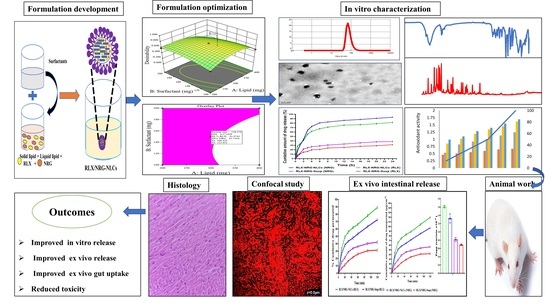
Nanostructured Lipid Carrier-Based Codelivery of Raloxifene and Naringin: Formulation, Optimization, In Vitro, Ex Vivo, In Vivo Assessment, and Acute Toxicity Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Excipients Screening
2.2.2. Experimentation Design
2.2.3. Formulation of RLX/NRG NLCs
2.2.4. Lyophilization of RLX/NRG NLCs Formulations
2.2.5. In Vitro Characterization of RLX/NRG NLCs
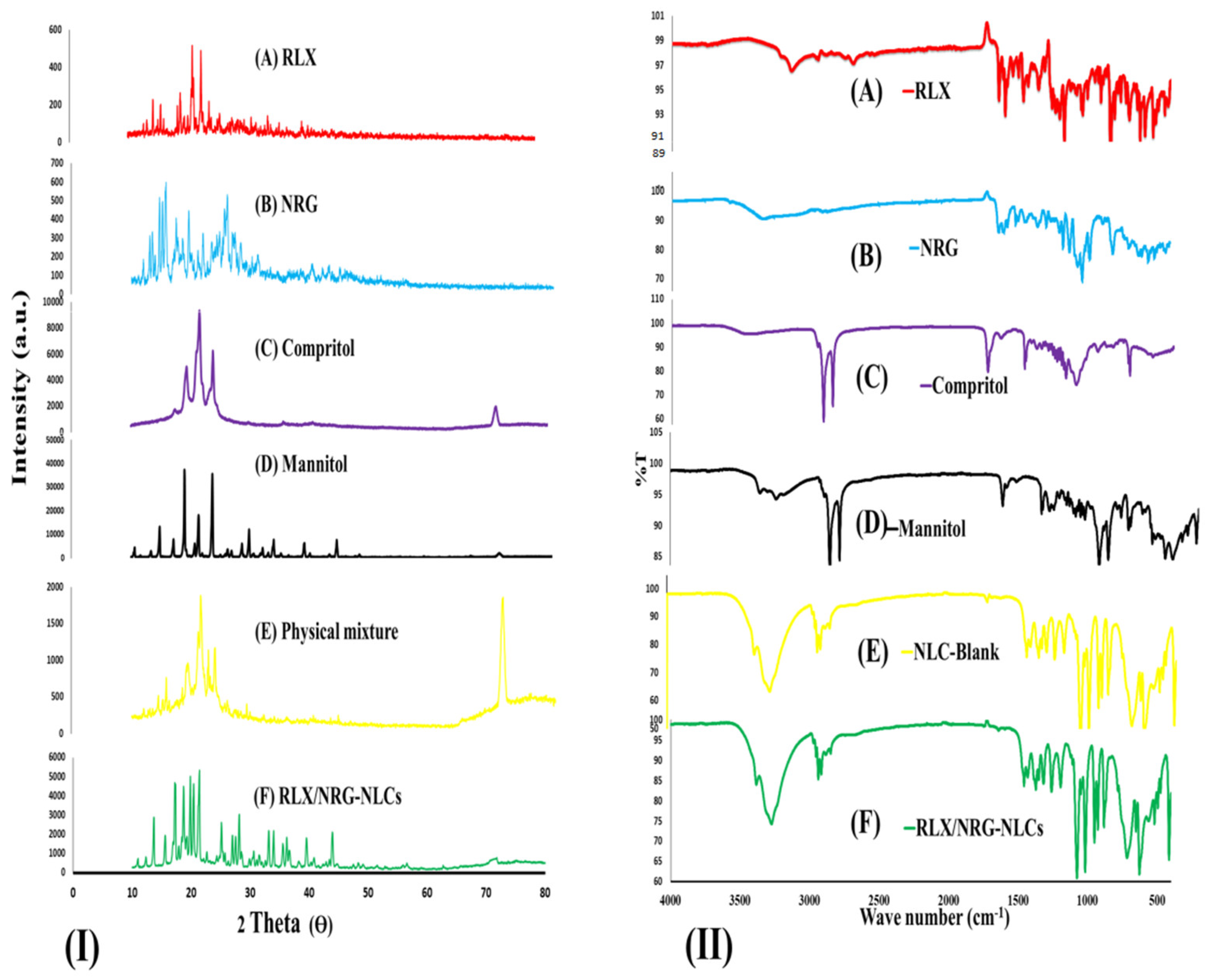
X-ray Diffraction (XRD)
Fourier-Transform Infrared Spectroscopy (FTIR)
Microscopic Evaluation
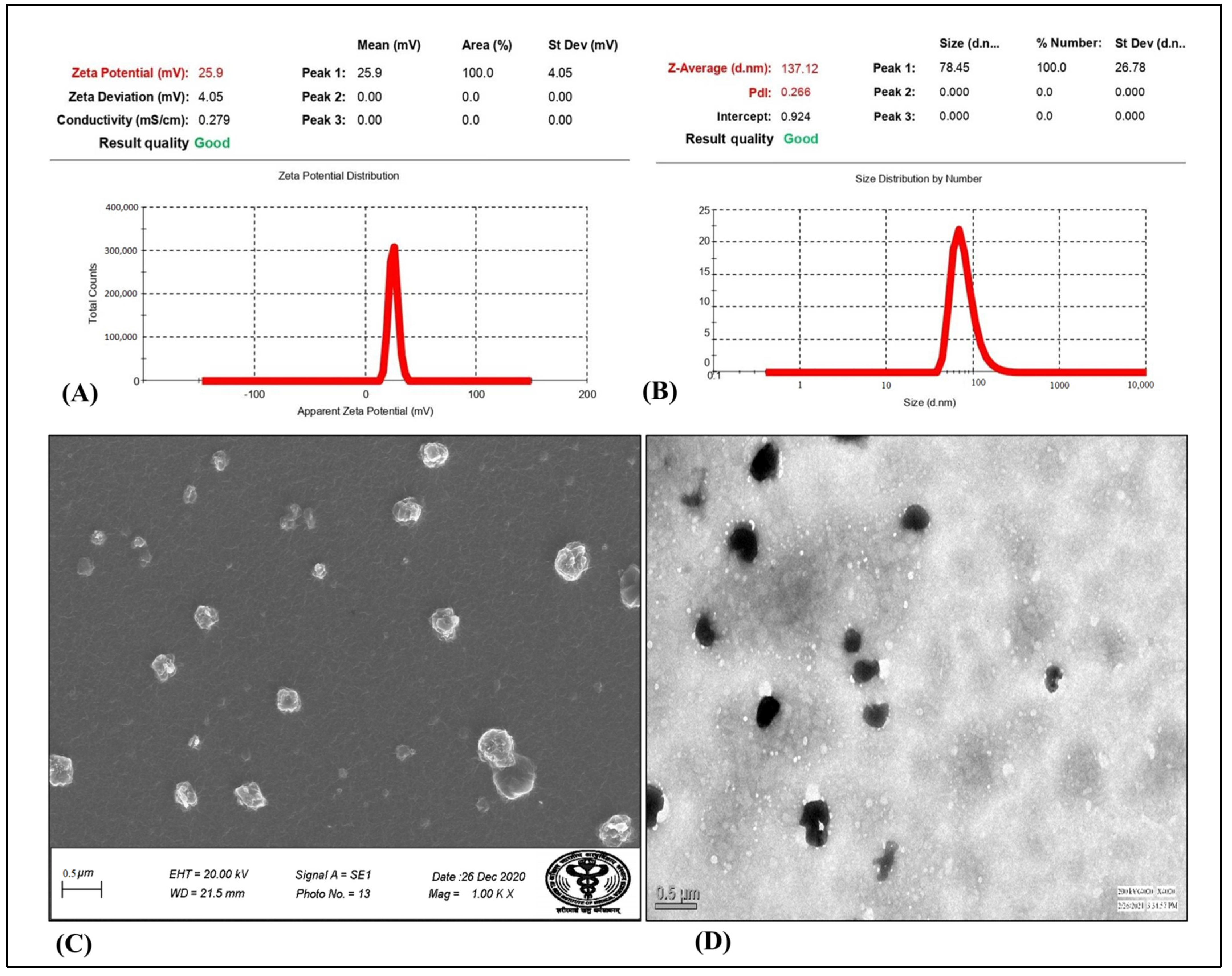
Particle Size, Polydispersity Index, and Zeta Potential Measurement
Entrapment Efficiency (EE)
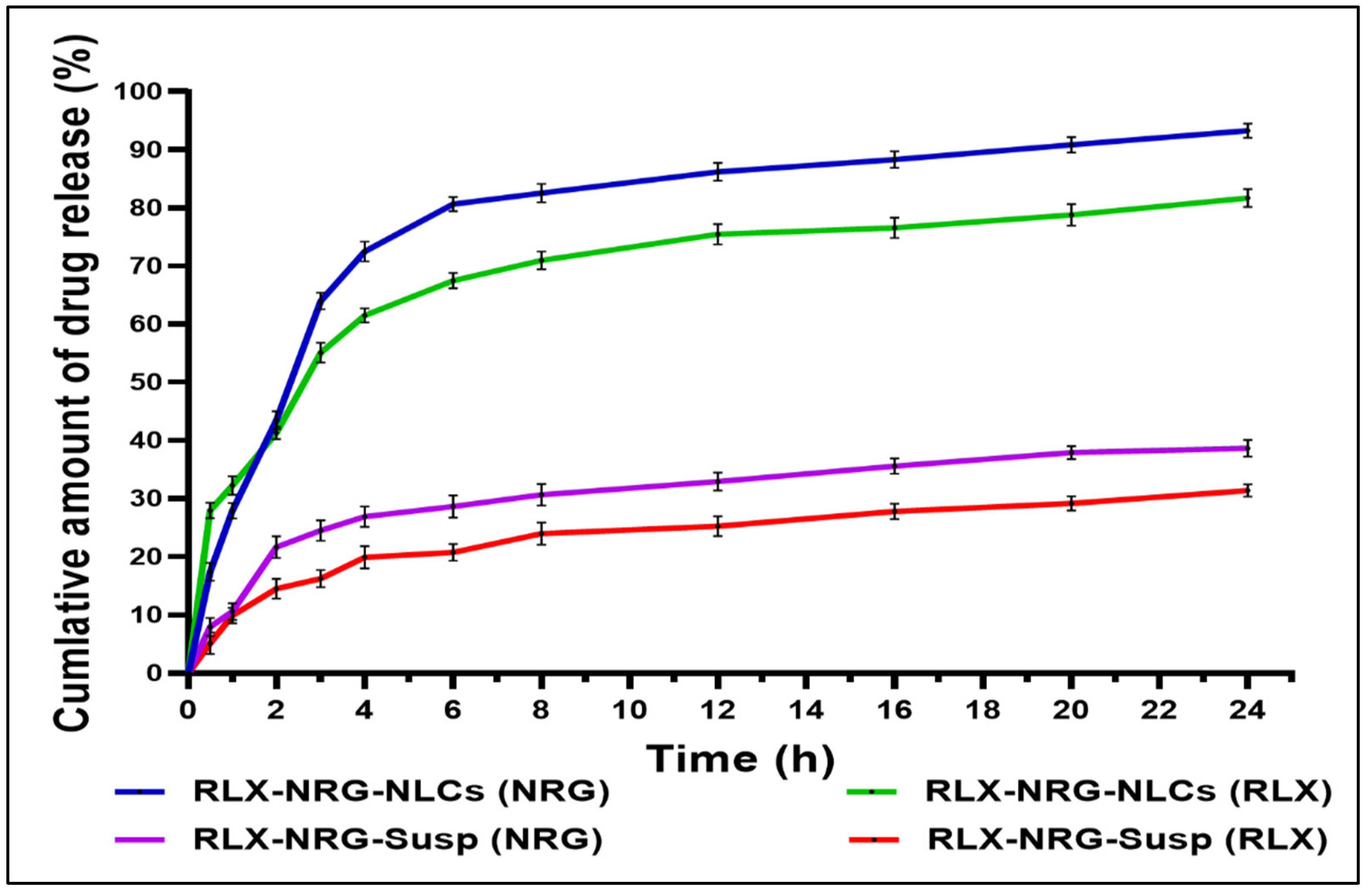
In Vitro Drug Release and Kinetics Modeling
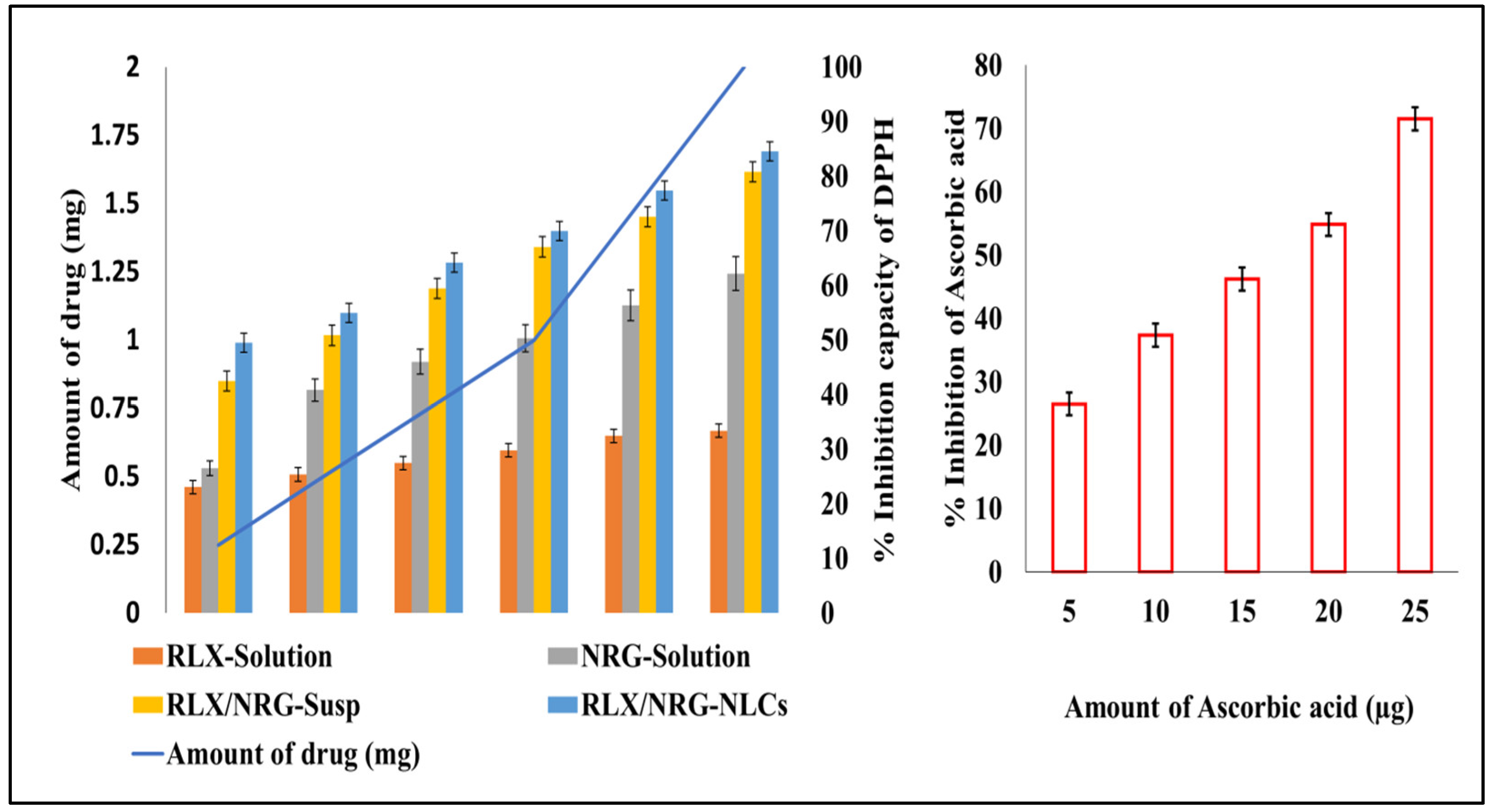
In Vitro Antioxidant Activity
2.3. Animal Studies
2.3.1. Ex Vivo Permeability Study
2.3.2. Confocal Laser Scanning Microscopy (CLSM)
2.3.3. In Vivo Acute Oral Toxicity
2.4. Storage Stability
2.5. Statistical Analysis
3. Results and Discussion
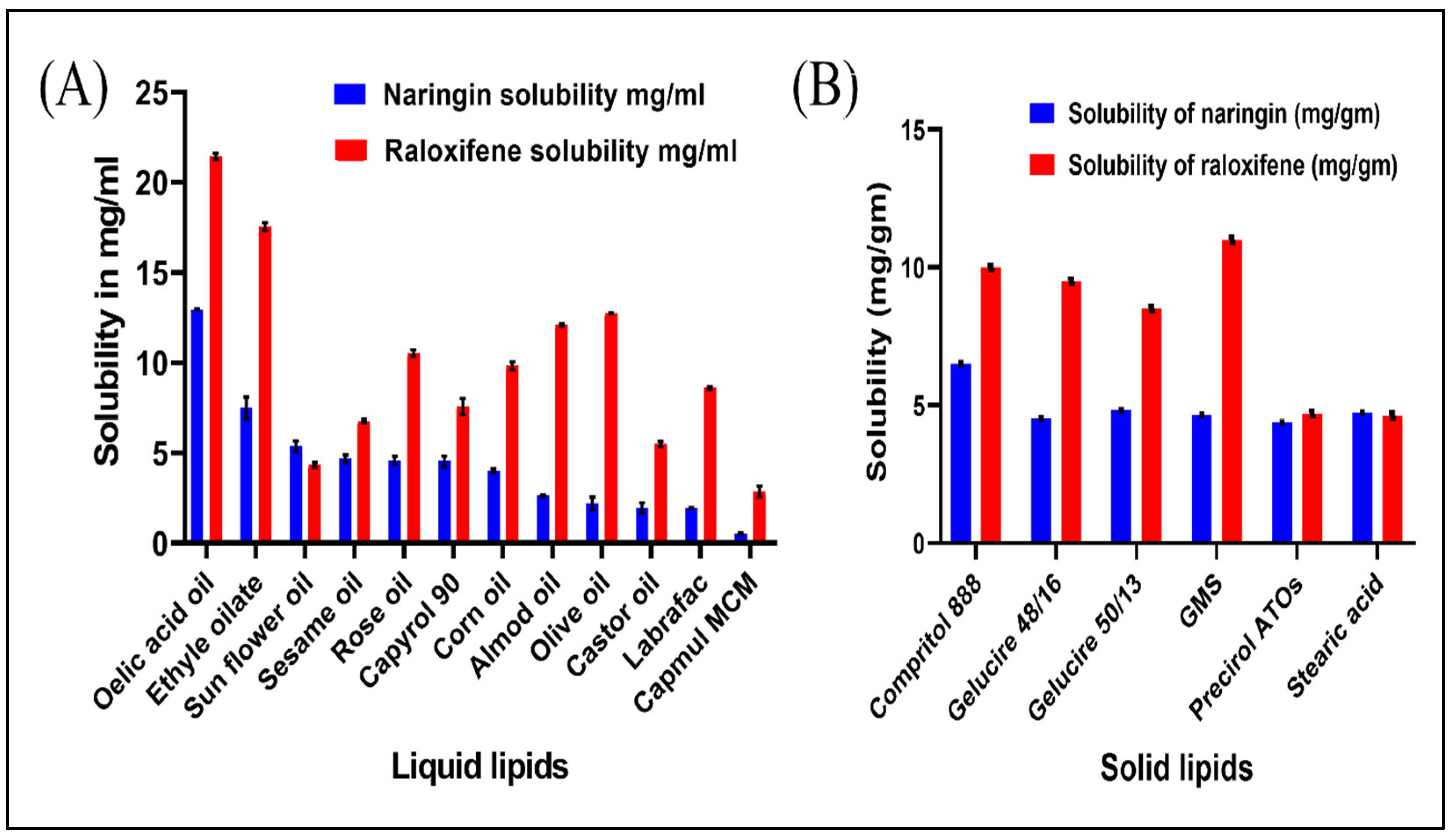
3.1. Excipients Selection
3.2. Experimentation Design
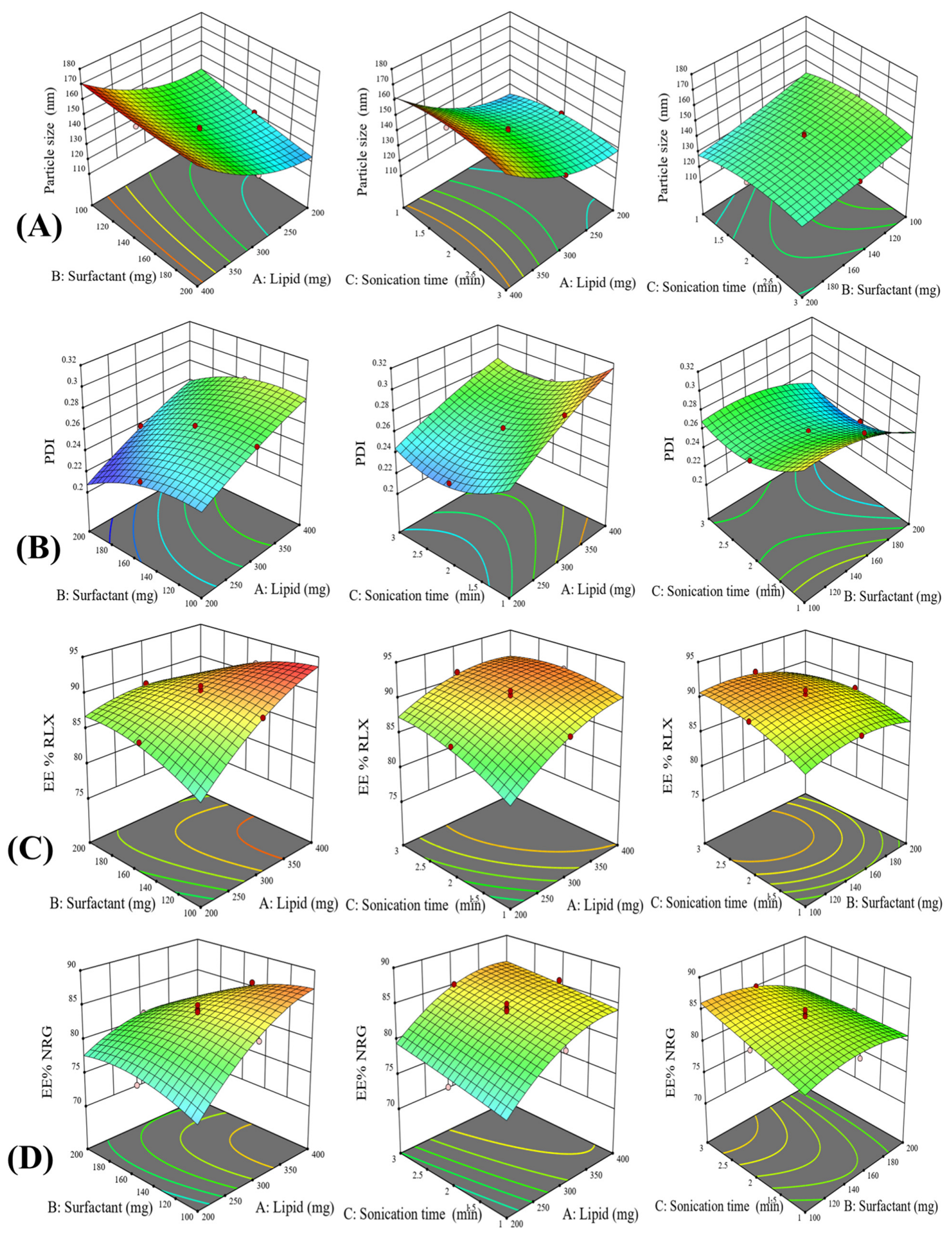
3.2.1. The Impact of Independent Variables on Particle Size
3.2.2. The Impact of Independent Variables on the PDI
3.2.3. The Impact of Independent Variables on EE% of RLX and NRG
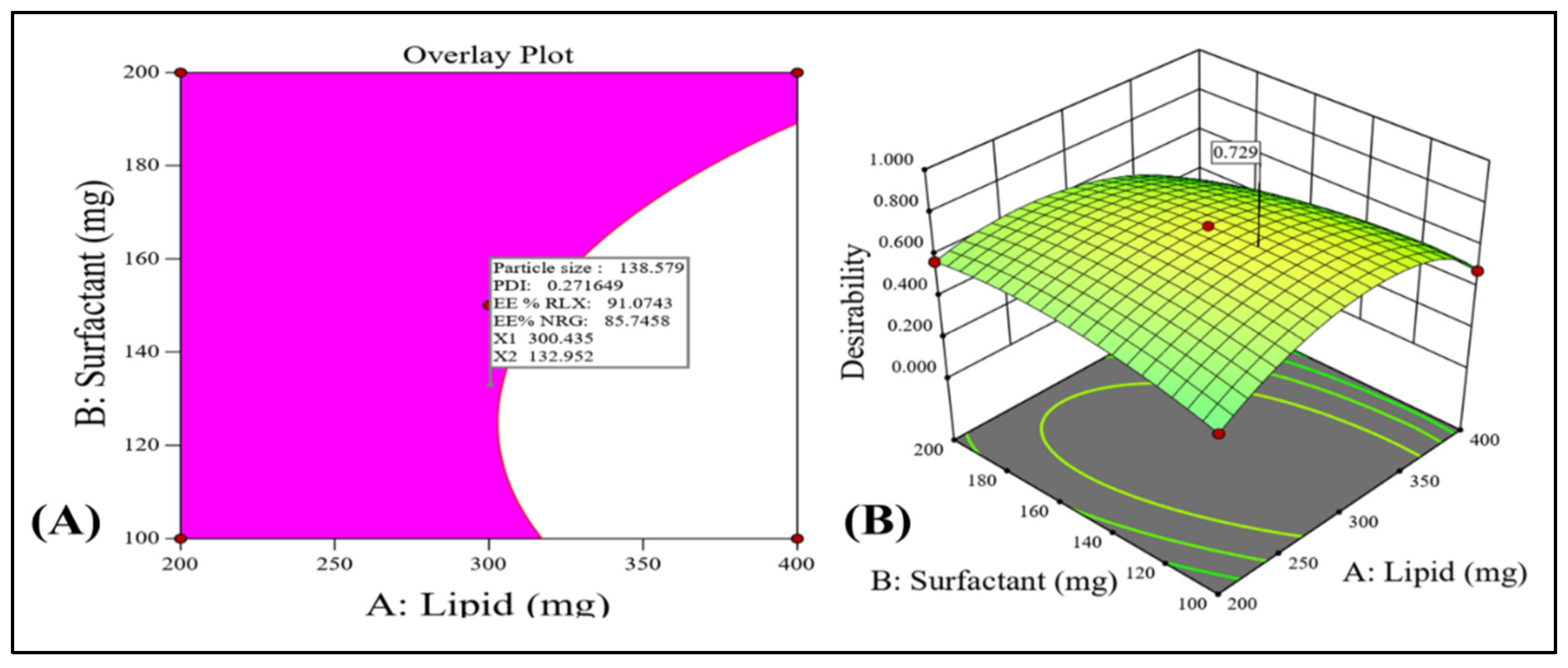
3.2.4. Checkpoint Selection of an Optimum Formulation
3.3. Characterization of Optimized RLX/NRG NLCs
3.3.1. XRD Analysis
3.3.2. FTIR Analysis
3.3.3. Particle Size, PDI, Zeta Potential
3.3.4. Surface Morphology Study
3.3.5. Determination of RLX and NRG Entrapment Efficiency
3.3.6. In Vitro Drug Release Study
3.3.7. Antioxidant Activity
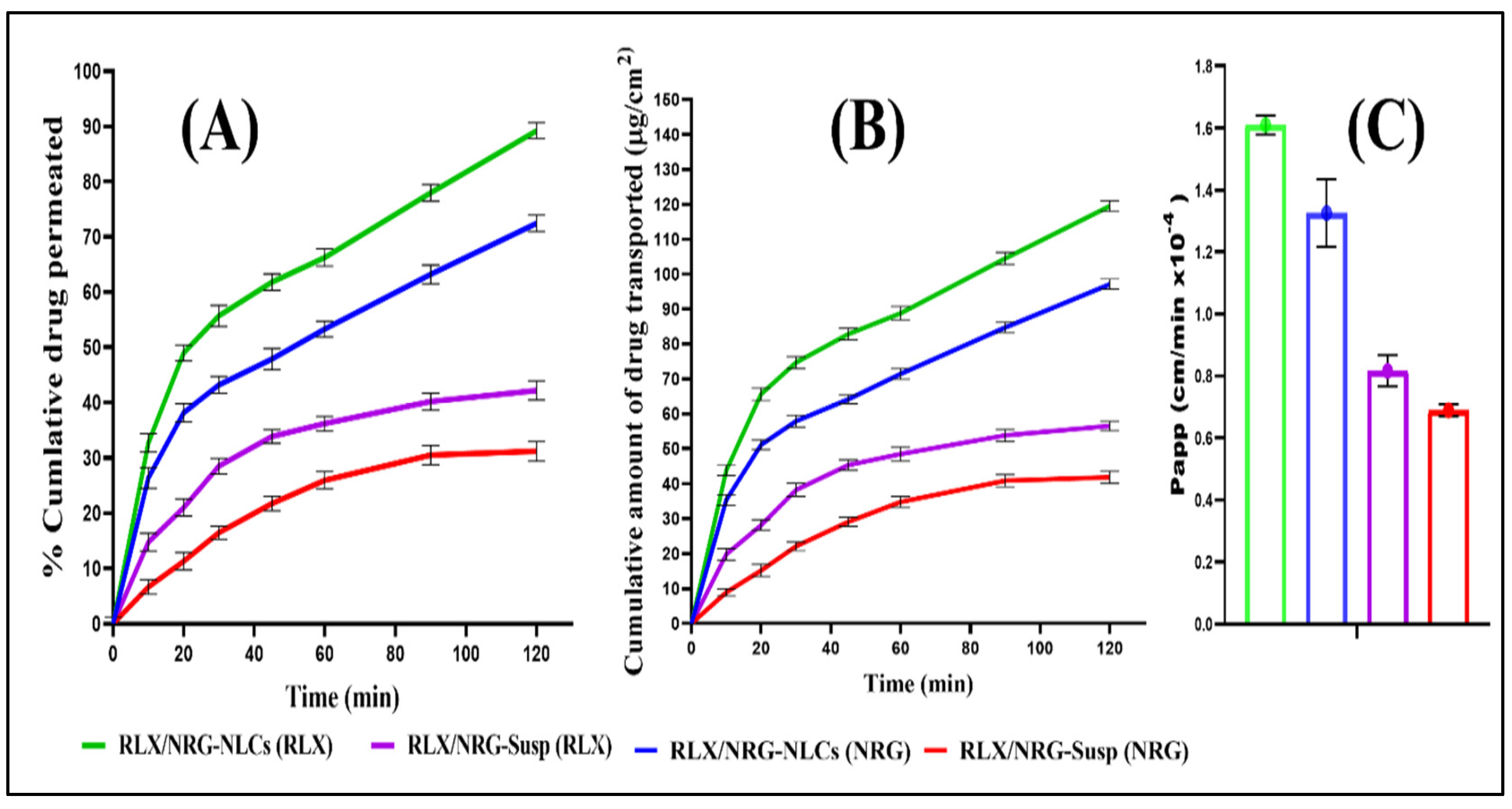
3.3.8. Ex Vivo Intestinal Permeation Study
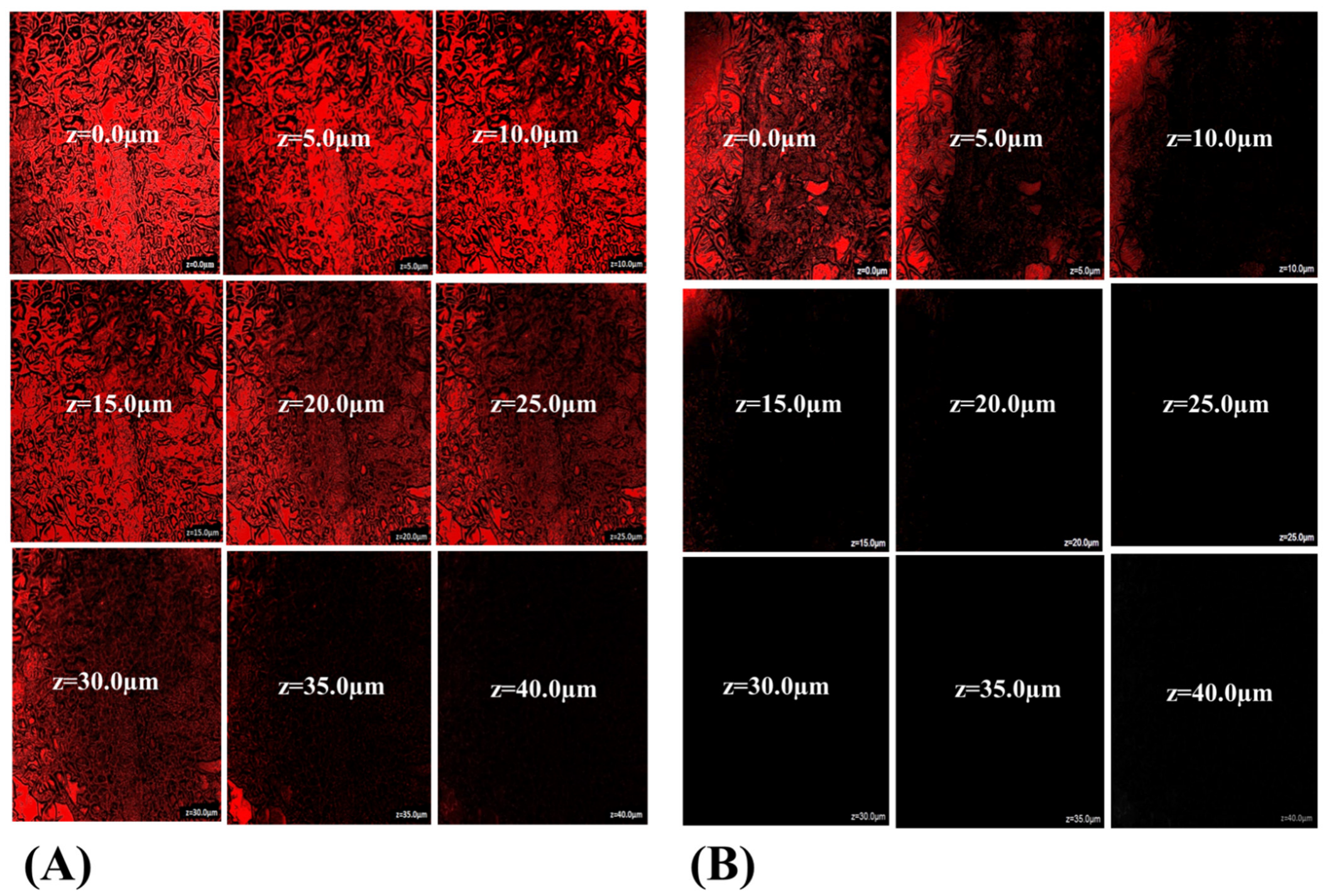
3.3.9. Intestinal Uptake by CLSM
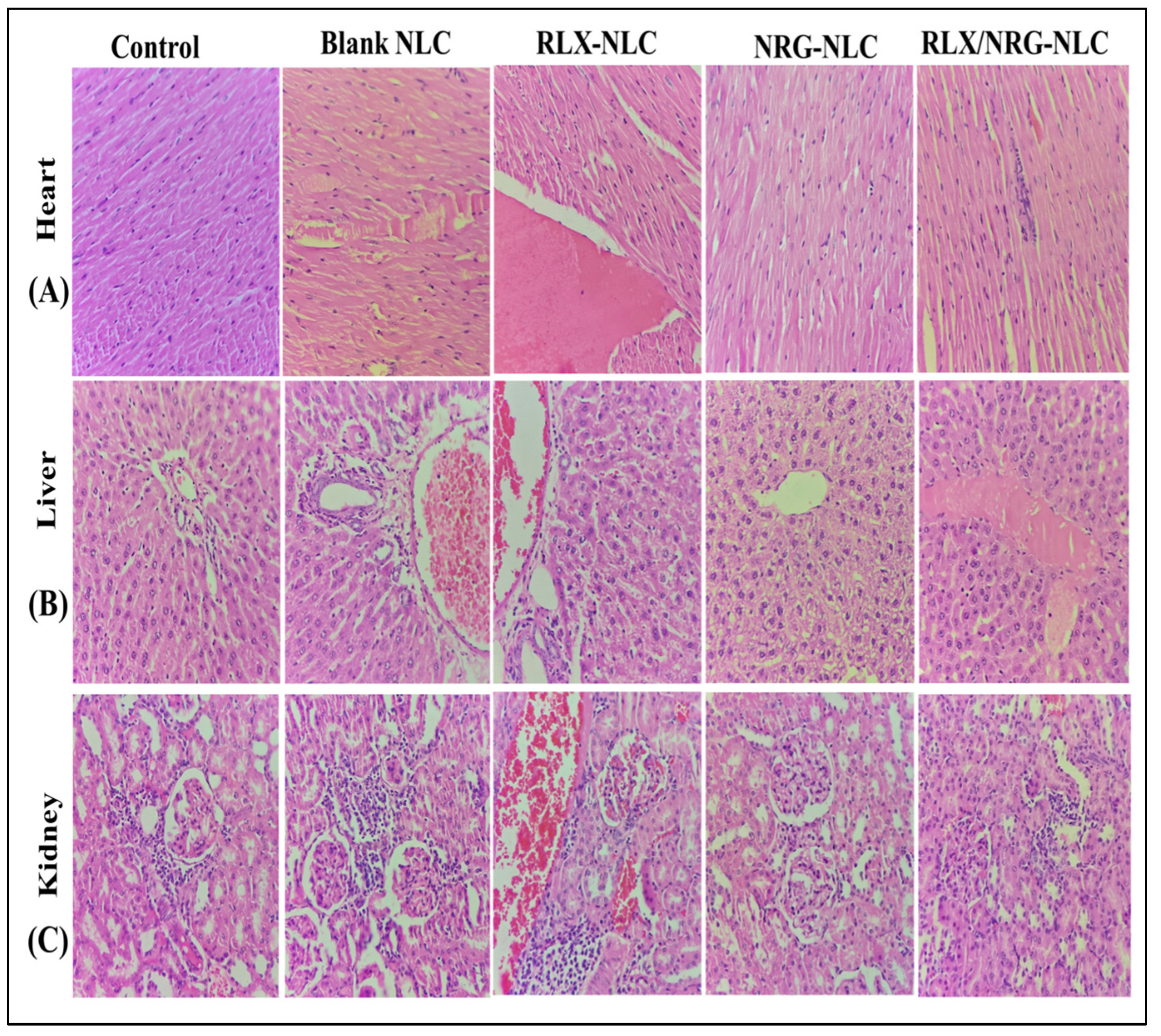
3.3.10. In Vivo Acute Toxicity Study
3.3.11. Storage Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gupta, P.; Neupane, Y.R.; Parvez, S.; Kohli, K. Recent Advances in Targeted Nanotherapeutic Approaches for Breast Cancer Management. Nanomedicine 2021, 16, 2605–2631. [Google Scholar] [CrossRef]
- Mangla, B.; Raj, Y.; Singh, A.; Kohli, K. Tamoxifen and Sulphoraphane for the Breast Cancer Management: A Synergistic Nanomedicine Approach. Med. Hypotheses 2019, 132, 109379. [Google Scholar] [CrossRef] [PubMed]
- Alhalmi, A.; Beg, S.; Almalki, W.H.; Alghamdi, S.; Kohli, K. Recent Advances in Nanotechnology-Based Targeted Therapeutics for Breast Cancer Management. Curr. Drug Metab. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.D.; Solanki, P.; Pandit, J.; Jahan, R.N. Fabrication and Optimization of Raloxifene Loaded Spanlastics Vesicle for Transdermal Delivery Journal of Drug Delivery Science and Technology Fabrication and Optimization of Raloxifene Loaded Spanlastics Vesicle for Transdermal Delivery. J. Drug Deliv. Sci. Technol. 2022, 68, 103102. [Google Scholar] [CrossRef]
