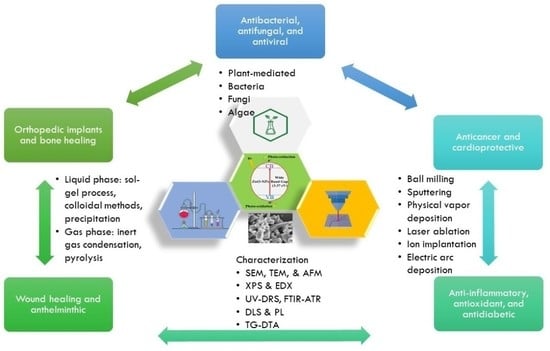
Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications
Abstract
1. Introduction
2. Biological Activities of ZnO-NPs
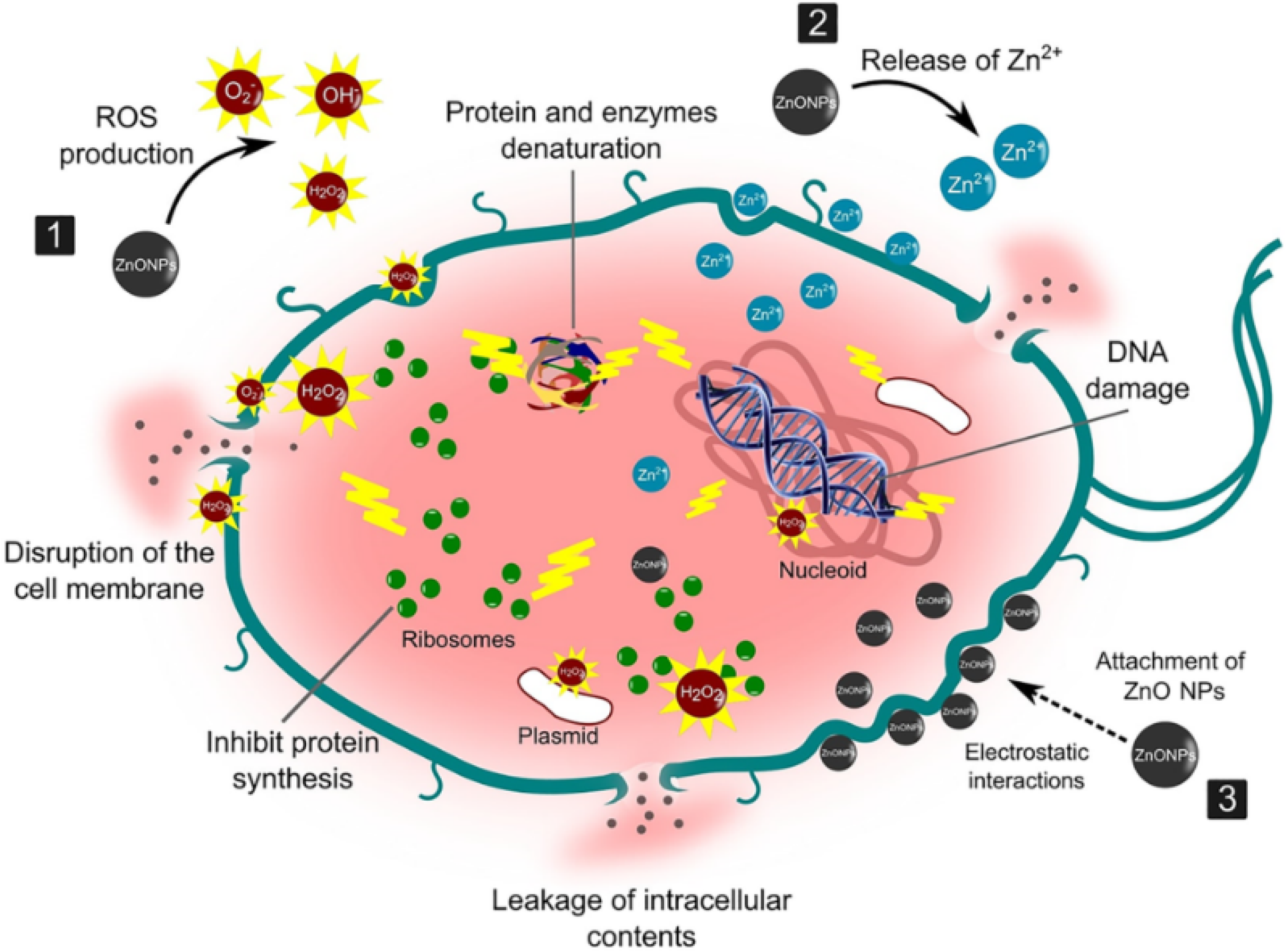
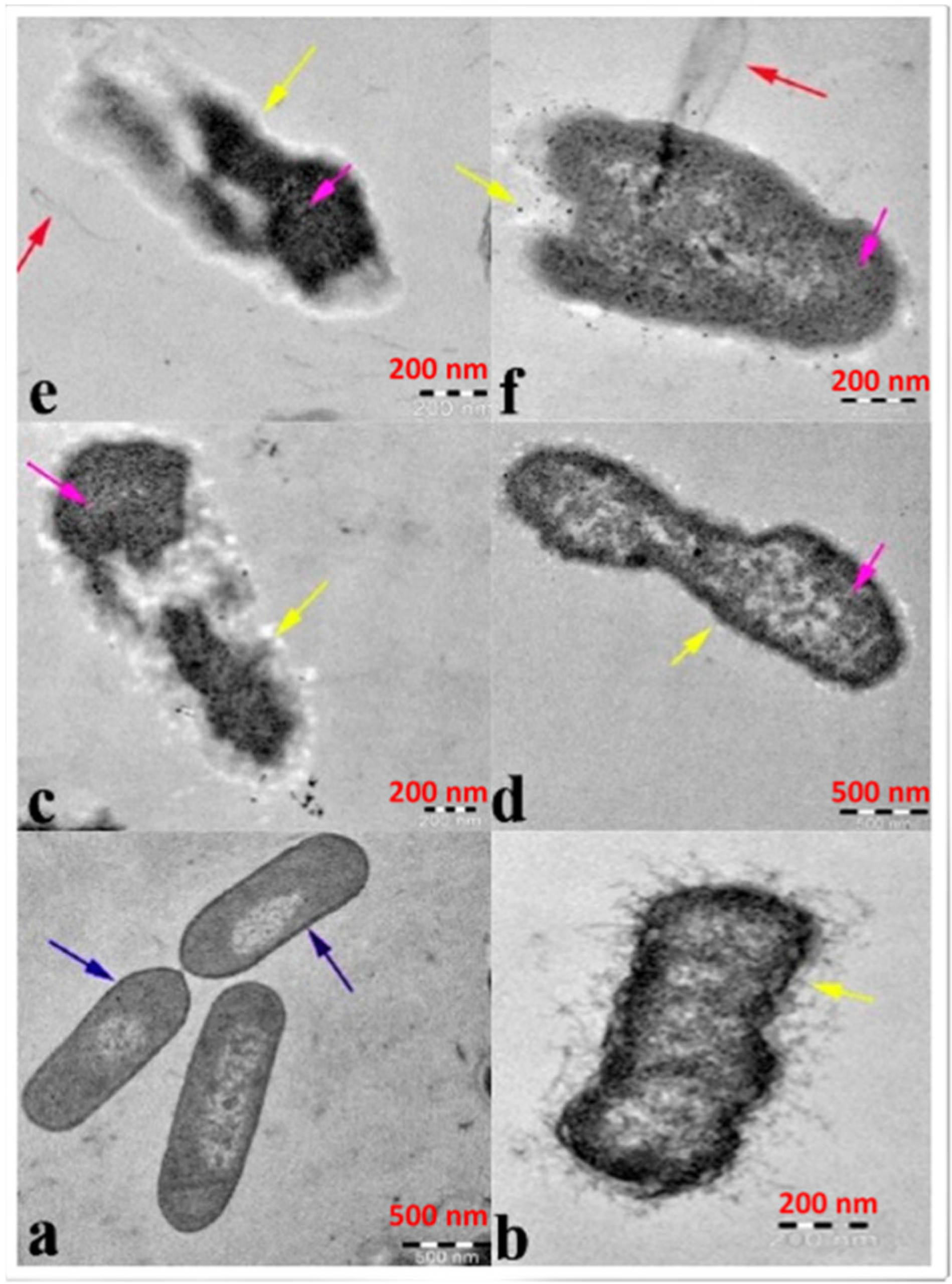
2.1. Antibacterial Action of ZnO-NPs
2.2. Antifungal Action of ZnO-NPs
2.3. Cytotoxic Effect of ZnO-NPs
2.4. Wound Healing Activity of ZnO-NPs
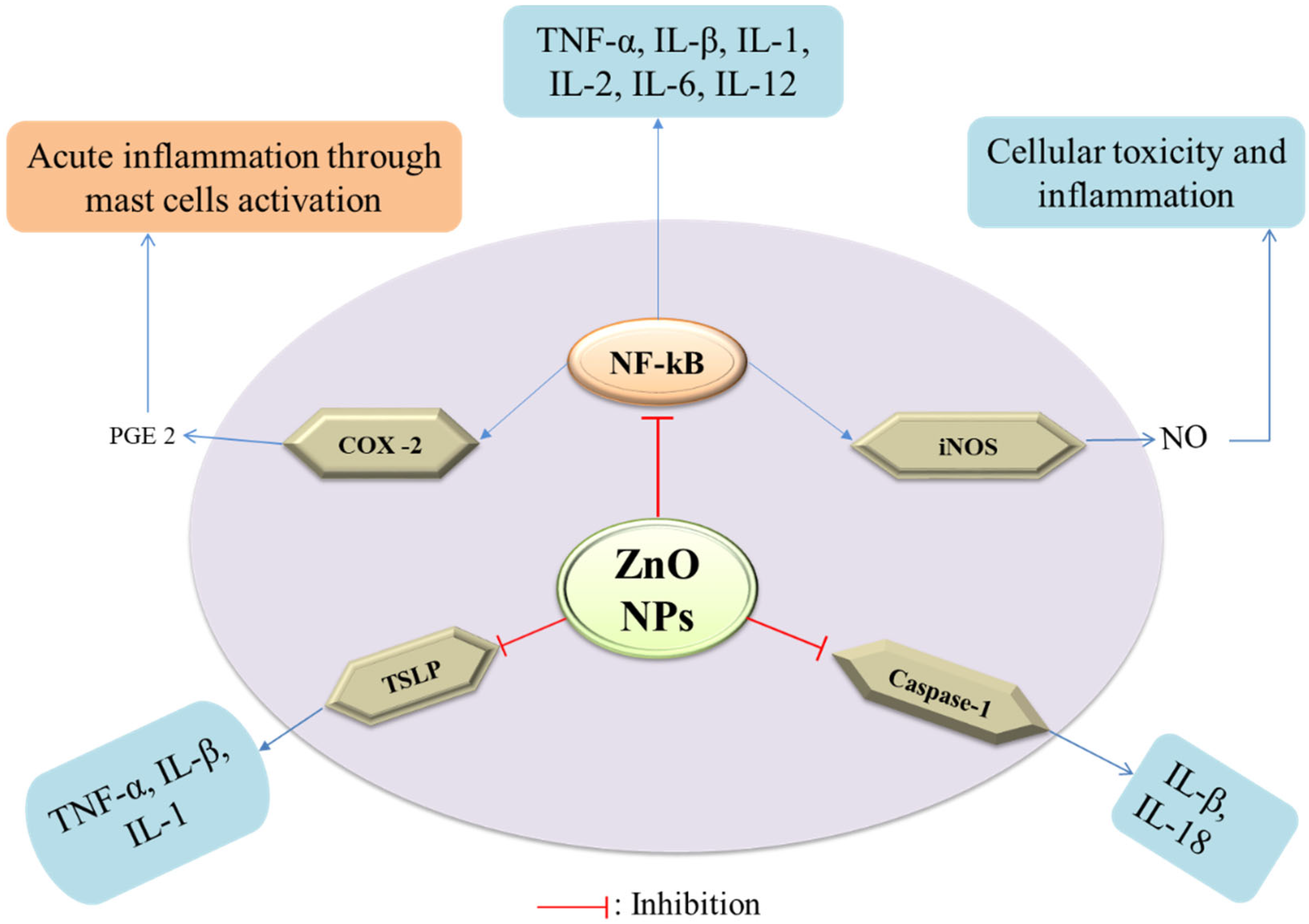
2.5. Anti-Inflammatory Activity of ZnO-NPs
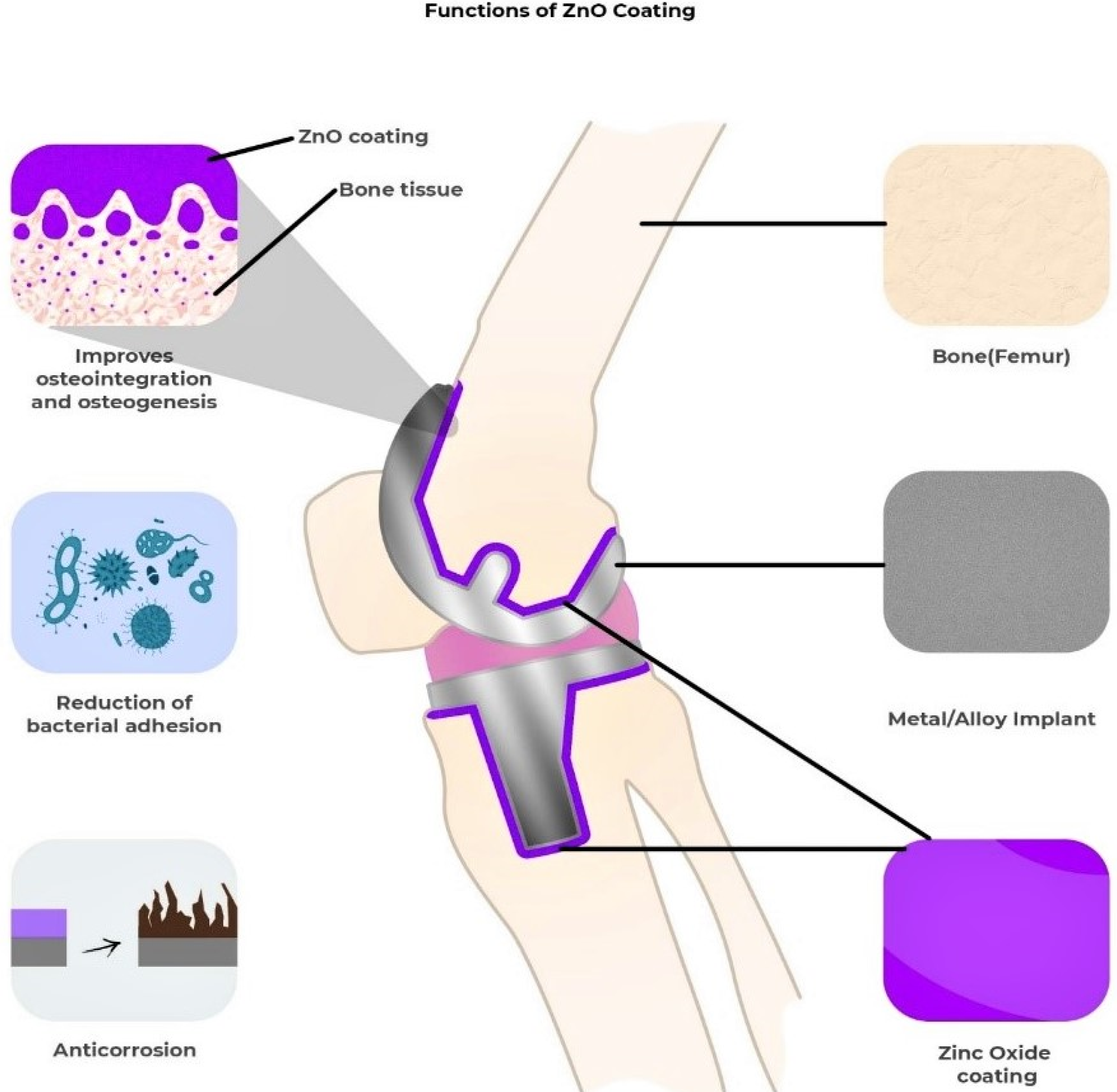
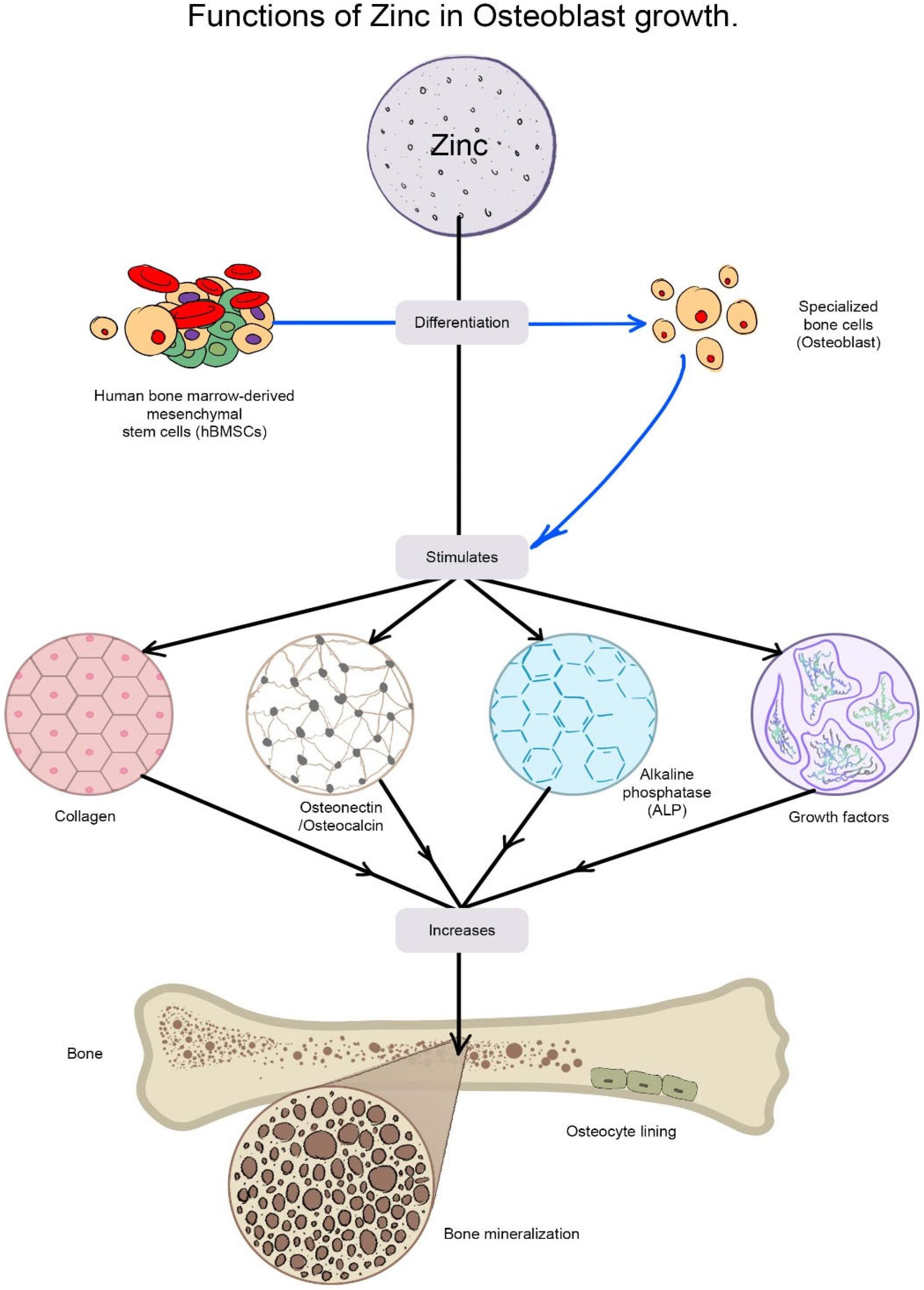
2.6. Orthopedic Implants and Bone Healing Activity of ZnO-NPs
2.7. Antidiabetic Action of ZnO-NPs
2.8. Antioxidant Activity of ZnO-NPs
2.9. Antiviral Action of ZnO-NPs
2.10. Cardioprotective Action of ZnO-NPs
2.11. Anthelminthic Action of ZnO-NPs
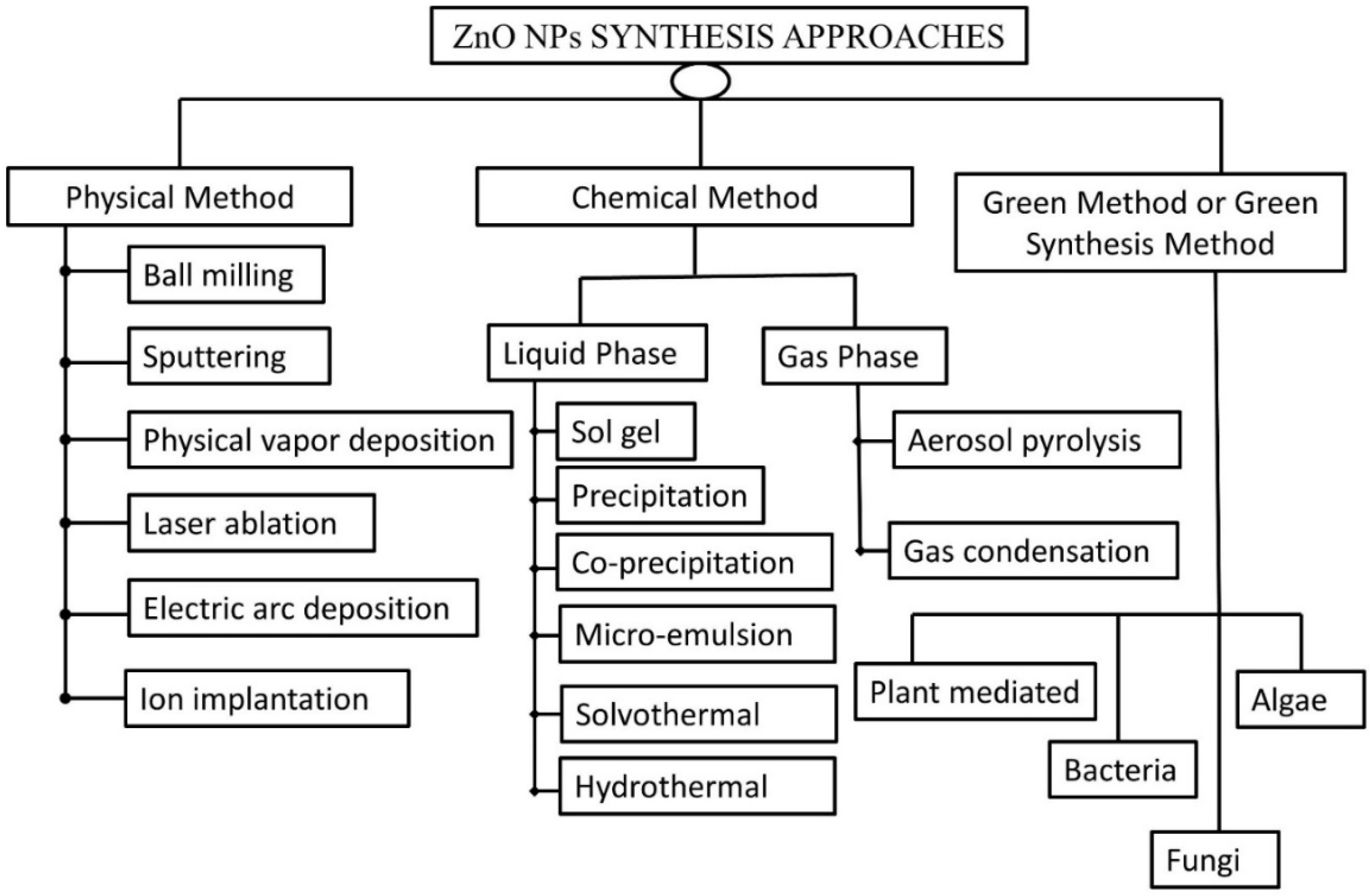
3. Approaches for Synthesizing ZnO-NPs
3.1. Physical Methods
3.2. Chemical Methods
3.2.1. Liquid-Phase Synthesis
3.2.2. Gas-Phase Synthesis
3.3. Green Synthesis
3.3.1. Plant-Mediated Synthesis of ZnO-NPs
3.3.2. Green Synthesis Using Bacterial Extracts
3.3.3. Green Synthesis Using Fungal Extracts
3.3.4. Green Synthesis Using Microalgae and Macroalgae
4. Characterization of ZnO-NPs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jayachandran, A.; Aswathy, T.R.; Nair, A.S. Green synthesis and characterization of zinc oxide nanoparticles using Cayratia pedata leaf extract. Biochem. Biophys. Rep. 2021, 26, 100995. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles—An eco-friendly approach. Resour. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Rodnyi, P.A.; Khodyuk, I.V. Optical and luminescence properties of zinc oxide (Review). Opt. Spectrosc. 2011, 111, 776–785. [Google Scholar] [CrossRef]
- Shaba, E.Y.; Jacob, J.O.; Tijani, J.O.; Suleiman, M.A.T. A critical review of synthesis parameters affecting the properties of zinc oxide nanoparticle and its application in wastewater treatment. Appl. Water Sci. 2021, 11, 48. [Google Scholar] [CrossRef]
- Kielbik, P.; Kaszewski, J.; Rosowska, J.; Wolska, E.; Witkowski, B.; Gralak, M.; Gajewski, Z.; Godlewski, M.; Godlewski, M.M. Biodegradation of the ZnO:Eu nanoparticles in the tissues of adult mouse after alimentary application. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 843–852. [Google Scholar] [CrossRef]
- Mandal, B.K. Scopes of green synthesized metal and metal oxide nanomaterials in antimicrobial therapy. In Nanobiomaterials in Antimicrobial Therapy; William Andrew Publishing: Cambridge, MA, USA, 2016; pp. 313–341. [Google Scholar] [CrossRef]
- Wiesmann, N.; Tremel, W.; Brieger, J. Zinc oxide nanoparticles for therapeutic purposes in cancer medicine. J. Mater. Chem. B 2020, 8, 4973–4989. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, J.; Wang, B.; Xu, G.; Yang, X.; Zou, Z.; Yu, C. Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy 2021, 17, 4266–4285. [Google Scholar] [CrossRef] [PubMed]
- Wiesmann, N.; Kluenker, M.; Demuth, P.; Brenner, W.; Tremel, W.; Brieger, J. Zinc overload mediated by zinc oxide nanoparticles as innovative anti-tumor agent. J. Trace Elem. Med. Biol. 2018, 51, 226–234. [Google Scholar] [CrossRef]
- Stepankova, H.; Swiatkowski, M.; Kruszynski, R.; Svec, P.; Michalkova, H.; Smolikova, V.; Ridoskova, A.; Splichal, Z.; Michalek, P.; Richtera, L.; et al. The Anti-Proliferative Activity of Coordination Compound-Based ZnO Nanoparticles as a Promising Agent against Triple Negative Breast Cancer Cells. Int. J. Nanomed. 2021, 16, 4431–4449. [Google Scholar] [CrossRef]
- Bai, K.-J.; Chuang, K.-J.; Ma, C.-M.; Chang, T.-Y.; Chuang, H.-C. Human lung adenocarcinoma cells with an EGFR mutation are sensitive to non-autophagic cell death induced by zinc oxide and aluminium-doped zinc oxide nanoparticles. J. Toxicol. Sci. 2017, 42, 437–444. [Google Scholar] [CrossRef]
- Alsagaby, S.A.; Vijayakumar, R.; Premanathan, M.; Mickymaray, S.; Alturaiki, W.; Al-Baradie, R.S.; AlGhamdi, S.; Aziz, M.A.; Alhumaydhi, F.A.; Alzahrani, F.A.; et al. Transcriptomics-Based Characterization of the Toxicity of ZnO Nanoparticles against Chronic Myeloid Leukemia Cells. Int. J. Nanomed. 2020, 15, 7901–7921. [Google Scholar] [CrossRef]
- He, T.; Long, J.; Li, J.; Liu, L.; Cao, Y. Toxicity of ZnO nanoparticles (NPs) to A549 cells and A549 epithelium in vitro: Interactions with dipalmitoyl phosphatidylcholine (DPPC). Environ. Toxicol. Pharmacol. 2017, 56, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Hatami, Z.; Ragheb, E.; Jalali, F.; Tabrizi, M.A.; Shamsipur, M. Zinc oxide-gold nanocomposite as a proper platform for label-free DNA biosensor. Bioelectrochemistry 2020, 133, 107458. [Google Scholar] [CrossRef]
- Nath, J.; Dror, I.; Landa, P.; Vanek, T.; Kaplan-Ashiri, I.; Berkowitz, B. Synthesis and characterization of isotopically-labeled silver, copper and zinc oxide nanoparticles for tracing studies in plants. Environ. Pollut. 2018, 242, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.W.; Cui, Z.M.; Liu, Z.Q.; Xu, F.; Chen, Y.S.; Luo, Y.L. Organic-Inorganic Nanohybrid Electrochemical Sensors from Multi-Walled Carbon Nanotubes Decorated with Zinc Oxide Nanoparticles and In-Situ Wrapped with Poly(2-methacryloyloxyethyl ferrocenecarboxylate) for Detection of the Content of Food Additives. Nanomaterials 2019, 9, 1388. [Google Scholar] [CrossRef]
- Eymard-Vernain, E.; Luche, S.; Rabilloud, T.; Lelong, C. Impact of nanoparticles on the Bacillus subtilis (3610) competence. Sci. Rep. 2018, 8, 2978. [Google Scholar] [CrossRef] [PubMed]
