Mechanical Circulatory Support Devices for the Treatment of Cardiogenic Shock Complicating Acute Myocardial Infarction—A Review
Abstract
:1. Introduction
2. Pathophysiology, Diagnosis, and Prognosis
3. Treatment
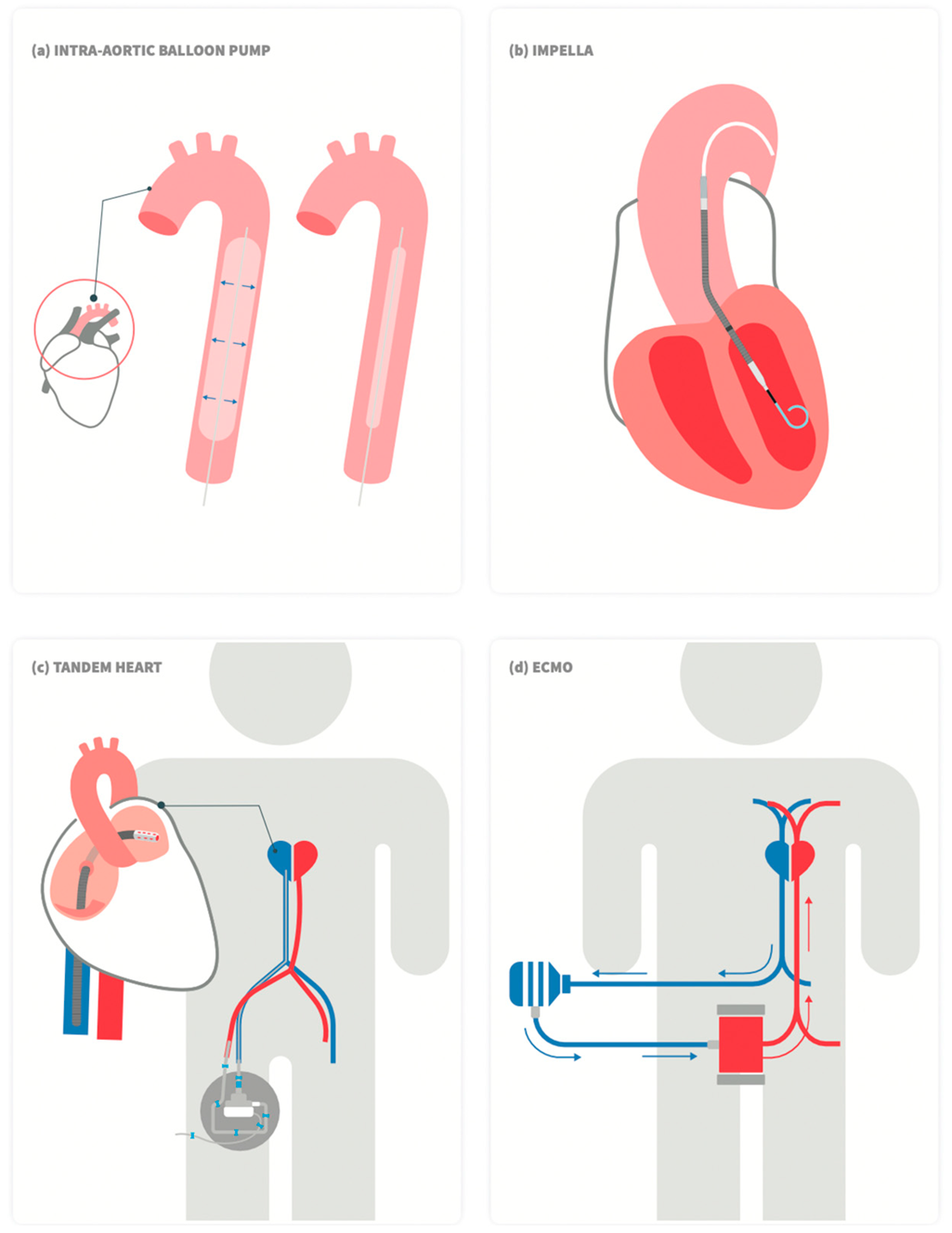
4. Mechanical Circulatory Support
5. Intra-Aortic Balloon Pump (IABP)
6. Impella
7. TendemHeart
8. Veno-Arterial Extracorporeal Membrane Oxygenation (VA-ECMO)
9. Combining MCS Devices in CS Management
10. The Use of MCS in the Setting of CS Due to Mechanical Complications of AMI
11. Structural Heart Interventions for Emergent Treatment of Patients with CS
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Thiele, H.; Ohman, E.M.; De Waha-Thiele, S.; Zeymer, U.; Desch, S. Management of cardiogenic shock complicating myocardial infarction: An update 2019. Eur. Heart J. 2019, 40, 2671–2683. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.; Syed, M.; Patibandla, S.; Sulaiman, S.; Kheiri, B.; Shah, M.K.; Bianco, C.; Balla, S.; Patel, B. Fifteen-Year Trends in Incidence of Cardiogenic Shock Hospitalization and In-Hospital Mortality in the United States. J. Am. Heart Assoc. 2021, 10, e021061. [Google Scholar] [CrossRef]
- Kolte, D.; Khera, S.; Aronow, W.S.; Mujib, M.; Palaniswamy, C.; Sule, S.; Jain, D.; Gotsis, W.; Ahmed, A.; Frishman, W.H.; et al. Trends in Incidence, Management, and Outcomes of Cardiogenic Shock Complicating ST-Elevation Myocardial Infarction in the United States. J. Am. Heart Assoc. 2014, 3, e000590. [Google Scholar] [CrossRef] [PubMed]
- Aissaoui, N.; Puymirat, E.; Delmas, C.; Ortuno, S.; Durand, E.; Bataille, V.; Drouet, E.; Bonello, L.; Bonnefoy-Cudraz, E.; Lesmeles, G.; et al. Trends in cardiogenic shock complicating acute myocardial infarction. Eur. J. Heart Fail. 2020, 22, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Jeger, R.; Radovanovic, D.; Hunziker, P.R.; Pfisterer, M.E.; Stauffer, J.-C.; Erne, P.; Urban, P. For the AMIS Plus Registry Investigators Ten-year trends in the incidence and treatment of cardiogenic shock. Ann. Intern. Med. 2008, 149, 618–626. [Google Scholar] [CrossRef]
- Van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation 2017, 136, e232–e268. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.D.; Tomey, M.I.; Tamis-Holland, J.E.; Thiele, H.; Rao, S.V.; Menon, V.; Klein, D.G.; Naka, Y.; Piña, I.L.; Kapur, N.K.; et al. Invasive Management of Acute Myocardial Infarction Complicated by Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e815–e829. [Google Scholar] [CrossRef]
- Goldberg, R.J.; Gore, J.M.; Alpert, J.S.; Osganian, V.; de Groot, J.; Bade, J.; Chen, Z.; Frid, D.; Dalen, J.E. Cardiogenic shock after acute myocardial infarction. Incidence and mortality from a community-wide perspective, 1975 to 1988. N. Engl. J. Med. 1991, 325, 1117–1122. [Google Scholar] [CrossRef]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Sanborn, T.A.; White, H.D.; Talley, J.D.; Christopher, E.B.; Jacobs, A.K.; Slater, J.N.; Col, J.; et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N. Engl. J. Med. 1999, 341, 625–634. [Google Scholar] [CrossRef]