- Costa, B.; Amorim, I.; Gärtner, F.; Vale, N. Understanding Breast Cancer: From Conventional Therapies to Repurposed Drugs. Eur. J. Pharm. Sci. 2020, 151, 105401. [Google Scholar] [CrossRef] [PubMed]
- Ağardan, N.B.M.; Değim, Z.; Yılmaz; Altıntaş, L.; Topal, T. Tamoxifen/Raloxifene Loaded Liposomes for Oral Treatment of Breast Cancer. J. Drug Deliv. Sci. Technol. 2020, 57, 101612. [Google Scholar] [CrossRef]
- Chen, R.; Qi, Q.L.; Wang, M.T.; Li, Q.Y. Therapeutic Potential of Naringin: An Overview. Pharm. Biol. 2016, 54, 3203–3210. [Google Scholar] [CrossRef]
- Alhalmi, A.; Amin, S.; Beg, S.; Al-Salahi, R.; Mir, S.R.; Kohli, K. Formulation and Optimization of Naringin Loaded Nanostructured Lipid Carriers Using Box-Behnken Based Design: In Vitro and Ex Vivo Evaluation. J. Drug Deliv. Sci. Technol. 2022, 74, 103590. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, L.; Zhang, Y. Naringin Inhibits Thyroid Cancer Cell Proliferation and Induces Cell Apoptosis through Repressing PI3K/AKT Pathway. Pathol. Res. Pract. 2019, 215, 152707. [Google Scholar] [CrossRef]
- Eanes, L.; Patel, Y.M. Inhibition of the MAPK Pathway Alone Is Insufficient to Account for All of the Cytotoxic Effects of Naringenin in MCF-7 Breast Cancer Cells. Biochim. Open 2016, 3, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Ajji, P.K.; Walder, K.; Puri, M. Combination of Balsamin and Flavonoids Induce Apoptotic Effects in Liver and Breast Cancer Cells. Front. Pharmacol. 2020, 11, 574496. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg Zand, R.S.; Jenkins, D.J.A.; Diamandis, E.P. Steroid Hormone Activity of Flavonoids and Related Compounds. Breast Cancer Res. Treat. 2000, 62, 35–49. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, P.; Pellegrini, M.; Totta, P.; Acconcia, F.; Marino, M. Xenoestrogens Alter Estrogen Receptor (ER) α Intracellular Levels. PLoS ONE 2014, 9, e88961. [Google Scholar] [CrossRef] [PubMed]
- Connor, K.; Howell, J.; Chen, I.; Safe, S. Naringenin: A Weakly Estrogenic Bioflavonoid That Exhibits Antiestrogenic Activity. Biochem. Pharmacol. 1995, 50, 1485–1493. [Google Scholar]
- Mir, I.A.; Tiku, A.B. Chemopreventive and Therapeutic Potential of “Naringenin,” a Flavanone Present in Citrus Fruits. Nutr. Cancer 2015, 67, 27–42. [Google Scholar] [CrossRef] [PubMed]
- So, F.V.; Guthrie, N.; Chambers, A.F.; Moussa, M.; Carroll, K.K.; So, F.V.; Guthrie, N.; Chambers, A.F.; Moussa, M.; So, F.V.; et al. Inhibition of Human Breast Cancer Cell Proliferation and Delay of Mammary Tumorigenesis by Flavonoids and Citrus Juices. Nutr. Cancer 2009, 5581, 167–181. [Google Scholar] [CrossRef]
- El-Leithy, E.S.; Hassan, S.A.; Abdel-Rashid, R.S. Tamoxifen Citrate/Coenzyme Q10 as Smart Nanocarriers Bitherapy for Breast Cancer: Cytotoxicity, Genotoxicity, and Antioxidant Activity. J. Drug Deliv. Sci. Technol. 2019, 51, 36–44. [Google Scholar] [CrossRef]