- Shakerimoghaddam, A.; Razavi, D.; Rahvar, F.; Khurshid, M.; Ostadkelayeh, S.M.; Esmaeili, S.-A.; Khaledi, A.; Eshraghi, M. Evaluate the Effect of Zinc Oxide and Silver Nanoparticles on Biofilm and icaA Gene Expression in Methicillin-Resistant Staphylococcus aureus Isolated from Burn Wound Infection. J. Burn Care Res. 2020, 41, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Bashandy, S.A.; Alaamer, A.; Moussa, S.; Omara, E.A. Role of zinc oxide nanoparticles in alleviating hepatic fibrosis and nephrotoxicity induced by thioacetamide in rats. Can. J. Physiol. Pharmacol. 2018, 96, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Anan, H.H.; Zidan, R.A.; El-Baset, S.A.A.; Ali, M.M. Ameliorative effect of zinc oxide nanoparticles on cyclophosphamide induced testicular injury in adult rat. Tissue Cell 2018, 54, 80–93. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, H.-R.; Dong, L.; Xu, M.-R.; Zhang, L.; Ding, W.-P.; Zhang, J.-Q.; Lin, J.; Zhang, Y.-J.; Qiu, B.-S.; et al. Enhancing tumor chemotherapy and overcoming drug resistance through autophagy-mediated intracellular dissolution of zinc oxide nanoparticles. Nanoscale 2019, 11, 11789–11807. [Google Scholar] [CrossRef]
- Świątek, Z.M.; Woźnicka, O.; Bednarska, A.J. Unravelling the ZnO-NPs mechanistic pathway: Cellular changes and altered morphology in the gastrointestinal tract of the earthworm Eisenia andrei. Ecotoxicol. Environ. Saf. 2020, 196, 110532. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Shah, M.; Hashmi, S.S.; Nazir, M.; Naz, S.; Ahmad, W.; Khan, I.U.; Hano, C. Green Bio-Assisted Synthesis, Characterization and Biological Evaluation of Biocompatible ZnO NPs Synthesized from Different Tissues of Milk Thistle (Silybum marianum). Nanomaterials 2019, 9, 1171. [Google Scholar] [CrossRef]
- Lakshmipriya, T.; Gopinath, S.C.B. Introduction to nanoparticles and analytical devices. In Nanoparticles in Analytical and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–29. [Google Scholar] [CrossRef]
- Kalpana, V.N.; Rajeswari, V.D. A Review on Green Synthesis, Biomedical Applications, and Toxicity Studies of ZnO NPs. Bioinorg. Chem. Appl. 2018, 2018, e3569758. [Google Scholar] [CrossRef]
- Sur, D.H.; Mukhopadhyay, M. Role of zinc oxide nanoparticles for effluent treatment using Pseudomonas putida and Pseudomonas aureofaciens. Bioprocess Biosyst. Eng. 2018, 42, 187–198. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro. Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.d.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Yusof, H.M.; Mohamad, R.; Zaidan, U.H.; Rahman, N.A.A. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: A review. J. Anim. Sci. Biotechnol. 2019, 10, 57. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Athinarayanan, J.; Periyasamy, V.S.; Alshuniaber, M.A.; Alshammari, G.; Hakeem, M.J.; Ahmed, M.A.; Alshatwi, A.A. Antibacterial Mechanisms of Zinc Oxide Nanoparticle against Bacterial Food Pathogens Resistant to Beta-Lactam Antibiotics. Molecules 2022, 27, 2489. [Google Scholar] [CrossRef]
- Abomuti, M.A.; Danish, E.Y.; Firoz, A.; Hasan, N.; Malik, M.A. Green Synthesis of Zinc Oxide Nanoparticles Using Salvia officinalis Leaf Extract and Their Photocatalytic and Antifungal Activities. Biology 2021, 10, 1075. [Google Scholar] [CrossRef]
- Xu, M.-N.; Li, L.; Pan, W.; Zheng, H.-X.; Wang, M.-L.; Peng, X.-M.; Dai, S.-Q.; Tang, Y.-M.; Zeng, K.; Huang, X.-W. Zinc Oxide Nanoparticles Prime a Protective Immune Response in Galleria mellonella to Defend against Candida albicans. Front. Microbiol. 2021, 12, 766138. [Google Scholar] [CrossRef]
- Sonia, S.; Ruckmani, K.; Sivakumar, M. Antimicrobial and antioxidant potentials of biosynthesized colloidal zinc oxide nanoparticles for a fortified cold cream formulation: A potent nanocosmeceutical application. Mater. Sci. Eng. C 2017, 79, 581–589. [Google Scholar] [CrossRef]
- Shobha, N.; Nanda, N.; Giresha, A.S.; Manjappa, P.; Sophiya, P.; Dharmappa, K.; Nagabhushana, B. Synthesis and characterization of Zinc oxide nanoparticles utilizing seed source of Ricinus communis and study of its antioxidant, antifungal and anticancer activity. Mater. Sci. Eng. C 2018, 97, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Al-Janabi, A.A.H.S.; Bashi, A.M. Development of a new synthetic xerogel nanoparticles of silver and zinc oxide against causative agents of dermatophytoses. J. Dermatol. Treat. 2018, 30, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, Q.; Huang, H.; Duan, Y.; Xiao, G.; Le, T. Enhanced physico-mechanical, barrier and antifungal properties of soy protein isolate film by incorporating both plant-sourced cinnamaldehyde and facile synthesized zinc oxide nanosheets. Colloids Surf. B Biointerfaces 2019, 180, 31–38. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef]
- Kairyte, K.; Kadys, A.; Luksiene, Z. Antibacterial and antifungal activity of photoactivated ZnO nanoparticles in suspension. J. Photochem. Photobiol. B Biol. 2013, 128, 78–84. [Google Scholar] [CrossRef]
- Sharma, D.; Rajput, J.; Kaith, B.; Kaur, M.; Sharma, S. Synthesis of ZnO nanoparticles and study of their antibacterial and antifungal properties. Thin Solid Films 2010, 519, 1224–1229. [Google Scholar] [CrossRef]
- Motazedi, R.; Rahaiee, S.; Zare, M. Efficient biogenesis of ZnO nanoparticles using extracellular extract of Saccharomyces cerevisiae: Evaluation of photocatalytic, cytotoxic and other biological activities. Bioorg. Chem. 2020, 101, 103998. [Google Scholar] [CrossRef]
- Dobrucka, R.; Dlugaszewska, J.; Kaczmarek, M. Cytotoxic and antimicrobial effects of biosynthesized ZnO nanoparticles using of Chelidonium majus extract. Biomed. Microdevices 2017, 20, 5. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, X.; Zheng, Y. Biosynthesis of polyphenols functionalized ZnO nanoparticles: Characterization and their effect on human pancreatic cancer cell line. J. Photochem. Photobiol. B Biol. 2018, 183, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.-C.; Wang, C.; Park, W.I. ZnO nanorods: Synthesis, characterization and applications. Semicond. Sci. Technol. 2005, 20, S22–S34. [Google Scholar] [CrossRef]