- Thiele, H.; de Waha-Thiele, S.; Freund, A.; Zeymer, U.; Desch, S.; Fitzgerald, S. Management of cardiogenic shock. EuroIntervention 2021, 17, 451–465. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Samsky, M.D.; Morrow, D.A.; Proudfoot, A.G.; Hochman, J.S.; Thiele, H.; Rao, S.V. Cardiogenic Shock After Acute Myocardial Infarction: A Review. JAMA 2021, 326, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Garan, A.R.; Kapur, N.K.; O’Neill, W.W.; Lindenfeld, J.; Pinney, S.P.; Uriel, N.; Burkhoff, D.; Kern, M. Value of Hemodynamic Monitoring in Patients With Cardiogenic Shock Undergoing Mechanical Circulatory Support. Circulation 2020, 141, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
- Baran, D.A.; Grines, C.L.; Bailey, S.; Burkhoff, D.; Hall, S.A.; Henry, T.D.; Hollenberg, S.M.; Kapur, N.K.; O’Neill, W.; Ornato, J.P.; et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter. Cardiovasc. Interv. 2019, 94, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, L.W.; Pagani, F.D.; Young, J.B.; Jessup, M.; Miller, L.; Kormos, R.L.; Naftel, D.C.; Ulisney, K.; Desvigne-Nickens, P.; Kirklin, J.K. INTERMACS Profiles of Advanced Heart Failure: The Current Picture. J. Heart Lung Transplant. 2009, 28, 535–541. [Google Scholar] [CrossRef]
- Reynolds, H.; Hochman, J. Cardiogenic Shock: Current concepts and improving outcomes. Circulation 2008, 117, 686–697. [Google Scholar] [CrossRef]
- Overgaard, C.B.; Džavík, V. Inotropes and Vasopressors: Review of Physiology and Clinical Use in Cardiovascular Disease. Circulation 2008, 118, 1047–1056. [Google Scholar] [CrossRef]
- Vahdatpour, C.; Collins, D.; Goldberg, S. Cardiogenic Shock. J. Am. Heart Assoc. 2019, 8, e011991. [Google Scholar] [CrossRef]
- Trpkov, C.; Gibson, J.D.; Miller, R.J.; Grant, A.D.; Schnell, G.; Har, B.J.; Clarke, B. Percutaneous Left Ventricular Assist Device in Cardiogenic Shock: A Five-Year Single Canadian Center Initial Experience. CJC Open 2020, 2, 370–378. [Google Scholar] [CrossRef]
- Rihal, C.S.; Naidu, S.S.; Givertz, M.M.; Szeto, W.Y.; Burke, J.A.; Kapur, N.K.; Kern, M.; Garratt, K.N.; Goldstein, J.A.; Dimas, V.; et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J. Am. Coll. Cardiol. 2015, 65, e7–e26. [Google Scholar] [CrossRef] [Green Version]
- Balthazar, T.; Vandenbriele, C.; Verbrugge, F.H.; Uil, C.D.; Engström, A.; Janssens, S.; Rex, S.; Meyns, B.; Van Mieghem, N.; Price, S.; et al. Managing Patients with Short-Term Mechanical Circulatory Support: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- den Uil, C.A.; Akin, S.; Jewbali, L.S.; dos Reis Miranda, D.; Brugts, J.J.; Constantinescu, A.A.; Kappetein, A.P.; Caliskan, K. Short-term mechanical circulatory support as a bridge to durable left ventricular assist device implantation in refractory cardiogenic shock: A systematic review and meta-analysis. Eur. J. Cardio-Thorac. Surg. 2017, 52, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, B.N.; Truesdell, A.G.; Psotka, M.A.; Rosner, C.; Singh, R.; Sinha, S.S.; Damluji, A.A.; Batchelor, W.B. A Standardized and Comprehensive Approach to the Management of Cardiogenic Shock. JACC Heart Fail. 2020, 8, 879–891. [Google Scholar] [CrossRef]
- Ba, M.R.F.; Kapur, N.K.; Patel, K.; Salam, M.A.; Schreiber, T.; Kaki, A.; Hanson, I.; Almany, S.; Timmis, S.; Dixon, S.; et al. Improved Outcomes Associated with the use of Shock Protocols: Updates from the National Cardiogenic Shock Initiative. Catheter. Cardiovasc. Interv. 2019, 93, 1173–1183. [Google Scholar] [CrossRef]