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Nanocarrier Mediated Combination Drug Delivery for Chemotherapy e A Review. J. Drug Deliv. Sci. Technol. 2017, 39, 362–371. [Google Scholar] [CrossRef]
- Jabri, T.; Imran, M.; Aziz, A.; Rao, K.; Kawish, M.; Irfan, M.; Malik, M.I.; Simjee, S.U.; Arfan, M.; Shah, M.R. Design and Synthesis of Mixed Micellar System for Enhanced Anticancer Efficacy of Paclitaxel through Its Co-Delivery with Naringin. Drug Dev. Ind. Pharm. 2019, 45, 703–714. [Google Scholar] [CrossRef]
- Erdogan, S.; Doganlar, O.; Doganlar, Z.B.; Turkekul, K. Naringin Sensitizes Human Prostate Cancer Cells to Paclitaxel Therapy. Prostate Int. 2018, 6, 126–135. [Google Scholar] [CrossRef]
- Teeter, J.S.; Meyerhoff, R.D. Environmental Fate and Chemistry of Raloxifene Hydrochloride. Environ. Toxicol. Chem. 2002, 21, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, R.; Gao, Y.; Zheng, Y.; Liao, L.; Cao, Y.; Li, J. Encapsulation of Hydrophobic and Low-Soluble Polyphenols into Nanoliposomes by PH-Driven Method: Naringenin and Naringin as Model Compounds. Foods 2021, 10, 963. [Google Scholar] [CrossRef] [PubMed]
- Murthy, A.; Ravi, P.R.; Kathuria, H.; Vats, R. Self-Assembled Lecithin-Chitosan Nanoparticles Improve the Oral Bioavailability and Alter the Pharmacokinetics of Raloxifene. Int. J. Pharm. 2020, 588, 119731. [Google Scholar] [CrossRef]
- Zeng, X.; Yao, H.; Zheng, Y.; He, Y.; He, Y.; Rao, H.; Li, P.; Su, W. Tissue Distribution of Naringin and Derived Metabolites in Rats after a Single Oral Administration. J. Chromatogr. B 2020, 1136, 121846. [Google Scholar] [CrossRef]
- Mahmood, S.; Chatterjee, B.; Mandal, U.K. Pharmacokinetic Evaluation of the Synergistic Effect of Raloxifene Loaded Transfersomes for Transdermal Delivery. J. Drug Deliv. Sci. Technol. 2021, 63, 102545. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, X.; Ma, J.; Kang, J.; Zhang, Y.; Guo, Y. Sustained Release of Naringin from Silk-Fibroin-Nanohydroxyapatite Scaffold for the Enhancement of Bone Regeneration. Mater. Today Bio 2022, 13, 100206. [Google Scholar] [CrossRef]
- Zhang, M.; Hagan, C.T.; Foley, H.; Tian, X.; Yang, F.; Au, K.M.; Mi, Y.; Medik, Y.; Roche, K.; Wagner, K.; et al. Co-Delivery of Etoposide and Cisplatin in Dual-Drug Loaded Nanoparticles Synergistically Improves Chemoradiotherapy in Non-Small Cell Lung Cancer Models. Acta Biomater. 2021, 124, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Ben Khalifa, R.; Cacciatore, I.; Dimmito, M.P.; Ciulla, M.; Grande, R.; Puca, V.; Robuffo, I.; de Laurenzi, V.; Chekir-Ghedira, L.; Di Stefano, A.; et al. Multiple Lipid Nanoparticles as Antimicrobial Drug Delivery Systems. J. Drug Deliv. Sci. Technol. 2022, 67, 102887. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Ahmad, M.Z.; Garg, A.; Ahmad, J. Advancement in Design of Nanostructured Lipid Carriers for Cancer Targeting and Theranostic Application. Biochim. Biophys. Acta-Gen. Subj. 2021, 1865, 129936. [Google Scholar] [CrossRef]