- Wang, H.; Wingett, D.; Engelhard, M.; Feris, K.; Reddy, K.M.; Turner, P.; Layne, J.; Hanley, C.; Bell, J.; Tenne, D.; et al. Fluorescent dye encapsulated ZnO particles with cell-specific toxicity for potential use in biomedical applications. J. Mater. Sci. Mater. Electron. 2008, 20, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.; Layne, J.; Punnoose, A.; Reddy, K.M.; Coombs, I.; Coombs, A.; Feris, K.; Wingett, D. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 2008, 19, 295103. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef]
- Chong, C.L.; Fang, C.M.; Pung, S.Y.; Ong, C.E.; Pung, Y.F.; Kong, C.; Pan, Y. Current Updates On the In vivo Assessment of Zinc Oxide Nanoparticles Toxicity Using Animal Models. BioNanoScience 2021, 11, 590–620. [Google Scholar] [CrossRef]
- Kaur, G.; Narayanan, G.; Garg, D.; Sachdev, A.; Matai, I. Biomaterials-Based Regenerative Strategies for Skin Tissue Wound Healing. ACS Appl. Bio Mater. 2022, 5, 2069–2106. [Google Scholar] [CrossRef]
- Khan, A.U.R.; Huang, K.; Jinzhong, Z.; Zhu, T.; Morsi, Y.; Aldalbahi, A.; El-Newehy, M.; Yan, X.; Mo, X. Exploration of the antibacterial and wound healing potential of a PLGA/silk fibroin based electrospun membrane loaded with zinc oxide nanoparticles. J. Mater. Chem. B 2021, 9, 1452–1465. [Google Scholar] [CrossRef]
- Hasannasab, M.; Nourmohammadi, J.; Dehghan, M.M.; Ghaee, A. Immobilization of bromelain and ZnO nanoparticles on silk fibroin nanofibers as an antibacterial and anti-inflammatory burn dressing. Int. J. Pharm. 2021, 610, 121227. [Google Scholar] [CrossRef]
- Kantipudi, S.; Sunkara, J.R.; Rallabhandi, M.; Thonangi, C.V.; Cholla, R.D.; Kollu, P.; Parvathaneni, M.K.; Pammi, S.V.N. Enhanced wound healing activity of Ag–ZnO composite NPs in Wistar Albino rats. IET Nanobiotechnol. 2018, 12, 473–478. [Google Scholar] [CrossRef]
- Metwally, A.A.; Abdel-Hady, A.-N.A.A.; Haridy, M.A.M.; Ebnalwaled, K.; Saied, A.A.; Soliman, A.S. Wound healing properties of green (using Lawsonia inermis leaf extract) and chemically synthesized ZnO nanoparticles in albino rats. Environ. Sci. Pollut. Res. 2021, 29, 23975–23987. [Google Scholar] [CrossRef]
- Vedhanayagam, M.; Nair, B.U.; Sreeram, K.J. Collagen-ZnO Scaffolds for Wound Healing Applications: Role of Dendrimer Functionalization and Nanoparticle Morphology. ACS Appl. Bio Mater. 2018, 1, 1942–1958. [Google Scholar] [CrossRef] [PubMed]
- Arshad, R.; Sohail, M.F.; Sarwar, H.S.; Saeed, H.; Ali, I.; Akhtar, S.; Hussain, S.Z.; Afzal, I.; Jahan, S.; Rehman, A.U.; et al. ZnO-NPs embedded biodegradable thiolated bandage for postoperative surgical site infection: In vitro and in vivo evaluation. PLoS ONE 2019, 14, e0217079. [Google Scholar] [CrossRef] [PubMed]
- Shahriari-Khalaji, M.; Hu, G.; Chen, L.; Cao, Z.; Andreeva, T.; Xiong, X.; Krastev, R.; Hong, F.F. Functionalization of Aminoalkylsilane-Grafted Bacterial Nanocellulose with ZnO-NPs-Doped Pullulan Electrospun Nanofibers for Multifunctional Wound Dressing. ACS Biomater. Sci. Eng. 2021, 7, 3933–3946. [Google Scholar] [CrossRef]
- Soubhagya, A.; Moorthi, A.; Prabaharan, M. Preparation and characterization of chitosan/pectin/ZnO porous films for wound healing. Int. J. Biol. Macromol. 2020, 157, 135–145. [Google Scholar] [CrossRef]
- Cleetus, C.M.; Primo, F.A.; Fregoso, G.; Raveendran, N.L.; Noveron, J.C.; Spencer, C.T.; Ramana, C.V.; Joddar, B. Alginate Hydrogels with Embedded ZnO Nanoparticles for Wound Healing Therapy. Int. J. Nanomed. 2020, 15, 5097–5111. [Google Scholar] [CrossRef]
- Chopra, M.; Bernela, M.; Kaur, P.; Manuja, A.; Kumar, B.; Thakur, R. Alginate/gum acacia bipolymeric nanohydrogels—Promising carrier for Zinc oxide nanoparticles. Int. J. Biol. Macromol. 2015, 72, 827–833. [Google Scholar] [CrossRef]
- Saddik, M.S.; Elsayed, M.M.A.; El-Mokhtar, M.A.; Sedky, H.; Abdel-Aleem, J.A.; Abu-Dief, A.M.; Al-Hakkani, M.F.; Hussein, H.L.; Al-Shelkamy, S.A.; Meligy, F.Y.; et al. Tailoring of Novel Azithromycin-Loaded Zinc Oxide Nanoparticles for Wound Healing. Pharmaceutics 2022, 14, 111. [Google Scholar] [CrossRef]
- Manuja, A.; Raguvaran, R.; Kumar, B.; Kalia, A.; Tripathi, B. Accelerated healing of full thickness excised skin wound in rabbits using single application of alginate/acacia based nanocomposites of ZnO nanoparticles. Int. J. Biol. Macromol. 2020, 155, 823–833. [Google Scholar] [CrossRef]
- Agren, M.S. Studies on zinc in wound healing. Acta Derm. Venereol. Suppl. 1990, 154, 1–36. [Google Scholar]
- Idzik, M.; Poloczek, J.; Skrzep-Poloczek, B.; Dróżdż, E.; Chełmecka, E.; Czuba, Z.; Jochem, J.; Stygar, D. The Effects of 21-Day General Rehabilitation after Hip or Knee Surgical Implantation on Plasma Levels of Selected Interleukins, VEGF, TNF-α, PDGF-BB, and Eotaxin-1. Biomolecules 2022, 12, 605. [Google Scholar] [CrossRef]
- Hanada, T.; Yoshimura, A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002, 13, 413–421. [Google Scholar] [CrossRef]
- Agarwal, H.; Shanmugam, V. A review on anti-inflammatory activity of green synthesized zinc oxide nanoparticle: Mechanism-based approach. Bioorg. Chem. 2019, 94, 103423. [Google Scholar] [CrossRef] [PubMed]
- Nagajyothi, P.; Cha, S.J.; Yang, I.J.; Sreekanth, T.; Kim, K.J.; Shin, H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B Biol. 2015, 146, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Seo, J.-H.; Kim, H.-M.; Jeong, H.-J. Aluminum-doped zinc oxide nanoparticles attenuate the TSLP levels via suppressing caspase-1 in activated mast cells. J. Biomater. Appl. 2016, 30, 1407–1416. [Google Scholar] [CrossRef]
- Olbert, M.; Argasińska, J.G.; Nowak, G.; Librowski, T. Beneficial effect of nanoparticles over standard form of zinc oxide in enhancing the anti-inflammatory activity of ketoprofen in rats. Pharmacol. Rep. 2017, 69, 679–682. [Google Scholar] [CrossRef]