- Telukuntla, K.S.; Estep, J.D. Acute Mechanical Circulatory Support for Cardiogenic Shock. Methodist DeBakey Cardiovasc. J. 2020, 16, 27–35. [Google Scholar] [CrossRef]
- Eckman, P.M.; Katz, J.N.; El Banayosy, A.; Bohula, E.A.; Sun, B.; van Diepen, S. Veno-Arterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock: An Introduction for the Busy Clinician. Circulation 2019, 140, 2019–2037. [Google Scholar] [CrossRef]
- Kimman, J.R.; Van Mieghem, N.M.; Endeman, H.; Brugts, J.J.; Constantinescu, A.A.; Manintveld, O.C.; Dubois, E.A.; Uil, C.A.D. Mechanical Support in Early Cardiogenic Shock: What Is the Role of Intra-aortic Balloon Counterpulsation? Curr. Heart Fail. Rep. 2020, 17, 247–260. [Google Scholar] [CrossRef]
- Patterson, T.; Perera, D.; Redwood, S.R. Intra-Aortic Balloon Pump for High-Risk Percutaneous Coronary Intervention. Circ. Cardiovasc. Interv. 2014, 7, 712–720. [Google Scholar] [CrossRef]
- Thiele, H.; Sick, P.; Boudriot, E.; Diederich, K.-W.; Hambrecht, R.; Niebauer, J.; Schuler, G. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur. Heart J. 2005, 26, 1276–1283. [Google Scholar] [CrossRef]
- Dangas, G.D.; Kini, A.S.; Sharma, S.K.; Henriques, J.P.; Claessen, B.; Dixon, S.R.; Massaro, J.M.; Palacios, I.; Popma, J.J.; Ohman, E.M.; et al. Impact of Hemodynamic Support With Impella 2.5 Versus Intra-Aortic Balloon Pump on Prognostically Important Clinical Outcomes in Patients Undergoing High-Risk Percutaneous Coronary Intervention (from the PROTECT II Randomized Trial). Am. J. Cardiol. 2013, 113, 222–228. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Neumann, F.-J.; Ferenc, M.; Olbrich, H.-G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Thelemann, N.; Neumann, F.-J.; Hausleiter, J.; Abdel-Wahab, M.; Meyer-Saraei, R.; Fuernau, G.; Eitel, I.; Hambrecht, R.; et al. Intraaortic Balloon Pump in Cardiogenic Shock Complicating Acute Myocardial Infarction: Long-Term 6-Year Outcome of the Randomized IABP-SHOCK II Trial. Circulation 2019, 139, 395–403. [Google Scholar] [CrossRef]
- Ahmad, Y.; Sen, S.; Shun-Shin, M.J.; Ouyang, J.; Finegold, J.A.; Al-Lamee, R.K.; Davies, J.E.R.; Cole, G.D.; Francis, D.P. Intra-aortic Balloon Pump Therapy for Acute Myocardial Infarction. JAMA Intern. Med. 2015, 175, 931–939. [Google Scholar] [CrossRef]
- Prondzinsky, R.; Unverzagt, S.; Russ, M.; Lemm, H.; Swyter, M.; Wegener, N.; Buerke, U.; Raaz, U.; Ebelt, H.; Schlitt, A.; et al. Hemodynamic Effects of Intra-aortic Balloon Counterpulsation in Patients With Acute Myocardial Infarction Complicated by Cardiogenic Shock. Shock 2012, 37, 378–384. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Cui, K.; Lyu, S.; Liu, H.; Song, X.; Yuan, F.; Xu, F.; Zhang, M.; Zhang, M.; Wang, W.; Zhang, D.; et al. Timing of initiation of intra-aortic balloon pump in patients with acute myocardial infarction complicated by cardiogenic shock: A meta-analysis. Clin. Cardiol. 2019, 42, 1126–1134. [Google Scholar] [CrossRef]
- Chhabra, L.; Chaudhry, H.; Chhabra, R.; Kayani, W.T. Impella device use in high-risk PCI. EuroIntervention 2019, 15, 731. [Google Scholar] [CrossRef]