- Medina-montano, C.; Berti, I.R.; Gambaro, C.; Svensson, M.; Padula, G.; Chain, C.Y.; Castro, G.R.; Grabbe, S.; Bros, M.; Gehring, S.; et al. Nanostructured Lipid Carriers Loaded with Dexamethasone Prevent Inflammatory Responses in Primary Non-Parenchymal Liver Cells. Pharmaceutics 2022, 14, 1611. [Google Scholar] [CrossRef]
- Nasirizadeh, S.; Malaekeh-Nikouei, B. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers in Oral Cancer Drug Delivery. J. Drug Deliv. Sci. Technol. 2020, 55, 101458. [Google Scholar] [CrossRef]
- Salvi, V.R.; Pawar, P. Nanostructured Lipid Carriers (NLC) System: A Novel Drug Targeting Carrier. J. Drug Deliv. Sci. Technol. 2019, 51, 255–267. [Google Scholar] [CrossRef]
- El-Leithy, E.S.; Abdel-Rashid, R.S. Lipid Nanocarriers for Tamoxifen Citrate/Coenzyme Q10 Dual Delivery. J. Drug Deliv. Sci. Technol. 2017, 41, 239–250. [Google Scholar] [CrossRef]
- Khames, A. Formulation and Characterization of Eplerenone Nanoemulsion Liquisolids, an Oral Delivery System with Higher Release Rate and Improved Bioavailability. Pharmaceutics 2019, 11, 40. [Google Scholar] [CrossRef]
- Marathe, S.; Shadambikar, G.; Mehraj, T.; Sulochana, S.P.; Dudhipala, N.; Majumdar, S. Development of α-Tocopherol Succinate-Based Nanostructured Lipid Carriers for Delivery of Paclitaxel. Pharmaceutics 2022, 14, 1034. [Google Scholar] [CrossRef] [PubMed]
- Rojekar, S.; Pai, R.; Abadi, L.F.; Mahajan, K.; Prajapati, M.K.; Kulkarni, S.; Vavia, P. Dual Loaded Nanostructured Lipid Carrier of Nano-Selenium and Etravirine as a Potential Anti-HIV Therapy. Int. J. Pharm. 2021, 607, 120986. [Google Scholar] [CrossRef]
- Garcês, A.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Formulations Based on Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for Cutaneous Use: A Review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef]
- Beg, S.; Hasnain, M.S.; Rahman, M.; Swain, S. Introduction to Quality by Design (QbD): Fundamentals, Principles, and Applications; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128157992. [Google Scholar]
- Mangla, B.; Neupane, Y.R.; Singh, A.; Kumar, P.; Shafi, S.; Kohli, K. Lipid-Nanopotentiated Combinatorial Delivery of Tamoxifen and Sulforaphane: Ex Vivo, In Vivo and Toxicity Studies. Nanomedicine 2020, 15, 2563–2583. [Google Scholar] [CrossRef]
- Dudhipala, N.; Gorre, T. Neuroprotective Effect of Ropinirole Lipid Nanoparticles Enriched Hydrogel for Parkinson’s Disease: In Vitro, Ex Vivo, Pharmacokinetic and Pharmacodynamic Evaluation. Pharmaceutics 2020, 12, 448. [Google Scholar] [CrossRef]
- Rajput, A.P.; Butani, S.B. Resveratrol Anchored Nanostructured Lipid Carrier Loaded In Situ Gel via Nasal Route: Formulation, Optimization and In Vivo Characterization. J. Drug Deliv. Sci. Technol. 2019, 51, 214–223. [Google Scholar] [CrossRef]