- Jan, H.; Shah, M.; Andleeb, A.; Faisal, S.; Khattak, A.; Rizwan, M.; Drouet, S.; Hano, C.; Abbasi, B.H. Plant-Based Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) Using Aqueous Leaf Extract of Aquilegia pubiflora: Their Antiproliferative Activity against HepG2 Cells Inducing Reactive Oxygen Species and Other In Vitro Properties. Oxid. Med. Cell. Longev. 2021, 2021, e4786227. [Google Scholar] [CrossRef]
- Basirun, W.J.; Nasiri-Tabrizi, B.; Baradaran, S. Overview of Hydroxyapatite–Graphene Nanoplatelets Composite as Bone Graft Substitute: Mechanical Behavior and in-vitro Biofunctionality. Crit. Rev. Solid State Mater. Sci. 2017, 43, 177–212. [Google Scholar] [CrossRef]
- Kamachimudali, U.; Sridhar, T.M.; Raj, B. Corrosion of bio implants. Sadhana 2003, 28, 601–637. [Google Scholar] [CrossRef]
- Filipović, U.; Dahmane, R.G.; Ghannouchi, S.; Zore, A.; Bohinc, K. Bacterial adhesion on orthopedic implants. Adv. Colloid Interface Sci. 2020, 283, 102228. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, R.; Kumar, S. Coatings on orthopedic implants to overcome present problems and challenges: A focused review. Mater. Today Proc. 2021, 45, 5269–5276. [Google Scholar] [CrossRef]
- Wang, N.; Fuh, J.Y.H.; Dheen, S.T.; Kumar, A.S. Functions and applications of metallic and metallic oxide nanoparticles in orthopedic implants and scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 109, 160–179. [Google Scholar] [CrossRef]
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, D.; Huang, S.; Wang, B.; Zhang, X.; Wang, W.; Wei, X. Biodegradable magnesium screws and vascularized iliac grafting for displaced femoral neck fracture in young adults. BMC Musculoskelet. Disord. 2015, 16, 329. [Google Scholar] [CrossRef]
- Han, P.; Cheng, P.; Zhang, S.; Zhao, C.; Ni, J.; Zhang, Y.; Zhong, W.; Hou, P.; Zhang, X.; Zheng, Y.; et al. In vitro and in vivo studies on the degradation of high-purity Mg (99.99 wt.%) screw with femoral intracondylar fractured rabbit model. Biomaterials 2015, 64, 57–69. [Google Scholar] [CrossRef]
- Navarro, M.; Aparicio, C.; Charles-Harris, M.; Ginebra, M.P.; Engel, E.; Planell, J.A. Development of a Biodegradable Composite Scaffold for Bone Tissue Engineering: Physicochemical, Topographical, Mechanical, Degradation, and Biological Properties. In Ordered Polymeric Nanostructures at Surfaces; Vancso, G.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 209–231. [Google Scholar] [CrossRef]
- Evans, C.H. Advances in Regenerative Orthopedics. Mayo Clin. Proc. 2013, 88, 1323–1339. [Google Scholar] [CrossRef]
- Day, R.M. Bioactive Glass Stimulates the Secretion of Angiogenic Growth Factors and Angiogenesis in Vitro. Tissue Eng. 2005, 11, 768–777. [Google Scholar] [CrossRef]
- Jin, G.; Qin, H.; Cao, H.; Qian, S.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X.; Chu, P.K. Synergistic effects of dual Zn/Ag ion implantation in osteogenic activity and antibacterial ability of titanium. Biomaterials 2014, 35, 7699–7713. [Google Scholar] [CrossRef]
- Wang, R.; He, X.; Gao, Y.; Zhang, X.; Yao, X.; Tang, B. Antimicrobial property, cytocompatibility and corrosion resistance of Zn-doped ZrO2/TiO2 coatings on Ti6Al4V implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 7–15. [Google Scholar] [CrossRef]
- Pang, S.; He, Y.; Zhong, R.; Guo, Z.; He, P.; Zhou, C.; Xue, B.; Wen, X.; Li, H. Multifunctional ZnO/TiO2 nanoarray composite coating with antibacterial activity, cytocompatibility and piezoelectricity. Ceram. Int. 2019, 45, 12663–12671. [Google Scholar] [CrossRef]
- Iqbal, N.; Kadir, M.R.A.; Mahmood, N.H.; Salim, N.; Froemming, G.; Balaji, H.; Kamarul, T. Characterization, antibacterial and in vitro compatibility of zinc–silver doped hydroxyapatite nanoparticles prepared through microwave synthesis. Ceram. Int. 2014, 40, 4507–4513. [Google Scholar] [CrossRef]
- Yamaguchi, M. Role of zinc in bone formation and bone resorption. J. Trace Elem. Exp. Med. 1998, 11, 119–135. [Google Scholar] [CrossRef]
- Seo, H.-J.; Cho, Y.-E.; Kim, T.; Shin, H.-I.; Kwun, I.-S. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 2010, 4, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Storrie, H.; Stupp, S.I. Cellular response to zinc-containing organoapatite: An in vitro study of proliferation, alkaline phosphatase activity and biomineralization. Biomaterials 2005, 26, 5492–5499. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, C.J.; Koh, J.-Y.; Bush, A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Hove, E.; Elvehjem, C.; Hart, E. The effect of zinc on alkaline phosphatases. J. Biol. Chem. 1940, 134, 425–442. [Google Scholar] [CrossRef]
- Coleman, J.E. Structure and Mechanism of Alkaline Phosphatase. Annu. Rev. Biophys. Biomol. Struct. 1992, 21, 441–483. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.C. Matrix vesicles and calcification. Curr. Rheumatol. Rep. 2003, 5, 222–226. [Google Scholar] [CrossRef]
- Clancaglini, P.; Plzauro, J.M.; Curti, C.; Tedesco, A.C.; Leone, F.A. Effect of membrane moiety and magnesium ions on the inhibition of matrix-induced alkaline phosphatase by zinc ions. Int. J. Biochem. 1990, 22, 747–751. [Google Scholar] [CrossRef]
- Ciancaglini, P.; Pizauro, J.; Rezende, A.; Leone, F. Solubilization of membrane-bound matrix-induced alkaline phosphatase with polyoxyethylene 9-lauryl ether (polidocanol): Purification and metalloenzyme properties. Int. J. Biochem. 1990, 22, 385–392. [Google Scholar] [CrossRef]
- Reginster, J.-Y.; Strause, L.G.; Saltman, P.; Franchimont, P. Trace elements and postmenopausal osteoporosis: A preliminary report of decreased serum manganese. Med. Sci. Res. 2022, 16, 1988. Available online: https://orbi.uliege.be/handle/2268/160100 (accessed on 10 August 2022).