- Schrage, B.; Ibrahim, K.; Loehn, T.; Werner, N.; Sinning, J.-M.; Pappalardo, F.; Pieri, M.; Skurk, C.; Lauten, A.; Landmesser, U.; et al. Impella Support for Acute Myocardial Infarction Complicated by Cardiogenic Shock. Circulation 2019, 139, 1249–1258. [Google Scholar] [CrossRef]
- Vetrovec, G.W.; Anderson, M.; Schreiber, T.; Popma, J.; Lombardi, W.; Maini, B.; Møller, J.E.; Schäfer, A.; Dixon, S.R.; Hall, S.; et al. The cVAD registry for percutaneous temporary hemodynamic support: A prospective registry of Impella mechanical circulatory support use in high-risk PCI, cardiogenic shock, and decompensated heart failure. Am. Heart J. 2018, 199, 115–121. [Google Scholar] [CrossRef]
- Seyfarth, M.; Sibbing, D.; Bauer, I.; Fröhlich, G.; Bott-Flügel, L.; Byrne, R.; Dirschinger, J.; Kastrati, A.; Schömig, A. A Randomized Clinical Trial to Evaluate the Safety and Efficacy of a Percutaneous Left Ventricular Assist Device Versus Intra-Aortic Balloon Pumping for Treatment of Cardiogenic Shock Caused by Myocardial Infarction. J. Am. Coll. Cardiol. 2008, 52, 1584–1588. [Google Scholar] [CrossRef] [Green Version]
- Ouweneel, D.M.; Eriksen, E.; Sjauw, K.D.; van Dongen, I.M.; Hirsch, A.; Packer, E.J.; Vis, M.M.; Wykrzykowska, J.J.; Koch, K.T.; Baan, J.; et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 69, 278–287. [Google Scholar] [CrossRef]
- Karami, M.; Eriksen, E.; Ouweneel, D.M.; Claessen, B.E.; Vis, M.M.; Baan, J.; Beijk, M.; Packer, E.J.S.; Sjauw, K.D.; Engstrom, A.; et al. Long-term 5-year outcome of the randomized IMPRESS in severe shock trial: Percutaneous mechanical circulatory support vs. intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 1009–1015. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Grines, C.; Schreiber, T.; Moses, J.; Maini, B.; Dixon, S.R.; Ohman, E.M. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am. Heart J. 2018, 202, 33–38. [Google Scholar] [CrossRef]
- Dhruva, S.S.; Ross, J.; Mortazavi, B.J.; Hurley, N.C.; Krumholz, H.M.; Curtis, J.P.; Berkowitz, A.; Masoudi, F.A.; Messenger, J.C.; Parzynski, C.S.; et al. Association of Use of an Intravascular Microaxial Left Ventricular Assist Device vs Intra-aortic Balloon Pump With In-Hospital Mortality and Major Bleeding Among Patients With Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA 2020, 323, 734. [Google Scholar] [CrossRef]
- Anderson, M.B.; Goldstein, J.; Milano, C.; Morris, L.D.; Kormos, R.L.; Bhama, J.; Kapur, N.K.; Bansal, A.; Garcia, J.; Baker, J.N.; et al. Benefits of a novel percutaneous ventricular assist device for right heart failure: The prospective RECOVER RIGHT study of the Impella RP device. J. Heart Lung Transplant. 2015, 34, 1549–1560. [Google Scholar] [CrossRef]
- Amin, A.P.; Spertus, J.A.; Curtis, J.P.; Desai, N.; Masoudi, F.A.; Bach, R.G.; McNeely, C.; Al-Badarin, F.; House, J.A.; Kulkarni, H.; et al. The Evolving Landscape of Impella Use in the United States Among Patients Undergoing Percutaneous Coronary Intervention With Mechanical Circulatory Support. Circulation 2020, 141, 273–284. [Google Scholar] [CrossRef]
- Burkhoff, D.; Cohen, H.; Brunckhorst, C.; O’Neill, W.W.; TandemHeart Investigators Group. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am. Heart J. 2006, 152, 469.e1–469.e8. [Google Scholar] [CrossRef]