- Pakdaman, P.; Bikhof, M.; Parivar, K.; Akbarzadeh, A.; Yousefi, M. Preparation and Evaluation of Gemcitabin and Cisplatin-Entrapped Folate-PEGylated Liposomes as Targeting Co-Drug Delivery System in Cancer Therapy. J. Drug Deliv. Sci. Technol. 2021, 65, 102756. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, R.; Song, X.; Gong, J.; Wang, J.; Lin, M.; Yang, F.; Cheng, X.; Gao, X.; Peng, L.; et al. Optimization of Maduramicin Ammonium-Loaded Nanostructured Lipid Carriers Using Box–Behnken Design for Enhanced Anticoccidial Effect against Eimeria Tenella in Broiler Chickens. Pharmaceutics 2022, 14, 1330. [Google Scholar] [CrossRef] [PubMed]
- Harshita; Barkat, A.; Beg, S.; Pottoo, F.H.; Siddiqui, S.; Ahmad, F.J. Paclitaxel-Loaded Nanolipidic Carriers with Improved Oral Bioavailability and Anticancer Activity against Human Liver Carcinoma. AAPS PharmSciTech 2019, 20, 87. [Google Scholar] [CrossRef]
- Feng, Q.; Zhu, Y.; Yuan, Y.; Li, W.; Yu, H.; Hu, M. Oral Administration Co-Delivery Nanoparticles of Docetaxel and Bevacizumab for Improving Intestinal Absorption and Enhancing Anticancer Activity. Mater. Sci. Eng. C 2021, 124, 112039. [Google Scholar] [CrossRef] [PubMed]
- Mishra, H.; Mishra, P.K.; Iqbal, Z.; Jaggi, M.; Madaan, A. Co-Delivery of Eugenol and Dacarbazine by Hyaluronic Acid-Coated Liposomes for Targeted Inhibition of Survivin in Treatment of Resistant Metastatic Melanoma. Pharmaceutics 2019, 11, 163. [Google Scholar] [CrossRef]
- Rahman, M.; Almalki, W.H.; Afzal, O.; Altamimi, A.S.A.; Kazmi, I.; Al-Abbasi, F.A.; Choudhry, H.; Alenezi, S.K.; Barkat, M.A.; Beg, S.; et al. Cationic Solid Lipid Nanoparticles of Resveratrol for Hepatocellular Carcinoma Treatment: Systematic Optimization, In Vitro Characterization and Preclinical Investigation. Int. J. Nanomed. 2020, 15, 9283–9299. [Google Scholar] [CrossRef]
- Kumar, S.P.; Birundha, K.; Kaveri, K.; Devi, K.T.R. Antioxidant Studies of Chitosan Nanoparticles Containing Naringenin and Their Cytotoxicity Effects in Lung Cancer Cells. Int. J. Biol. Macromol. 2015, 78, 87–95. [Google Scholar] [CrossRef]
- Qizilbash, F.F.; Ashhar, M.U.; Zafar, A.; Qamar, Z. Thymoquinone-Enriched Naringenin-Loaded Nanostructured Lipid Carrier for Brain Delivery via Nasal Route: In Vitro Prospect and In Vivo Therapeutic Efficacy for the Treatment of Depression. Pharmaceutics 2022, 14, 656. [Google Scholar] [CrossRef]
- Singh, A.; Neupane, Y.R.; Shafi, S.; Mangla, B.; Kohli, K. PEGylated Liposomes as an Emerging Therapeutic Platform for Oral Nanomedicine in Cancer Therapy: In Vitro and in Vivo Assessment. J. Mol. Liq. 2020, 303, 112649. [Google Scholar] [CrossRef]
- Soni, K.; Rizwanullah, M.; Kohli, K. Development and Optimization of Sulforaphane- Loaded Nanostructured Lipid Carriers by the Box-Behnken Design for Improved Oral Efficacy against Cancer: In Vitro, Ex Vivo and In Vivo Assessments. Artif. Cells Nanomed. Biotechnol. 2018, 46, S15–S31. [Google Scholar] [CrossRef]