- Yusa, K.; Yamamoto, O.; Iino, M.; Takano, H.; Fukuda, M.; Qiao, Z.; Sugiyama, T. Eluted zinc ions stimulate osteoblast differentiation and mineralization in human dental pulp stem cells for bone tissue engineering. Arch. Oral Biol. 2016, 71, 162–169. [Google Scholar] [CrossRef]
- Guo, B.; Yang, M.; Liang, D.; Yang, L.; Cao, J.; Zhang, L. Cell apoptosis induced by zinc deficiency in osteoblastic MC3T3-E1 cells via a mitochondrial-mediated pathway. Mol. Cell. Biochem. 2011, 361, 209–216. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, W.; Qiao, Y.; Jiang, X.; Liu, X.; Ding, C. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta Biomater. 2011, 8, 904–915. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Lai, C.-H.; Hsu, J.-T.; Tang, C.-H.; Liao, W.-C.; Huang, H.-L. Antibacterial properties and human gingival fibroblast cell compatibility of TiO2/Ag compound coatings and ZnO films on titanium-based material. Clin. Oral Investig. 2011, 16, 95–100. [Google Scholar] [CrossRef]
- Roknian, M.; Fattah-Alhosseini, A.; Gashti, S.O.; Keshavarz, M.K. Study of the effect of ZnO nanoparticles addition to PEO coatings on pure titanium substrate: Microstructural analysis, antibacterial effect and corrosion behavior of coatings in Ringer’s physiological solution. J. Alloys Compd. 2018, 740, 330–345. [Google Scholar] [CrossRef]
- Colon, G.; Ward, B.C.; Webster, T.J. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J. Biomed. Mater. Res. Part A 2006, 78A, 595–604. [Google Scholar] [CrossRef]
- Lin, M.-H.; Wang, Y.-H.; Kuo, C.-H.; Ou, S.-F.; Huang, P.-Z.; Song, T.-Y.; Chen, Y.-C.; Chen, S.-T.; Wu, C.-H.; Hsueh, Y.-H.; et al. Hybrid ZnO/chitosan antimicrobial coatings with enhanced mechanical and bioactive properties for titanium implants. Carbohydr. Polym. 2021, 257, 117639. [Google Scholar] [CrossRef]
- Walker, J.; Shadanbaz, S.; Woodfield, T.B.F.; Staiger, M.P.; Dias, G.J. Magnesium biomaterials for orthopedic application: A review from a biological perspective. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1316–1331. [Google Scholar] [CrossRef]
- Adhikari, U.; Rijal, N.P.; Khanal, S.; Pai, D.; Sankar, J.; Bhattarai, N. Magnesium and Calcium-Containing Scaffolds for Bone Tissue Regeneration. In Proceedings of the ASME 2016 International Mechanical Engineering Congress and Exposition, Phoenix, AZ, USA, 11–17 November 2016. [Google Scholar] [CrossRef]
- Khader, A.; Arinzeh, T.L. Biodegradable zinc oxide composite scaffolds promote osteochondral differentiation of mesenchymal stem cells. Biotechnol. Bioeng. 2019, 117, 194–209. [Google Scholar] [CrossRef]
- Chausmer, A.B. Zinc, Insulin and Diabetes. J. Am. Coll. Nutr. 1998, 17, 109–115. [Google Scholar] [CrossRef]
- Jansen, J.; Karges, W.; Rink, L. Zinc and diabetes—Clinical links and molecular mechanisms. J. Nutr. Biochem. 2009, 20, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Hiromura, M.; Sakurai, H. Action mechanism of metallo-allixin complexes as antidiabetic agents. Pure Appl. Chem. 2008, 80, 2727–2733. [Google Scholar] [CrossRef]
- Bayrami, A.; Parvinroo, S.; Habibi-Yangjeh, A.; Pouran, S.R. Bio-extract-mediated ZnO nanoparticles: Microwave-assisted synthesis, characterization and antidiabetic activity evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 46, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Richards-Williams, C.; Contreras, J.L.; Berecek, K.H.; Schwiebert, E.M. Extracellular ATP and zinc are co-secreted with insulin and activate multiple P2X purinergic receptor channels expressed by islet beta-cells to potentiate insulin secretion. Purinergic Signal. 2008, 4, 393–405. [Google Scholar] [CrossRef]
- Arvanag, F.M.; Bayrami, A.; Habibi-Yangjeh, A.; Pouran, S.R. A comprehensive study on antidiabetic and antibacterial activities of ZnO nanoparticles biosynthesized using Silybum marianum L. seed extract. Mater. Sci. Eng. C 2018, 97, 397–405. [Google Scholar] [CrossRef]
- Meydan, I.; Burhan, H.; Gür, T.; Seçkin, H.; Tanhaei, B.; Sen, F. Characterization of Rheum ribes with ZnO nanoparticle and its antidiabetic, antibacterial, DNA damage prevention and lipid peroxidation prevention activity of in vitro. Environ. Res. 2021, 204, 112363. [Google Scholar] [CrossRef]
- Vinotha, V.; Iswarya, A.; Thaya, R.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Al-Anbr, M.N.; Vaseeharan, B. Synthesis of ZnO nanoparticles using insulin-rich leaf extract: Anti-diabetic, antibiofilm and anti-oxidant properties. J. Photochem. Photobiol. B Biol. 2019, 197, 111541. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Anbazhagan, V.; Anusuya, S. Green route synthesis of ZnO nanoparticles using Senna auriculata aqueous flower extract as reducing agent and evaluation of its antimicrobial, antidiabetic and cytotoxic activity. Appl. Biochem. Biotechnol. 2022; in press. [Google Scholar]
- Rajakumar, G.; Thiruvengadam, M.; Mydhili, G.; Gomathi, T.; Chung, I.-M. Green approach for synthesis of zinc oxide nanoparticles from Andrographis paniculata leaf extract and evaluation of their antioxidant, anti-diabetic, and anti-inflammatory activities. Bioprocess Biosyst. Eng. 2017, 41, 21–30. [Google Scholar] [CrossRef]
- Malaikozhundan, B.; Vinodhini, J.; Kalanjiam, M.A.R.; Vinotha, V.; Palanisamy, S.; Vijayakumar, S.; Vaseeharan, B.; Mariyappan, A. High synergistic antibacterial, antibiofilm, antidiabetic and antimetabolic activity of Withania somnifera leaf extract-assisted zinc oxide nanoparticle. Bioprocess Biosyst. Eng. 2020, 43, 1533–1547. [Google Scholar] [CrossRef]
- Winston, G.W.; Regoli, F.; Dugas, A.J.; Fong, J.H.; Blanchard, K.A. A Rapid Gas Chromatographic Assay for Determining Oxyradical Scavenging Capacity of Antioxidants and Biological Fluids. Free Radic. Biol. Med. 1998, 24, 480–493. [Google Scholar] [CrossRef]
- Ryu, C.S.; Kim, C.H.; Lee, S.Y.; Lee, K.S.; Choung, K.J.; Song, G.Y.; Kim, B.-H.; Ryu, S.Y.; Lee, H.S.; Kim, S.K. Evaluation of the total oxidant scavenging capacity of saponins isolated from Platycodon grandiflorum. Food Chem. 2012, 132, 333–337. [Google Scholar] [CrossRef]
- Regoli, F.; Nigro, M.; Bompadre, S.; Winston, G.W. Total oxidant scavenging capacity (TOSC) of microsomal and cytosolic fractions from Antarctic, Arctic and Mediterranean scallops: Differentiation between three potent oxidants. Aquat. Toxicol. 2000, 49, 13–25. [Google Scholar] [CrossRef]
- Colvin, V.L. The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 2003, 21, 1166–1170. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y. Toxicological and biological effects of nanomaterials. Int. J. Nanotechnol. 2007, 4, 179. [Google Scholar] [CrossRef]
- Das, D.; Nath, B.C.; Phukon, P.; Kalita, A.; Dolui, S.K. Synthesis of ZnO nanoparticles and evaluation of antioxidant and cytotoxic activity. Colloids Surfaces B Biointerfaces 2013, 111, 556–560. [Google Scholar] [CrossRef]
- Loganathan, S.; Shivakumar, M.S.; Karthi, S.; Nathan, S.S.; Selvam, K. Metal oxide nanoparticle synthesis (ZnO-NPs) of Knoxia sumatrensis (Retz.) DC. Aqueous leaf extract and It’s evaluation of their antioxidant, anti-proliferative and larvicidal activities. Toxicol. Rep. 2020, 8, 64–72. [Google Scholar] [CrossRef]
- Asif, N.; Fatima, S.; Aziz, N.; Shehzadi; Zaki, A.; Fatma, T. Biofabrication and characterization of cyanobacteria derived ZnO NPs for their bioactivity comparison with commercial chemically synthesized nanoparticles. Bioorg. Chem. 2021, 113, 104999. [Google Scholar] [CrossRef]
- Rabiee, N.; Bagherzadeh, M.; Ghadiri, A.M.; Kiani, M.; Aldhaher, A.; Ramakrishna, S.; Tahriri, M.; Tayebi, L.; Webster, T.J. Green Synthesis of ZnO NPs via Salvia hispanica: Evaluation of Potential Antioxidant, Antibacterial, Mammalian Cell Viability, H1N1 Influenza Virus Inhibition and Photocatalytic Activities. J. Biomed. Nanotechnol. 2020, 16, 456–466. [Google Scholar] [CrossRef]
- Kalaimurugan, D.; Lalitha, K.; Durairaj, K.; Sivasankar, P.; Park, S.; Nithya, K.; Shivakumar, M.S.; Liu, W.-C.; Balamuralikrishnan, B.; Venkatesan, S. Biogenic synthesis of ZnO nanoparticles mediated from Borassus flabellifer (Linn): Antioxidant, antimicrobial activity against clinical pathogens, and photocatalytic degradation activity with molecular modeling. Environ. Sci. Pollut. Res. 2022; in press. [Google Scholar]
- Singh, M.; Lee, K.E.; Vinayagam, R.; Kang, S.G. Antioxidant and Antibacterial Profiling of Pomegranate-pericarp Extract Functionalized-zinc Oxide Nanocomposite. Biotechnol. Bioprocess Eng. BBE 2021, 26, 728–737. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Moniri, M.; Azizi, S.; Rahim, R.A.; Ariff, A.B.; Saad, W.Z.; Namvar, F.; Navaderi, M.; Mohamad, R. Biosynthesis of ZnO Nanoparticles by a New Pichia kudriavzevii Yeast Strain and Evaluation of Their Antimicrobial and Antioxidant Activities. Molecules 2017, 22, 872. [Google Scholar] [CrossRef]