- Cheng, J.M.; Uil, C.A.D.; Hoeks, S.E.; Van Der Ent, M.; Jewbali, L.S.; Van Domburg, R.T.; Serruys, P.W. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: A meta-analysis of controlled trials. Eur. Heart J. 2009, 30, 2102–2108. [Google Scholar] [CrossRef]
- Grandin, E.W.; Nunez, J.I.; Willar, B.; Kennedy, K.; Rycus, P.; Tonna, J.E.; Kapur, N.K.; Shaefi, S.; Garan, A.R. Mechanical Left Ventricular Unloading in Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation. J. Am. Coll. Cardiol. 2022, 79, 1239–1250. [Google Scholar] [CrossRef]
- Ouweneel, D.M.; Schotborgh, J.V.; Limpens, J.; Sjauw, K.D.; Engström, A.E.; Lagrand, W.K.; Cherpanath, T.G.V.; Driessen, A.H.G.; De Mol, B.A.J.M.; Henriques, J.P.S. Extracorporeal life support during cardiac arrest and cardiogenic shock: A systematic review and meta-analysis. Intensiv. Care Med. 2016, 42, 1922–1934. [Google Scholar] [CrossRef] [Green Version]
- Tsangaris, A.; Alexy, T.; Kalra, R.; Kosmopoulos, M.; Elliott, A.; Bartos, J.A.; Yannopoulos, D. Overview of Veno-Arterial Extracorporeal Membrane Oxygenation (VA-ECMO) Support for the Management of Cardiogenic Shock. Front. Cardiovasc. Med. 2021, 8, 686558. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Tahara, Y.; Koga, H.; Noguchi, T.; Inoue, S.; Yasuda, S. The association between time to extracorporeal cardiopulmonary resuscitation and outcome in patients with out-of-hospital cardiac arrest. Eur. Heart Journal. Acute Cardiovasc. Care 2022, 11, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-H.; Kim, H.C.; Ahn, C.-M.; Lee, S.-J.; Hong, S.-J.; Yang, J.H.; Kim, J.-S.; Kim, B.-K.; Ko, Y.-G.; Choi, D.; et al. Association Between Timing of Extracorporeal Membrane Oxygenation and Clinical Outcomes in Refractory Cardiogenic Shock. JACC Cardiovasc. Interv. 2021, 14, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Schrage, B.; Becher, P.M.; Bernhardt, A.; Bezerra, H.; Blankenberg, S.; Brunner, S.; Colson, P.; Deseda, G.C.; Dabboura, S.; Eckner, D.; et al. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: Results from an international, multicenter cohort study. Circulation 2020, 142, 2095–2106. [Google Scholar] [CrossRef]
- Hochman, J.S.; Buller, C.E.; Sleeper, L.A.; Boland, J.; Dzavik, V.; Sanborn, T.A.; Godfrey, E.; White, H.D.; Lim, J.; LeJemtel, T. Cardiogenic shock complicating acute myocardial infarction—Etiologies, management and outcome: A report from the SHOCK Trial Registry. J. Am. Coll. Cardiol. 2000, 36 (Suppl. 3), 1063–1070. [Google Scholar] [CrossRef]
- Damluji, A.A.; van Diepen, S.; Katz, J.N.; Menon, V.; Tamis-Holland, J.E.; Bakitas, M.; Cohen, M.G.; Balsam, L.B.; Chikwe, J. Mechanical Complications of Acute Myocardial Infarction: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e16–e35. [Google Scholar] [CrossRef]
- Nakamura, M.; Imamura, T.; Hori, M.; Yokoyama, S.; Doi, T.; Fukahara, K.; Kinugawa, K. Prognostic implication of risk scoring systems in patients with cardiogenic shock supported by ECMO and Impella. J. Artif. Organs 2021, 24, 372–376. [Google Scholar] [CrossRef]