- Saifi, Z.; Rizwanullah, M.; Mir, S.R.; Amin, S. Bilosomes Nanocarriers for Improved Oral Bioavailability of Acyclovir: A Complete Characterization through In Vitro, Ex-Vivo and In Vivo Assessment. J. Drug Deliv. Sci. Technol. 2020, 57, 101634. [Google Scholar] [CrossRef]
- Singh, A.; Neupane, Y.R.; Mangla, B.; Kohli, K. Nanostructured Lipid Carriers for Oral Bioavailability Enhancement of Exemestane: Formulation Design, In Vitro, Ex Vivo, and In Vivo Studies. J. Pharm. Sci. 2019, 108, 3382–3395. [Google Scholar] [CrossRef] [PubMed]
- Tom, G.; Philip, S.; Isaac, R.; Praseetha, P.K.; Jiji, S.G.; Asha, V.V. Preparation of an Efficient and Safe Polymeric-Magnetic Nanoparticle Delivery System for Sorafenib in Hepatocellular Carcinoma. Life Sci. 2018, 206, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.S.; Yazan, L.S.; Ng, W.K.; Noordin, M.M.; Sapuan, S.; Foo, J.B.; Tor, Y.S. Acute and Subacute Toxicity Profiles of Thymoquinone-Loaded Nanostructured Lipid Carrier in BALB/c Mice. Int. J. Nanomed. 2016, 11, 5905–5915. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Pradhan, M.; Patel, R.J.; Singhvi, G. Design and Optimization of Curcumin Loaded Nano Lipid Carrier System Using Box-Behnken Design. Biomed. Pharmacother. 2021, 141, 111919. [Google Scholar] [CrossRef]
- Lin, Y.; Shen, Q.; Katsumi, H.; Okada, N.; Fujita, T.; Jiang, X.; Yamamoto, A. Effects of Labrasol and Other Pharmaceutical Excipients on the Intestinal Transport and Absorption of Rhodamine123, a P-Glycoprotein Substrate, in Rats. Biol. Pharm. Bull. 2007, 30, 1301–1307. [Google Scholar] [CrossRef]
- Collnot, E.M.; Baldes, C.; Wempe, M.F.; Kappl, R.; Hüttermann, J.; Hyatt, J.A.; Edgar, K.J.; Schaefer, U.F.; Lehr, C.M. Mechanism of Inhibition of P-Glycoprotein Mediated Efflux by Vitamin E TPGS: Influence on ATPase Activity and Membrane Fluidity. Mol. Pharm. 2007, 4, 465–474. [Google Scholar] [CrossRef]
- Aburahma, M.H.; Badr-Eldin, S.M. Compritol 888 ATO: A Multifunctional Lipid Excipient in Drug Delivery Systems and Nanopharmaceuticals. Expert Opin. Drug Deliv. 2014, 11, 1865–1883. [Google Scholar] [CrossRef]
- Abdel-Salam, F.S.; Elkheshen, S.A.; Mahmoud, A.A.; Basalious, E.B.; Amer, M.S.; Mostafa, A.A.; Elkasabgy, N.A. In-Situ Forming Chitosan Implant-Loaded with Raloxifene Hydrochloride and Bioactive Glass Nanoparticles for Treatment of Bone Injuries: Formulation and Biological Evaluation in Animal Model. Int. J. Pharm. 2020, 580, 119213. [Google Scholar] [CrossRef]
- Gollavilli, H.; Hegde, A.R.; Managuli, R.S.; Bhaskar, K.V.; Dengale, S.J.; Reddy, M.S.; Kalthur, G.; Mutalik, S. Naringin Nano-Ethosomal Novel Sunscreen Creams: Development and Performance Evaluation. Colloids Surf. B Biointerfaces 2020, 193, 111122. [Google Scholar] [CrossRef]
- Hakim, A.; Jamaluddin; Loka, I.N.; Sukib; Prastiwi, N.W.W.S. New Method for Isolation of Naringin Compound from Citrus maxima. Nat. Resour. 2019, 10, 299–304. [Google Scholar] [CrossRef] [Green Version]
