses in the United States, 2018–2019. Clin. Infect. Dis. 2021, 72, 1992–1999. [Google Scholar] [CrossRef]
- Roy, S.; Sandhu, A.; Medina, A.; Clawson, D.S.; Wilson, J.M. Adenoviruses in fecal samples from asymptomatic rhesus macaques, United States. Emerg. Infect. Dis. 2012, 18, 1081. [Google Scholar] [CrossRef]
- Banatvala, J.E.; Griffiths, P.; Schoub, B.; Mortimer, P. Principles and Practice of Clinical Virology; Wiley: New York, NY, USA, 2009. [Google Scholar]
- Singh-Naz, N.; Rodriguez, W.; Kidd, A.; Brandt, C. Monoclonal antibody enzyme-linked immunosorbent assay for specific identification and typing of subgroup F adenoviruses. J. Clin. Microbiol. 1988, 26, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Guerrant, R.L.; Hughes, J.M.; Lima, N.L.; Crane, J. Diarrhea in developed and developing countries: Magnitude, special settings, and etiologies. Rev. Infect. Dis. 1990, 12, S41–S50. [Google Scholar] [CrossRef] [PubMed]
- Lamps, L.W. Infective disorders of the gastrointestinal tract. Histopathology 2007, 50, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, A.; Baumgart, D. Viral gastroenteritis in adults. Recent Pat. Antiinfect. Drug Discov. 2011, 6, 54–63. [Google Scholar] [CrossRef]
- Thewainy, H.T.; Hasony, H. Enteric adenovirus associated with acute gastroenteritis among hospitalized and healthy childern under five-years of age in Basrah, Iraq. Med. J. Basrah Univ. 2019, 37, 37–44. [Google Scholar]
- Wu, B.; Qi, X.; Xu, K.; Ji, H.; Zhu, Y.; Tang, F.; Zhou, M. Genetic characteristics of the coxsackievirus A24 variant causing outbreaks of acute hemorrhagic conjunctivitis in Jiangsu, China, 2010. PLoS ONE 2014, 9, e86883. [Google Scholar] [CrossRef]
| Region | Country | Study | Study Design | Targeted Children | Sample Size | Sample Location | Disease | Prevalence Rate |
|---|---|---|---|---|---|---|---|---|
| Middle East | Iraq | Ali Harb et al., 2019 | A descriptive cross-sectional observational study | Children below five years with acute diarrhea | 320 children | Stool specimens | Gastroenteritis | 4.5% |
| Iraq | Dilshad Jaff et al., 2016 | A descriptive cross-sectional observational study | Children below five years of age with gastroenteritis | 100 children | Stool specimens | Gastroenteritis | 3.0% | |
| Jordan | Nasser Kaplan et al., 2008 | A descriptive prospective cross-sectional study | Children below five years with an acute respiratory infection | 326 children | Nasopharyngeal aspirates | Acute respiratory infection | 18.0% | |
| Jordan | Mamdoh Meqdam et al., 2001 | A descriptive cross-sectional observational study | Children below thirteen years old with respiratory tract infections | 350 children | Nasopharyngeal aspirates | Acute respiratory infection | 15.4% | |
| Kuwait | Hawraa Mohammad et al., 2020 | A 4-year descriptive retrospective study | Children below ten years with gastroenteritis | 84 children | Stool samples | Gastroenteritis | 23.2% | |
| Kuwait | Wassim Chehadeh et al., 2018 | A 4-year descriptive retrospective study | Children below four years with severe respiratory disease | 743 children | Nasopharyngeal aspirate and Nasopharyngeal swab | Severe respiratory infections | 3.6% | |
| Yemen | Khaled Al-Moyed et al., 2015 | A descriptive cross-sectional observational study | Children below five years with acute gastroenteritis | 326 children | Stool samples | Gastroenteritis | 11% | |
| Yemen | Mohammad Al Amad et al., 2019 | A descriptive retrospective study | Children below fifteen years of age with severe acute respiratory infections | 1413 children | Nasopharyngeal and oropharyngeal swabs | Acute respiratory infection | 7.0% | |