- Fraccaro, C.; Teles, R.C.; Tchétché, D.; Saia, F.; Bedogni, F.; Montorfano, M.; Fiorina, C.; Meucci, F.; De Benedictis, G.M.; Leonzi, O.; et al. Transcatheter aortic valve implantation (TAVI) in cardiogenic shock: TAVI-shock registry results. Catheter. Cardiovasc. Interv. 2020, 96, 1128–1135. [Google Scholar] [CrossRef]
- Jung, R.G.; Simard, T.; Kovach, C.; Flint, K.; Don, C.; Di Santo, P.; Adamo, M.; Branca, L.; Valentini, F.; Benito-González, T.; et al. Transcatheter Mitral Valve Repair in Cardiogenic Shock and Mitral Regurgitation: A Patient-Level, Multicenter Analysis. JACC: Cardiovasc. Interv. 2020, 14, 1–11. [Google Scholar] [CrossRef]
- Tang, G.H.L.; Estevez-Loureiro, R.; Yu, Y.; Prillinger, J.B.; Zaid, S.; Psotka, M.A. Survival Following Edge-to-Edge Transcatheter Mitral Valve Repair in Patients With Cardiogenic Shock: A Nationwide Analysis. J. Am. Heart Assoc. 2021, 10, e019882. [Google Scholar] [CrossRef]
- Haberman, D.; Estévez-Loureiro, R.; Benito-Gonzalez, T.; Denti, P.; Arzamendi, D.; Adamo, M.; Freixa, X.; Nombela-Franco, L.; Villablanca, P.; Krivoshei, L.; et al. Conservative, surgical, and percutaneous treatment for mitral regurgitation shortly after acute myocardial infarction. Eur. Heart J. 2021, 43, 641–650. [Google Scholar] [CrossRef]
- Estévez-Loureiro, R.; Shuvy, M.; Taramasso, M.; Benito-Gonzalez, T.; Denti, P.; Arzamendi, D.; Adamo, M.; Freixa, X.; Villablanca, P.; Krivoshei, L.; et al. Use of MitraClip for mitral valve repair in patients with acute mitral regurgitation following acute myocardial infarction: Effect of cardiogenic shock on outcomes (IREMMI Registry). Catheter. Cardiovasc. Interv. 2021, 97, 1259–1267. [Google Scholar] [CrossRef]
| Stage | Description | Hemodynamics | Biochemical Markers |
|---|---|---|---|
| A At risk | No signs or symptoms of CS but at risk for CS development. May include patients with large acute myocardial infarction. | Normotensive (SBP ≥ 100 or normal for patient) If hemodynamics done: - Cardiac index ≥ 2.5 - CVP < 10 - PA sat ≥ 65% | Normal labs - Normal renal function - Normal lactic acid |
| B Beginning CS | A patient who has clinical evidence of relative hypotension or tachycardia without hypoperfusion. | SBP <90 or MAP <60 or >30 mmHg drop from baseline. - Pulse ≥ 100 - If hemodynamics done - Cardiac index ≥ 2.2 - PA sat ≥ 65% | - Normal lactate - Minimal renal function impairment - Elevated BNP |
| C Classic CS | A patient that manifests with hypoperfusion that requires intervention (inotrope, pressor, or mechanical support, including ECMO) beyond volume resuscitation to restore perfusion. These patients typically present with relative hypotension. | May include any of: SBP <90 or MAP <60 or >30 mmHg drop from baseline and drugs/device used to maintain BP above these targets Hemodynamics: - Cardiac index < 2.2 - PCWP >15 - RAP/PCWP ≥ 0.8 - PAPI < 1.85 - Cardiac power output ≤ 0.6 | May include any of the following: - Lactate ≥2 - Creatinine doubling OR >50% drop in GFR - Increased LFTs - Elevated BNP |
| D Deteriorating | A patient that is similar to category C but is getting worse. They have failure to respond to initial interventions. | Any of Stage C and: Requiring multiple pressors OR addition of mechanical circulatory support devices to maintain perfusion | Any of Stage C and: Deteriorating |