- Pedro, I.D.R.; Almeida, O.P.; Martins, H.R.; de Alcântara Lemos, J.; Branco de Barros, A.L.; Leite, E.A.; Carneiro, G. Optimization and In Vitro/In Vivo Performance of Paclitaxel-Loaded Nanostructured Lipid Carriers for Breast Cancer Treatment. J. Drug Deliv. Sci. Technol. 2019, 54, 101370. [Google Scholar] [CrossRef]
- Soni, N.K.; Sonali, L.J.; Singh, A.; Mangla, B.; Neupane, Y.R.; Kohli, K. Nanostructured Lipid Carrier Potentiated Oral Delivery of Raloxifene for Breast Cancer Treatment. Nanotechnology 2020, 31, 475101. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Lu, Y.; Jv, M.; Hu, J.; Li, Q.; Tu, J. Enhancing Effect of Labrasol on the Intestinal Absorption of Ganciclovir in Rats. Drug Dev. Ind. Pharm. 2011, 37, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Azmin, M.N.; Stuart, J.F.B.; Calman, K.C.; Florence, A.T. Effects of Polysorbate 80 on the Absorption and Distribution of Oral Methotrexate (MTX) in Mice. Cancer Chemother. Pharmacol. 1982, 9, 161–164. [Google Scholar] [CrossRef]
- Thakkar, H.; Desai, J. Influence of Excipients on Drug Absorption via Modulation of Intestinal Transporters Activity. Asian J. Pharm. 2015, 9, 69–82. [Google Scholar] [CrossRef]
- Shah, B.; Khunt, D.; Misra, M.; Padh, H. Non-Invasive Intranasal Delivery of Quetiapine Fumarate Loaded Microemulsion for Brain Targeting: Formulation, Physicochemical and Pharmacokinetic Consideration. Eur. J. Pharm. Sci. 2016, 91, 196–207. [Google Scholar] [CrossRef]
- Beloqui, A.; del Pozo-Rodríguez, A.; Isla, A.; Rodríguez-Gascón, A.; Solinís, M.Á. Nanostructured Lipid Carriers as Oral Delivery Systems for Poorly Soluble Drugs. J. Drug Deliv. Sci. Technol. 2017, 42, 144–154. [Google Scholar] [CrossRef]
- Qamar, Z.; Usama Ashhar, M.; Annu; Fatima Qizilibash, F.; Kumar Sahoo, P.; Ali, A.; Ali, J.; Baboota, S. Lipid Nanocarrier of Selegiline Augmented Anti-Parkinson’s Effect via P-Gp Modulation Using Quercetin. Int. J. Pharm. 2021, 609, 121131. [Google Scholar] [CrossRef]


| Independent Variables | High Level (+1) | Medium Level (0) | Low Level (−1) |
|---|---|---|---|
| A = lipid weight (mg) | 400 | 300 | 200 |
| B = surfactant weight (mg) | 200 | 150 | 100 |
| C = sonication time (min) | 3 | 2 | 1 |
| Dependent variables | Desired outcomes | ||
| Y1 = particle size (nm) | Minimize | ||
| Y2 = polydisperisbility index (PDI) | Minimize | ||
| Y3 = entrapment efficiency of RLX (%) | Maximize | ||
| Y4 = entrapment efficiency of NRG (%) | Maximize | ||
| Factor 1 | Factor 2 | Factor 3 | Response 1 | Response 2 | Response 3 | Response 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Run | Lipid (mg) | Surfactant (mg) | Sonication Time (mn) | Particle Size (nm) | PDI | EE%, RLX | EE%, NRG | ||||
| Actual | Predicted | Actual | Predicted | Actual | Predicted | Actual | Predicted | ||||
| 1 | −1 | 0 | 0 | 131.6 | 131.56 | 0.238 | 0.2341 | 86.45 | 85.95 | 76.87 | 78.03 |
| 2 | 1 | 1 | −1 | 159.38 | 159.82 | 0.291 | 0.2892 | 85.72 | 86.09 | 79.69 | 80.25 |