- Gupta, J.; Irfan, M.; Ramgir, N.; Muthe, K.P.; Debnath, A.K.; Ansari, S.; Gandhi, J.; Ranjith-Kumar, C.T.; Surjit, M. Antiviral Activity of Zinc Oxide Nanoparticles and Tetrapods against the Hepatitis E and Hepatitis C Viruses. Front. Microbiol. 2022, 13, 881595. Available online: https://www.frontiersin.org/articles/10.3389/fmicb.2022.881595 (accessed on 25 August 2022). [CrossRef] [PubMed]
- Briknarová, K.; Thomas, C.J.; York, J.; Nunberg, J.H. Structure of a Zinc-binding Domain in the Junín Virus Envelope Glycoprotein. J. Biol. Chem. 2011, 286, 1528–1536. [Google Scholar] [CrossRef]
- Byk, L.A.; Iglesias, N.G.; De Maio, F.A.; Gebhard, L.G.; Rossi, M.; Gamarnik, A.V. Dengue Virus Genome Uncoating Requires Ubiquitination. mBio 2016, 7, e00804-16. [Google Scholar] [CrossRef] [PubMed]
- Te Velthuis, A.J.W.; van den Worm, S.H.E.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; Van Hemert, M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef] [PubMed]
- Erk, I.; Huet, J.-C.; Duarte, M.; Duquerroy, S.; Rey, F.; Cohen, J.; Lepault, J. A Zinc Ion Controls Assembly and Stability of the Major Capsid Protein of Rotavirus. J. Virol. 2003, 77, 3595–3601. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Turpin, J.A.; Terpening, S.J.; Schaeffer, C.A.; Yu, G.; Glover, C.J.; Felsted, R.L.; Sausville, E.A.; Rice, W.G. Inhibitors of human immunodeficiency virus type 1 zinc fingers prevent normal processing of gag precursors and result in the release of noninfectious virus particles. J. Virol. 1996, 70, 6180–6189. [Google Scholar] [CrossRef]
- Brieger, A.; Rink, L.; Haase, H. Differential Regulation of TLR-Dependent MyD88 and TRIF Signaling Pathways by Free Zinc Ions. J. Immunol. 2013, 191, 1808–1817. [Google Scholar] [CrossRef]
- Haase, H.; Ober-Blöbaum, J.L.; Engelhardt, G.; Hebel, S.; Heit, A.; Heine, H.; Rink, L. Zinc Signals Are Essential for Lipopolysaccharide-Induced Signal Transduction in Monocytes. J. Immunol. 2008, 181, 6491–6502. [Google Scholar] [CrossRef]
- Lishchynskyi, O.; Shymborska, Y.; Stetsyshyn, Y.; Raczkowska, J.; Skirtach, A.G.; Peretiatko, T.; Budkowski, A. Passive antifouling and active self-disinfecting antiviral surfaces. Chem. Eng. J. 2022, 446, 137048. [Google Scholar] [CrossRef]
- Jana, B.; Chatterjee, A.; Roy, D.; Ghorai, S.; Pan, D.; Pramanik, S.K.; Chakraborty, N.; Ganguly, J. Chitosan/benzyloxy-benzaldehyde modified ZnO nano template having optimized and distinct antiviral potency to human cytomegalovirus. Carbohydr. Polym. 2021, 278, 118965. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tavakoli, A.; Moradi, A.; Tabarraei, A.; Bokharaei-Salim, F.; Zahmatkeshan, M.; Farahmand, M.; Javanmard, D.; Kiani, S.J.; Esghaei, M.; et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: Another emerging application of nanomedicine. J. Biomed. Sci. 2019, 26, 70. [Google Scholar] [CrossRef] [PubMed]
- El-Megharbel, S.; Alsawat, M.; Al-Salmi, F.; Hamza, R. Utilizing of (Zinc Oxide Nano-Spray) for Disinfection against “SARS-CoV-2” and Testing Its Biological Effectiveness on Some Biochemical Parameters during (COVID-19 Pandemic)—”ZnO Nanoparticles Have Antiviral Activity against (SARS-CoV-2)”. Coatings 2021, 11, 388. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, G.; Pandey, K.; Nayak, M.; Topno, R.; Rabidas, V.; Das, P. Virostatic potential of zinc oxide (ZnO) nanoparticles on capsid protein of cytoplasmic side of chikungunya virus. Int. J. Infect. Dis. 2018, 73, 368. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Al-Salm, F.A.; El-Shen, N.S. Nanoparticles Effects on Zinc Oxide/green Tea Complex on the Lipid Profile and Liver Functions of Rats after Monosodium Glutamate Treatment. J. Appl. Sci. 2018, 18, 65–70. [Google Scholar] [CrossRef]
- Hamza, R.Z.; El-Shenawy, N.S. The Interaction of Zinc Oxide/Green Tea Extract Complex Nanoparticles and its Effect on Monosodium Glutamate Toxicity in Liver of Rats. Curr. Pharm. Biotechnol. 2019, 20, 465–475. [Google Scholar] [CrossRef]
- El-Shenawy, N.S.; Hamza, R.Z.; Al-Salmi, F.A.; Al-Eisa, R.A. Evaluation of the Effect of Nanoparticles Zinc Oxide/Camellia sinensis Complex on the Kidney of Rats Treated with Monosodium Glutamate: Antioxidant and Histological Approaches. Curr. Pharm. Biotechnol. 2019, 20, 542–550. [Google Scholar] [CrossRef] [PubMed]
- El-Shenawy, N.S.; Hamza, R.Z.; Al-Salmi, F.A. Cardioprotective Effect of Zinc Oxide Nanoparticles/Green Tea Extract Complex on Monosodium Glutamate Toxicity. J. Biol. Eng. Med. 2019, 2019, 1–5. [Google Scholar] [CrossRef]
- Li, X.; Xing, Y.; Jiang, Y.; Ding, Y.; Li, W. Antimicrobial activities of ZnO powder-coated PVC film to inactivate food pathogens. Int. J. Food Sci. Technol. 2009, 44, 2161–2168. [Google Scholar] [CrossRef]
- Sawai, J.; Kawada, E.; Kanou, F.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M. Detection of active oxygen generated from ceramic powders having antibacterial activity. J. Chem. Eng. Jpn. 1996, 29, 627–633. [Google Scholar] [CrossRef]
- Khan, Y.A.; Singh, B.R.; Ullah, R.; Shoeb, M.; Naqvi, A.H.; Abidi, S.M.A. Anthelmintic Effect of Biocompatible Zinc Oxide Nanoparticles (ZnO NPs) on Gigantocotyle explanatum, a Neglected Parasite of Indian Water Buffalo. PLoS ONE 2015, 10, e0133086. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, A.K.; Singh, S.S.; Shanker, R.; Dhawan, A. Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radic. Biol. Med. 2011, 51, 1872–1881. [Google Scholar] [CrossRef]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 59–81. [Google Scholar] [CrossRef]
- Rashid, M.I.; Shahzad, T.; Shahid, M.; Ismail, I.M.; Shah, G.M.; Almeelbi, T. Zinc oxide nanoparticles affect carbon and nitrogen mineralization of Phoenix dactylifera leaf litter in a sandy soil. J. Hazard. Mater. 2017, 324, 298–305. [Google Scholar] [CrossRef]
- Mintcheva, N.; Aljulaih, A.A.; Wunderlich, W.; Kulinich, S.A.; Iwamori, S. Laser-Ablated ZnO Nanoparticles and Their Photocatalytic Activity toward Organic Pollutants. Materials 2018, 11, 1127. [Google Scholar] [CrossRef]
- Sergievskaya, A.; Chauvin, A.; Konstantinidis, S. Sputtering onto liquids: A critical review. Beilstein J. Nanotechnol. 2022, 13, 10–53. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Chapter 5—Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites. In Synthesis of Inorganic Nanomaterials; Woodhead Publishing: Cambridge, UK, 2018; pp. 121–139. [Google Scholar] [CrossRef]
- Islam, F.; Shohag, S.; Uddin, J.; Islam, R.; Nafady, M.H.; Akter, A.; Mitra, S.; Roy, A.; Bin Emran, T.; Cavalu, S. Exploring the Journey of Zinc Oxide Nanoparticles (ZnO-NPs) toward Biomedical Applications. Materials 2022, 15, 2160. [Google Scholar] [CrossRef]
- Navas, D.; Fuentes, S.; Castro-Alvarez, A.; Chavez-Angel, E. Review on Sol-Gel Synthesis of Perovskite and Oxide Nanomaterials. Gels 2021, 7, 275. [Google Scholar] [CrossRef]
- Khan, M.F.; Ansari, A.H.; Hameedullah, M.; Ahmad, E.; Husain, F.M.; Zia, Q.; Baig, U.; Zaheer, M.R.; Alam, M.M.; Khan, A.M.; et al. Sol-gel synthesis of thorn-like ZnO nanoparticles endorsing mechanical stirring effect and their antimicrobial activities: Potential role as nano-antibiotics. Sci. Rep. 2016, 6, 27689. [Google Scholar] [CrossRef]
- Bekele, B.; Degefa, A.; Tesgera, F.; Jule, L.T.; Shanmugam, R.; Dwarampudi, L.P.; Nagaprasad, N.; Ramasamy, K. Green versus Chemical Precipitation Methods of Preparing Zinc Oxide Nanoparticles and Investigation of Antimicrobial Properties. J. Nanomater. 2021, 2021, e9210817. [Google Scholar] [CrossRef]
- Gersten, B. Solvothermal Synthesis of Nanoparticles—PDF Free Download. 2005. Available online: https://docplayer.net/35339574-Solvothermal-synthesis-of-nanoparticles.html (accessed on 10 August 2022).
- Singh, T.A.; Sharma, A.; Tejwan, N.; Ghosh, N.; Das, J.; Sil, P.C. A state of the art review on the synthesis, antibacterial, antioxidant, antidiabetic and tissue regeneration activities of zinc oxide nanoparticles. Adv. Colloid Interface Sci. 2021, 295, 102495. [Google Scholar] [CrossRef]
- Iskandar, F. Nanoparticle processing for optical applications—A review. Adv. Powder Technol. 2009, 20, 283–292. [Google Scholar] [CrossRef]
- Uhm, Y.; Han, B.; Lee, M.; Hong, S.; Rhee, C. Synthesis and characterization of nanoparticles of ZnO by levitational gas condensation. Mater. Sci. Eng. A 2007, 449–451, 813–816. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998; Available online: http://catdir.loc.gov/catdir/enhancements/fy0635/98036292-t.html (accessed on 12 August 2022).