| E Extrimis | A patient that is experiencing cardiac arrest with ongoing CPR and/or ECMO being supported by multiple interventions. | No SBP without resuscitation PEA or refractory VT/VF hypotension despite maximal support | “Trying to die” - CPR (A-modifier) - pH ≤7.2 - Lactate ≥5 |
| Device | IABP | Impella (2.5, CP, 5.0, 5.5, ECP) | TendemHeart LA-FA | Impella RP | TendemHeart RA-PA | VA-ECMO |
|---|---|---|---|---|---|---|
| Flow | - | 2.5–5.5 L/min | Max 4 L/min | Max 4 L/min | Max 4 L/min | Max 7 L/min |
| Pump speed | - | Max 51,000 rpm | Max 7500 rpm | Max 33,000 rpm | Max 7500 rpm | Max 5000 rpm |
| Mechanism | Cardiac cycle timed balloon inflation-deflation | Axial flow continuous pump (LV to Ao) | Centrifugal flow continuous pump (LA to Ao) | Axial flow continuous pump (RA to PA) | Centrifugal flow continuous pump | Centrifugal flow continuous pump (RA to Ao) |
| Cannula size | 7–8F arterial | 9–14F arterial | 12–19F arterial 21F venous | 22F venous | 29F venous | 14–19F arterial 17–21F venous |
| Insertion | Femoral artery | Femoral artery | Femoral vein Femoral artery | Femoral vein | Internal jugular vein | Femoral vein Femoral artery |
| LV unloading | + | + to +++ | ++ | − | − | − |
| RV unloading | − | − | - | + | + | ++ |
| Cardiac power | − | ↑↑ | ↑↑ | - | - | ↑↑ |
| Afterload | ↓ | ↓↓ | ↑ | - | - | ↑↑ |
| Coronary perfusion | ↑ | ↑ | - | - | - | - |
| Complications | Cannula migration from LA to RA, tamponade, stroke, limb ischemia | Bleeding, hemolysis, vascular injuries, stroke, aortic valve injury | Dislodging of cannula, limb ischemia, femoral arteriovenous fistula, thromboembolism | Bleeding, hemolysis, vascular injuries, RV perforation, arrhythmia | Dislodging of cannula, vascular injury | Bleeding, thromboembolism, limb ischemia, renal failure, infections (including access site) lung edema, bleeding and hemoptysis |
| Contraindications | Severe aortic regurgitation, severe peripheral vascular disease precluding use | Severe peripheral vascular disease precluding use, LV thrombus, mechanical aortic valve, severe RV failure, aortic valve orifice area of 0.6 cm2 or less | Ventricular septal defect, significant aortic regurgitation | Inferior vena cava filter, severe tricuspis and/or pulmonic valve stenosis, mechanical right sided valves, thrombi in vena cava, right atrium | Ventricular septal defect | Expected lack of benefit (short life expectancy, terminal illness) |
| Study | Description | Sample Size | Primary Endpoint |
|---|---|---|---|
| Study on Early Intra-aortic Balloon Pump Placement in Acute Decompensated Heart Failure Complicated by Cardiogenic Shock (Altshock-2) | Patients will be randomized 1:1 to early IABP (within six hours of onset of cardiogenic shock) versus standard of care (vasoactive therapy). | 200 | 60-day patients’ survival or successful bridge to heart replacement therapy. |
| Danish-German Cardiogenic Shock Trial (DanGer Shock) | Patients will be randomized to receive conventional circulatory support or support with the Impella CP device for a minimum of 48 h and inotropic support if needed. | 360 | All-cause mortality |
| Acute Impact of the Impella CP Assist Device in Pts. With Cardiogenic Shock on the Patients Hemodynamic (JenaMACS) | Assessment of the acute hemodynamic effects following implantation of the IMPELLA CP cardiac support device | 20 | Surrogate endpoint |