| Bahrain | Aysha Agab et al., 2016 | A case study | A seven-year-old male and a five-year-old female | Two Bahraini siblings | Conjunctival swabs | Acute hemorrhagic conjunctivitis | NM | |
| Lebanon | Rasha Zaraket et al., 2020 | A 12-months descriptive retrospective study | Children below twelve years with acute gastroenteritis | 308 children | Stool samples | Gastroenteritis | 25.3% | |
| United Arab Emirates | Ahmed Alsuwaidi et al., 2021 | A descriptive case-control observational study | Children below five years with diarrhea | 203 children as a case | Stool samples | Acute diarrhea | 17.2% | |
| North Africa | Egypt | Abdou Kamal Allayeh et al., 2018 | A descriptive cross-sectional observational study | Children below five years with gastroenteritis | 119 children | Fecal diarrhea samples | Gastroenteritis | 6.7% |
| Egypt | Maysaa El Sayed Zaki et al., 2017 | A descriptive cross-sectional observational study | Children below five years with acute diarrhea | 100 children | Stool sample | Acute diarrhea | 20.0% | |
| Egypt | Elmahdy Elmahdy et al., 2019 | A descriptive cross-sectional observational study | Children below five years with gastroenteritis | 60 children | Stool samples | Gastroenteritis | 28.3% | |
| Morocco | Imane Jroundi et al., 2014 | A descriptive prospective cross-sectional study | Children below five years with respiratory symptomatology | 700 children | Nasopharyngeal aspirates | Acute respiratory infection | 17.0% | |
| Morocco | Marcil Sarrah et al., 2018 | A descriptive prospective cross-sectional study | Children below fourteen years with severe acute viral respiratory infections | 103 children | Nasopharyngeal aspirates | Acute respiratory infection | 16.5% | |
| Sudan | Wafa Elhag et al., 2013 | A descriptive cross-sectional observational study | Children below fourteen years old with acute diarrhea | 511 children | Stool samples | Acute diarrhea | 1.5% | |
| Sudan | Mosab Adam et al., 2018 | A descriptive cross-sectional observational study | Children below five years with acute diarrhea | 437 children | Stool samples | Acute diarrhea | 1.6% | |
| Algeria | Fawzi Derrar et al., 2019 | A descriptive prospective cross-sectional study | Children below two years with respiratory tract infections | 117 children | Nasal or nasopharyngeal aspiration | Acute respiratory infection | 7.5% | |
| Libya | Amal Rahouma et al., 2011 | A descriptive cross-sectional observational study | Children below five years with acute diarrhea | 239 children | Stool specimens | Gastroenteritis | 7.1% | |
| Tunisia | Ines Brini et al., 2020 | A descriptive cross-sectional observational study | Children below five years with acute respiratory infections | 583 children | Nasopharyngeal aspirate | Acute respiratory infection | 19.6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qashqari, F.S.I. Human Mastadenovirus Infections in Children: A Review of the Current Status in the Arab World in the Middle East and North Africa. Children 2022, 9, 1356. https://doi.org/10.3390/children9091356
Qashqari FSI. Human Mastadenovirus Infections in Children: A Review of the Current Status in the Arab World in the Middle East and North Africa. Children. 2022; 9(9):1356. https://doi.org/10.3390/children9091356
Chicago/Turabian StyleQashqari, Fadi S. I. 2022. "Human Mastadenovirus Infections in Children: A Review of the Current Status in the Arab World in the Middle East and North Africa" Children 9, no. 9: 1356. https://doi.org/10.3390/children9091356
APA StyleQashqari, F. S. I. (2022). Human Mastadenovirus Infections in Children: A Review of the Current Status in the Arab World in the Middle East and North Africa. Children, 9(9), 1356. https://doi.org/10.3390/children9091356