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J. Nanobiotechnol. 2021, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef]
- Madan, H.; Sharma, S.; Udayabhanu; Suresh, D.; Vidya, Y.; Nagabhushana, H.; Rajanaik, H.; Anantharaju, K.; Prashantha, S.; Maiya, P.S. Facile green fabrication of nanostructure ZnO plates, bullets, flower, prismatic tip, closed pine cone: Their antibacterial, antioxidant, photoluminescent and photocatalytic properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 152, 404–416. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Bharath, L. Mechanism of plant-mediated synthesis of silver nanoparticles—A review on biomolecules involved, characterisation and antibacterial activity. Chem. Interact. 2017, 273, 219–227. [Google Scholar] [CrossRef]
- Küünal, S.; Rauwel, P.; Rauwel, E. Plant extract mediated synthesisof nanoparticles. In Emerging Applications of Nanoparticles and Architecture Nanostructures, 1st ed.; Barhoum, A., Makhlouf, A.S.H., Eds.; Elsevier: Cambridge, MA, USA, 2018; pp. 411–446. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A. Multimodal applications of phytonanoparticles. In Phytonanotechnology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 195–219. [Google Scholar] [CrossRef]
- Umar, H.; Kavaz, D.; Rizaner, N. Biosynthesis of zinc oxide nanoparticles using Albizia lebbeck stem bark, and evaluation of its antimicrobial, antioxidant, and cytotoxic activities on human breast cancer cell lines. Int. J. Nanomed. 2018, 14, 87–100. [Google Scholar] [CrossRef]
- Prashanth, G.K.; Prashanth, P.A.; Nagabhushana, B.M.; Ananda, S.; Krishnaiah, G.M.; Nagendra, H.G.; Sathyananda, H.M.; Singh, C.R.; Yogisha, S.; Tejabhiram, Y. Comparison of anticancer activity of biocompatible ZnO nanoparticles prepared by solution combustion synthesis using aqueous leaf extracts of Abutilon indicum, Melia azedarach and Indigofera tinctoria as biofuels. Artif. Cells Nanomed. Biotechnol. 2017, 46, 968–979. [Google Scholar] [CrossRef]
- Chandra, H.; Patel, D.; Kumari, P.; Jangwan, J.; Yadav, S. Phyto-mediated synthesis of zinc oxide nanoparticles of Berberis aristata: Characterization, antioxidant activity and antibacterial activity with special reference to urinary tract pathogens. Mater. Sci. Eng. C 2019, 102, 212–220. [Google Scholar] [CrossRef]
- Hassan, S.S.; Abdel-Shafy, H.I.; Mansour, M.S. Removal of pharmaceutical compounds from urine via chemical coagulation by green synthesized ZnO-nanoparticles followed by microfiltration for safe reuse. Arab. J. Chem. 2019, 12, 4074–4083. [Google Scholar] [CrossRef]
- Naseer, M.; Aslam, U.; Khalid, B.; Chen, B. Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 2020, 10, 9055. [Google Scholar] [CrossRef]
- Salem, M.S.E.-D.; Mahfouz, A.Y.; Fathy, R.M. The antibacterial and antihemolytic activities assessment of zinc oxide nanoparticles synthesized using plant extracts and gamma irradiation against the uro-pathogenic multidrug resistant Proteus vulgaris. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2020, 34, 175–196. [Google Scholar] [CrossRef]
- Sana, S.S.; Kumbhakar, D.V.; Pasha, A.; Pawar, S.C.; Grace, A.N.; Singh, R.P.; Nguyen, V.-H.; Van Le, Q.; Peng, W. Crotalaria verrucosa Leaf Extract Mediated Synthesis of Zinc Oxide Nanoparticles: Assessment of Antimicrobial and Anticancer Activity. Molecules 2020, 25, 4896. [Google Scholar] [CrossRef]
- Patil, B.N.; Taranath, T. Limonia acidissima L. leaf mediated synthesis of silver and zinc oxide nanoparticles and their antibacterial activities. Microb. Pathog. 2018, 115, 227–232. [Google Scholar] [CrossRef]
- Rad, S.S.; Sani, A.M.; Mohseni, S. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Mentha pulegium (L.). Microb. Pathog. 2019, 131, 239–245. [Google Scholar] [CrossRef]
- Jayappa, M.D.; Ramaiah, C.K.; Kumar, M.A.P.; Suresh, D.; Prabhu, A.; Devasya, R.P.; Sheikh, S. Green synthesis of zinc oxide nanoparticles from the leaf, stem and in vitro grown callus of Mussaenda frondosa L.: Characterization and their applications. Appl. Nanosci. 2020, 10, 3057–3074. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N.; et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous Fruit Extracts of Myristica fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef]
- Al-Mohaimeed, A.M.; Al-Onazi, W.A.; El-Tohamy, M.F. Multifunctional Eco-Friendly Synthesis of ZnO Nanoparticles in Biomedical Applications. Molecules 2022, 27, 579. [Google Scholar] [CrossRef]
- Raja, A.; Ashokkumar, S.; Marthandam, R.P.; Jayachandiran, J.; Khatiwada, C.P.; Kaviyarasu, K.; Raman, R.G.; Swaminathan, M. Eco-friendly preparation of zinc oxide nanoparticles using Tabernaemontana divaricata and its photocatalytic and antimicrobial activity. J. Photochem. Photobiol. B Biol. 2018, 181, 53–58. [Google Scholar] [CrossRef]
- Kundu, D.; Hazra, C.; Chatterjee, A.; Chaudhari, A.; Mishra, S. Extracellular biosynthesis of zinc oxide nanoparticles using Rhodococcus pyridinivorans NT2: Multifunctional textile finishing, biosafety evaluation and in vitro drug delivery in colon carcinoma. J. Photochem. Photobiol. B Biol. 2014, 140, 194–204. [Google Scholar] [CrossRef]
- Tripathi, R.; Bhadwal, A.S.; Gupta, R.K.; Singh, P.; Shrivastav, A.; Shrivastav, B. ZnO nanoflowers: Novel biogenic synthesis and enhanced photocatalytic activity. J. Photochem. Photobiol. B Biol. 2014, 141, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Rawat, A.K.S.; Khan, W.; Naqvi, A.H.; Singh, B.R. Biosynthesis of Stable Antioxidant ZnO Nanoparticles by Pseudomonas aeruginosa Rhamnolipids. PLoS ONE 2014, 9, e106937. [Google Scholar] [CrossRef] [PubMed]
- Abdo, A.M.; Fouda, A.; Eid, A.M.; Fahmy, N.M.; Elsayed, A.M.; Khalil, A.M.A.; Alzahrani, O.M.; Ahmed, A.F.; Soliman, A.M. Green Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) by Pseudomonas aeruginosa and Their Activity against Pathogenic Microbes and Common House Mosquito, Culex pipiens. Materials 2021, 14, 6983. [Google Scholar] [CrossRef] [PubMed]
- Jayaseelan, C.; Rahuman, A.A.; Kirthi, A.V.; Marimuthu, S.; Santhoshkumar, T.; Bagavan, A.; Gaurav, K.; Karthik, L.; Rao, K.B. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 90, 78–84. [Google Scholar] [CrossRef]
- Prasad, K.; Jha, A.K. ZnO Nanoparticles: Synthesis and Adsorption Study. Nat. Sci. 2009, 1, 129–135. [Google Scholar] [CrossRef]
- Dhandapani, P.; Siddarth, A.S.; Kamalasekaran, S.; Maruthamuthu, S.; Rajagopal, G. Bio-approach: Ureolytic bacteria mediated synthesis of ZnO nanocrystals on cotton fabric and evaluation of their antibacterial properties. Carbohydr. Polym. 2014, 103, 448–455. [Google Scholar] [CrossRef]
- El-Belely, E.F.; Farag, M.M.S.; Said, H.A.; Amin, A.S.; Azab, E.; Gobouri, A.A.; Fouda, A. Green Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) Using Arthrospira platensis (Class: Cyanophyceae) and Evaluation of their Biomedical Activities. Nanomaterials 2021, 11, 95. [Google Scholar] [CrossRef]
- Ebadi, M.; Zolfaghari, M.R.; Aghaei, S.S.; Zargar, M.; Noghabi, K.A. Desertifilum sp. EAZ03 cell extract as a novel natural source for the biosynthesis of zinc oxide nanoparticles and antibacterial, anticancer and antibiofilm characteristics of synthesized zinc oxide nanoparticles. J. Appl. Microbiol. 2021, 132, 221–236. [Google Scholar] [CrossRef]
- Barani, M.; Masoudi, M.; Mashreghi, M.; Makhdoumi, A.; Eshghi, H. Cell-free extract assisted synthesis of ZnO nanoparticles using aquatic bacterial strains: Biological activities and toxicological evaluation. Int. J. Pharm. 2021, 606, 120878. [Google Scholar] [CrossRef]
- Pati, R.; Mehta, R.K.; Mohanty, S.; Padhi, A.; Sengupta, M.; Vaseeharan, B.; Goswami, C.; Sonawane, A. Topical application of zinc oxide nanoparticles reduces bacterial skin infection in mice and exhibits antibacterial activity by inducing oxidative stress response and cell membrane disintegration in macrophages. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1195–1208. [Google Scholar] [CrossRef]
- Shamsuzzaman; Mashrai, A.; Khanam, H.; Aljawfi, R.N. Biological synthesis of ZnO nanoparticles using C. albicans and studying their catalytic performance in the synthesis of steroidal pyrazolines. Arab. J. Chem. 2017, 10, S1530–S1536. [Google Scholar] [CrossRef]
- Pavani, K.V.; Kumar, N.S.; Sangameswaran, B.B. Synthesis of lead nanoparticles by Aspergillus species. Pol. J. Microbiol. 2012, 61, 61–63. [Google Scholar] [CrossRef]
- Kalpana, V.; Kataru, B.A.S.; Sravani, N.; Vigneshwari, T.; Panneerselvam, A.; Rajeswari, V.D. Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus niger: Antimicrobial textiles and dye degradation studies. OpenNano 2018, 3, 48–55. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C. ZnO Nanoparticle Biosynthesis and Its Effect on Phosphorous-Mobilizing Enzyme Secretion and Gum Contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef]
- Pavani, K.V.; Balakrishna, K.; Cheemarla, N.K. Biosynthesis of zinc nanoparticles by Aspergillus species. Int. J. Nanotechnol. Appl. 2011, 5, 27–36. [Google Scholar]
- Sumanth, B.; Lakshmeesha, T.R.; Ansari, M.A.; Alzohairy, M.A.; Udayashankar, A.C.; Shobha, B.; Niranjana, S.R.; Srinivas, C.; Almatroudi, A. Mycogenic Synthesis of Extracellular Zinc Oxide Nanoparticles from Xylaria acuta and Its Nanoantibiotic Potential. Int. J. Nanomed. 2020, 15, 8519–8536. [Google Scholar] [CrossRef]
- Gray, D.J.P. Butterworths Medical Dictionary, 2nd ed. J. R. Coll. Gen. Pract. 1978, 28, 762. [Google Scholar]
- Azizi, S.; Ahmad, M.B.; Namvar, F.; Mohamad, R. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater. Lett. 2014, 116, 275–277. [Google Scholar] [CrossRef]