| Impella CP With VA ECMO for Cardiogenic Shock (REVERSE) | VA-ECMO with Impella CP (LV venting) versus VA-ECMO alone in cardiogenic shock. | 96 | Surrogate endpoint |
| Transient Circulatory Support in Cardiogenic Shock (ALLOASSIST) | Transient circulatory support (VA-ECMO, Impella) vs. standard therapy. | 240 | In-hospital mortality (from inclusion day to day 180) |
| Assessment of ECMO in Acute Myocardial Infarction Cardiogenic Shock (ANCHOR) | VA-ECMO via the femoral route, with IABP in the contralateral femoral artery versus ESC guidelines management. (i.e., no devices). | 400 | Treatment failure at 30 days: Death in the ECMO group and death OR rescue ECMO in the control group |
| Extracorporeal Life Support in Cardiogenic Shock (ECLS-SHOCK) | Extracorporeal life support and revascularization (PCI or CABG; ECLS insertion should be performed preferably before revascularization) versus revascularization alone. | 420 | 30-day mortality |
| ExtraCorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock (ECMO-CS) | VA-ECMO versus control in cardiogenic shock complicating myocardial infarction. | 120 | Composite of death from any cause, resuscitated circulatory arrest, and implantation of another mechanical circulatory support device within 30 days |
| Testing the Value of Novel Strategy and Its Cost Efficacy in Order to Improve the Poor Outcomes in Cardiogenic Shock (EUROSHOCK) | Early intervention with ECMO therapy vs. standard treatment (no ECMO). | 428 | All-cause mortality |
| ECMOsorb Trial-Impact of a VA-ECMO in Combination With CytoSorb in Critically Ill Patients With Cardiogenic Shock (ECMOsorb) | VA-ECMO and CytoSorb (An extracorporeal cytokine hemoadsorption system is integrated in the VA-ECMO circuit) vs. VA-ECMO without CytoSorb. | 54 | Surrogate endpoint |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruoha, S.; Yosefy, C.; Taha, L.; Dvir, D.; Shuvy, M.; Jubeh, R.; Carasso, S.; Glikson, M.; Asher, E. Mechanical Circulatory Support Devices for the Treatment of Cardiogenic Shock Complicating Acute Myocardial Infarction—A Review. J. Clin. Med. 2022, 11, 5241. https://doi.org/10.3390/jcm11175241
Bruoha S, Yosefy C, Taha L, Dvir D, Shuvy M, Jubeh R, Carasso S, Glikson M, Asher E. Mechanical Circulatory Support Devices for the Treatment of Cardiogenic Shock Complicating Acute Myocardial Infarction—A Review. Journal of Clinical Medicine. 2022; 11(17):5241. https://doi.org/10.3390/jcm11175241
Chicago/Turabian StyleBruoha, Sharon, Chaim Yosefy, Louay Taha, Danny Dvir, Mony Shuvy, Rami Jubeh, Shemy Carasso, Michael Glikson, and Elad Asher. 2022. "Mechanical Circulatory Support Devices for the Treatment of Cardiogenic Shock Complicating Acute Myocardial Infarction—A Review" Journal of Clinical Medicine 11, no. 17: 5241. https://doi.org/10.3390/jcm11175241
APA StyleBruoha, S., Yosefy, C., Taha, L., Dvir, D., Shuvy, M., Jubeh, R., Carasso, S., Glikson, M., & Asher, E. (2022). Mechanical Circulatory Support Devices for the Treatment of Cardiogenic Shock Complicating Acute Myocardial Infarction—A Review. Journal of Clinical Medicine, 11(17), 5241. https://doi.org/10.3390/jcm11175241