- Nagarajan, S.; Kuppusamy, K.A. Extracellular synthesis of zinc oxide nanoparticle using seaweeds of gulf of Mannar, India. J. Nanobiotechnol. 2013, 11, 39. [Google Scholar] [CrossRef]
- Rao, M.D.; Gautam, P. Synthesis and characterization of ZnO nanoflowers using Chlamydomonas reinhardtii: A green approach. Environ. Prog. Sustain. Energy 2016, 35, 1020–1026. [Google Scholar] [CrossRef]
- Subramanian, H.; Krishnan, M.; Mahalingam, A. Photocatalytic dye degradation and photoexcited anti-microbial activities of green zinc oxide nanoparticles synthesized via Sargassum muticum extracts. RSC Adv. 2021, 12, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Ishwarya, R.; Vaseeharan, B.; Kalyani, S.; Banumathi, B.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Al-Anbr, M.N.; Khaled, J.M.; Benelli, G. Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, antibiofilm and insecticidal activity. J. Photochem. Photobiol. B Biol. 2018, 178, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.B.; Dobrovolskaia, M.A.; Patri, A.K.; McNeil, S.E. Characterization of nanoparticles for therapeutics. Nanomedicine 2007, 2, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Lee, H.J.; Wark, A.W.; Corn, R.M. Attomole Microarray Detection of MicroRNAs by Nanoparticle-Amplified SPR Imaging Measurements of Surface Polyadenylation Reactions. J. Am. Chem. Soc. 2006, 128, 14044–14046. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Malarkodi, C.; Vanaja, M.; Annadurai, G. Anticancer and enhanced antimicrobial activity of biosynthesizd silver nanoparticles against clinical pathogens. J. Mol. Struct. 2016, 1116, 165–173. [Google Scholar] [CrossRef]
- Yasmin, A.; Ramesh, K.; Rajeshkumar, S. Optimization and stabilization of gold nanoparticles by using herbal plant extract with microwave heating. Nano Converg. 2014, 1, 12. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Prodpran, T.; Sumpavapol, P.; Songtipya, P. Properties and antimicrobial activity of fish protein isolate/fish skin gelatin film containing basil leaf essential oil and zinc oxide nanoparticles. Food Hydrocoll. 2014, 41, 265–273. [Google Scholar] [CrossRef]
- Ali, A.; Phull, A.-R.; Zia, M. Elemental zinc to zinc nanoparticles: Is ZnO NPs crucial for life? Synthesis, toxicological, and environmental concerns. Nanotechnol. Rev. 2018, 7, 413–441. [Google Scholar] [CrossRef]
- Wang, H.; Xie, J.; Yan, K.; Duan, M. Growth Mechanism of Different Morphologies of ZnO Crystals Prepared by Hydrothermal Method. J. Mater. Sci. Technol. 2011, 27, 153–158. [Google Scholar] [CrossRef]
- Doustkhah, E.; Esmat, M.; Fukata, N.; Ide, Y.; Hanaor, D.A.; Assadi, M.H.N. MOF-derived nanocrystalline ZnO with controlled orientation and photocatalytic activity. Chemosphere 2022, 303, 134932. [Google Scholar] [CrossRef]
- Ozgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]

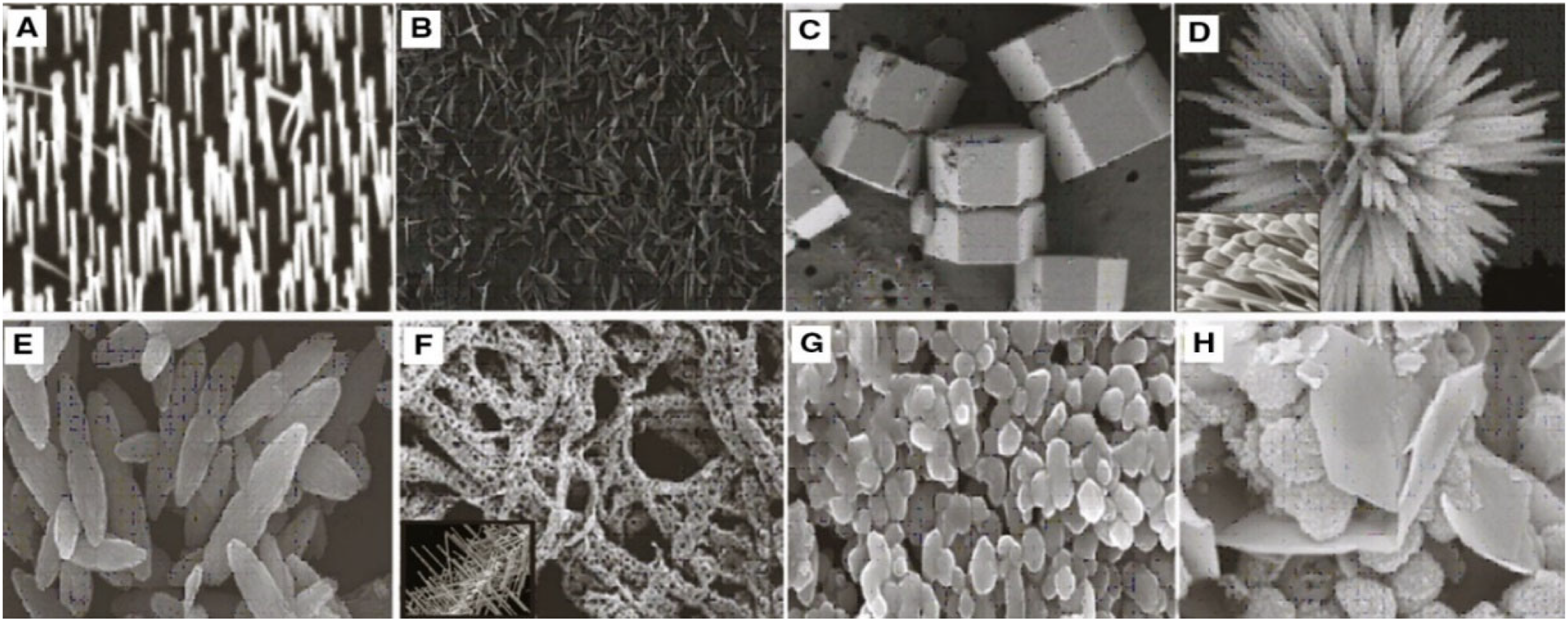
| Biological Source | Used Plant Parts | Extraction Technique | Zinc Precursors; Condition | Size of Nanoparticles Synthesized (nm) | Morphology of Nanoparticles | References |
|---|---|---|---|---|---|---|
| Albizia lebbeck | Stem bark | Decoction at 60 °C | Zinc nitrate hexahydrate and sodium hydroxide, calcined at 350 °C | DLS: 82.31 at 0.05 molar and 110 at 0.01 molar SEM: 66.25, 82.52, 112.87 at 0.1, 0.05, and 0.01 molar concentration | Rod and hexagonal | [168] |
| Abutilon indicum | Leaf | Solvent extraction at 90–95 °C | Zinc nitrate hexahydrate | XRD: 16.72 | Spheroid or rodlike | [169] |
| Azadirachta indica | Leaf | Soxhlet extraction at 350 °C | Zinc nitrate | XRD: 11–40 | Hexagonal disk | [164] |
| Berberis aristata | Leaf | Boil | Zinc acetate dehydrate, sodium hydroxide | XRD: 5–25 DLS: 90–110 | Needle | [170] |
| Camellia sinensis | Solid waste | Decoction | Zinc acetate, pH 12 | XRD: 19.5 | Rod | [171] |
| Cassia fistula | Leaf | Decoction at 70 °C | Zinc acetate dihydrate; 70 °C | XRD: 2.72 DLS: 68.1 | Spherical | [172] |
| Citrus limon | Leaf | Decoction at 60 °C | Zinc nitrate | TEM: 37.05 ± 18.27 DLS: 50.8 | Spherical | [173] |
| Crotalaria verrucosa | Leaf | Boil | Zinc nitrate hexahydrate | TEM: 27 XRD: 17.47 DLS: 27 | Hexagonal wurtzite | [174] |
| Limonia acidissima | Leaf | Decoction at 60 °C | Zinc nitrate: pH 10 | HRTEM: 12–53 | Spherical | [175] |
| Melia azadarach | Leaf | Decoction at 70 °C | Zinc acetate dihydrate; 70 °C | XRD: 2.72 DLS: 3.62 | Spherical | [172] |
| Mentha pulegium | Leaf | Boil | Zinc nitrate hexahydrate | TEM: 40 FE-SEM: 38–49 XRD: 44.94 | Hexagonal, quasispherical | [176] |
| Mussaenda frondosa | leaf, callus, and stem | Reflux at 100 °C | Zinc nitrate hexahydrate, calcined at 400 °C | XRD L-ZnO-NP: 8 and 15 C-ZnO-NP: 5 and 7 S-ZnO-NP: 9 and 12 | L-ZnO-NP: hexagonal wurtzite C-ZnO-NP and S-ZnO-NP: spherical | [177] |
| Myristica fragrans | Fruit | Decoction at 150 °C | Zinc acetate dihydrate; calcined at 500 °C | TEM: 35.5 SEM: 43.3–83.1 XRD: 41.23 | Spherical and hexagonal | [178] |
| Oats | Oat biomass | Boil | Zinc nitrate hexahydrate, calcined at 400 °C | DLS, SEM, TEM: 100 XRD: 17.52 | Wurtzite and hexagonal | [179] |
| Tabernaemontana divaricata | Leaf | Decoction at 80 °C | Zinc nitrate hexahydrate at 450 °C | TEM: 20–50 XRD: 36.82 | Hexagonal wurtzite | [180] |
| Strain of Bacteria | Family | Size of Nanoparticles Synthesized (nm) | Morphology of Nanoparticles | References |
|---|---|---|---|---|
| Rhodococcus pyridinivorans | Nocardiaceae | FE-SEM: 100–120 XRD: 120–130 | Hexagonal phase and roughly spherical | [181] |
| Pseudomonas aeruginosa | Pseudomonadaceae | TEM: 35–80 XRD: 27, DLS: 81 | Spherical | [183] |
| Pseudomonas aeruginosa NMJ15 | Pseudomonadaceae | TEM: 6–21 XRD: 21 | Spherical | [184] |
| Aeromonas hydrophila | Pseudomonadaceae | AFM: 57.72 XRD: 42–64 | Oval and spherical | [185] |
| Lactobacillus sporogens | Bacillaceae | TEM: 5–15 XRD: 11 | Hexagonal | [186] |
| B. licheniformis | Bacillaceae | TEM: 200 (nanopetal 40 nm width and 400 nm length) | Nanoflower | [182] |
| Serratia ureilytica (HM475278) | Enterobacteriaceae | SEM: 170–250 (at 30 min), 300–600 (at 60 min), 185–365 (at 90 min) | Spherical and nanoflower | [187] |
| Arthrospira platensis | Microcoleaceae | TEM: 30–55 XRD: ≈45 | Spherical | [188] |
| Desertifilum sp. EAZ03 | Desertifilaceae | TEM: 88 XRD: 60–80 | Rod | [189] |
| Marinobacter sp. 2C8 and Vibrio sp. VLA (cell-free extract) | Alteromonadaceae Vibrionaceae | 2C8-TEM: 10.23 ± 2.48 VLA-TEM: 20.26 ± 4.44 | Hexagonal wurtzite | [190] |
| Fungal Strain | Family | Size of Nanoparticles Synthesized (nm) | Morphology | References |
|---|---|---|---|---|
| Aspergillus niger | Trichocomaceae | SEM: 61 ± 0.65 XRD: 41 | Spherical Crystalline wurtzite | [194] |
| Candida albicans | Saccharomycetaceae | XRD: 25, SEM: 15–25, TEM: ~20 | Hexagonal wurtzite, quasispherical | [192] |
| Aspergillus fumigatus TFR-8 | Trichocomaceae | DLS: 1.2–6.8 | Oblate spherical and hexagonal | [195] |
| Aspergillus strain | Trichocomaceae | SEM: 50–120 | Spherical | [196] |
| Xylaria acuta | Xylariaceae | TEM: 30–50, average: 34 SEM: 40–55 DLS: 30–50 XRD: 35–45 | Rod and hexagonal | [197] |
| Algae Strain | Family | Size of As-Synthesized Nanoparticles (nm) | Morphology of the Nanoparticles | Surface Functional Groups | References |
|---|---|---|---|---|---|
| Sargassum muticum | Sargassaceae | FE-SEM: 30–57 XRD: 42 | Hexagonal wurtzite | Sulfate group asymmetric with stretching band, asymmetric C–O band coupled with C-O-SO3 and -OH group, sulfated polysaccharides | [199] |
| Sargassum muticum | Sargassaceae | SEM: 50 DLS: 25–50 XRD: 15–50 | Spherical | 3432 and 1609 cm−1 presence of O–H stretching, 500 cm−1 below suggests a Zn–O stretching vibration | [202] |
| Chlamydomonas reinhardtii | Chlamydomonaceae | HR-SEM: 55–80 XRD: 21 | Rod | N–H bending band of amide I and amide II, C=O stretching of zinc acetate C=O, and C–O–C stretch of polysaccharide | [201] |
| S. myriocystum | Sargassaceae | DLS: 46.6 AFM: 20–36 TEM: 76–186 | Rectangular, triangle, radial hexagonal, rod, and spherical shape | Carboxylic acid, with O–H and C=O stretching bands | [200] |
| Ulva lactuca | Ulvaceae | TEM: 10–50, av.: 15 XRD: 5–15 | Triangle, hexagon, rod | 420 cm−1 suggests ZnO, peaks at 1634.00, and 620.93 cm−1 suggests ZnO stretching and deformation vibration | [203] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandal, A.K.; Katuwal, S.; Tettey, F.; Gupta, A.; Bhattarai, S.; Jaisi, S.; Bhandari, D.P.; Shah, A.K.; Bhattarai, N.; Parajuli, N. Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications. Nanomaterials 2022, 12, 3066. https://doi.org/10.3390/nano12173066
Mandal AK, Katuwal S, Tettey F, Gupta A, Bhattarai S, Jaisi S, Bhandari DP, Shah AK, Bhattarai N, Parajuli N. Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications. Nanomaterials. 2022; 12(17):3066. https://doi.org/10.3390/nano12173066
Chicago/Turabian StyleMandal, Ashok Kumar, Saurav Katuwal, Felix Tettey, Aakash Gupta, Salyan Bhattarai, Shankar Jaisi, Devi Prasad Bhandari, Ajay Kumar Shah, Narayan Bhattarai, and Niranjan Parajuli. 2022. "Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications" Nanomaterials 12, no. 17: 3066. https://doi.org/10.3390/nano12173066
APA StyleMandal, A. K., Katuwal, S., Tettey, F., Gupta, A., Bhattarai, S., Jaisi, S., Bhandari, D. P., Shah, A. K., Bhattarai, N., & Parajuli, N. (2022). Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications. Nanomaterials, 12(17), 3066. https://doi.org/10.3390/nano12173066