| 3 | 0 | 0 | 1 | 137.12 | 137.04 | 0.266 | 0.2691 | 91.05 | 90.77 | 85.07 | 85.05 |
| 4 | 0 | 0 | 0 | 143.2 | 140.73 | 0.261 | 0.2620 | 90.05 | 90.43 | 84.21 | 83.91 |
| 5 | −1 | 1 | −1 | 115.6 | 114.40 | 0.225 | 0.2297 | 84.22 | 83.88 | 78.03 | 77.22 |
| 6 | 0 | 0 | 0 | 141.05 | 140.73 | 0.261 | 0.2620 | 90.32 | 90.43 | 84.08 | 83.91 |
| 7 | 0 | 0 | 0 | 139.04 | 140.73 | 0.262 | 0.2620 | 90.16 | 90.43 | 84.42 | 83.91 |
| 8 | 1 | 0 | 0 | 166.03 | 167.64 | 0.282 | 0.2831 | 91.53 | 91.71 | 85.66 | 85.23 |
| 9 | −1 | 1 | 1 | 121.7 | 122.98 | 0.224 | 0.2212 | 86.82 | 87.42 | 77.55 | 77.90 |
| 10 | −1 | −1 | 1 | 138.4 | 137.57 | 0.244 | 0.2466 | 84.72 | 84.43 | 78.81 | 78.07 |
| 11 | 0 | 0 | 2 | 142.15 | 140.73 | 0.261 | 0.2620 | 89.92 | 90.43 | 85.05 | 83.91 |
| 12 | 0 | 1 | 2 | 135.6 | 136.26 | 0.238 | 0.2369 | 88.63 | 88.45 | 81.01 | 81.13 |
| 13 | 1 | −1 | −1 | 169.02 | 167.34 | 0.317 | 0.3206 | 91.82 | 91.31 | 85.64 | 85.11 |
| 14 | 1 | 1 | 1 | 164.2 | 163.02 | 0.265 | 0.2662 | 85.83 | 85.38 | 79.73 | 79.51 |
| 15 | 0 | −1 | 0 | 146.4 | 147.32 | 0.267 | 0.2653 | 89.72 | 89.57 | 83.03 | 83.64 |
| 16 | 0 | 0 | 0 | 142.15 | 140.73 | 0.259 | 0.2620 | 91.05 | 90.43 | 83.02 | 83.91 |
| 17 | −1 | −1 | −1 | 134.44 | 135.22 | 0.268 | 0.2676 | 77.23 | 77.76 | 72.29 | 72.33 |
| 18 | 1 | −1 | 1 | 163.5 | 164.31 | 0.289 | 0.2851 | 93.32 | 93.74 | 88.81 | 89.44 |
| 19 | 0 | 0 | −1 | 132.6 | 134.26 | 0.297 | 0.2911 | 87.83 | 87.79 | 81.79 | 82.54 |
| 20 | 0 | 0 | 0 | 139.94 | 140.73 | 0.262 | 0.2620 | 90.45 | 90.43 | 84.15 | 83.91 |
| Model | Adjusted R2 | Predicted R2 | R2 | SD | CV% | Adeq. Precision |
|---|---|---|---|---|---|---|
| Response 1 | ||||||
| Linear | 0.8715 | 0.8069 | 0.8918 | 5.21 | ||
| 2FI | 0.8864 | 0.7053 | 0.9223 | 4.90 | ||
| Quadratic | 0.9859 | 0.9342 | 0.9926 | 1.73 | 1.21 | 43.6130 |
| Cubic | 0.9860 | −0.7498 | 0.9956 | 1.72 | ||
| Response 2 | ||||||
| Linear | 0.8524 | 0.8023 | 0.8757 | 0.0090 | ||
| 2FI | 0.8467 | 0.7585 | 0.8951 | 0.0092 | ||
| Quadratic | 0.9735 | 0.8204 | 0.9861 | 0.0038 | 1.45 | 36.6952 |
| Cubic | 0.9924 | −1.2337 | 0.9976 | 0.0020 | ||
| Response 3 | ||||||
| Linear | 0.3190 | −0.2172 | 0.4266 | 3.02 | ||
| 2FI | 0.6125 | −1.1119 | 0.7349 | 2.27 | ||
| Quadratic | 0.9783 | 0.8410 | 0.9886 | 0.5387 | 0.6098 | 41.9474 |
| Cubic | 0.9869 | −0.1771 | 0.9959 | 0.4184 | ||
| Response 4 | ||||||
| Linear | 0.4686 | 0.1005 | 0.5525 | 2.85 | ||
| 2FI | 0.6540 | −0.5362 | 0.7633 | 2.30 | ||
| Quadratic | 0.9520 | 0.7665 | 0.9747 | 0.8581 | 1.05 | 28.2023 |
| Cubic | 0.9633 | −4.1038 | 0.9884 | 0.7501 |
| Parameters (Unit) | Control | Blank NLCs | NRG NLCs | RLX NLCs | RLX/NRG NLCs |
|---|---|---|---|---|---|
| Hemoglobin (gm/dl) | 14.3 ± 1.02 | 15.8 ± 0.68 | 12.7 ± 0.5 | 13.9 ± 0.62 | 12.2 ± 0.8 |
| TLC (total leucocyte count) (th/cumm) | 8.5 ± 0.2 | 8.4 ± 0.3 | 7.8 ± 0.2 | 4.4 ± 1.2 | 7.6 ± 1.01 |
| Polymorphs (%) | 50 ± 3.2 | 60 ± 2.2 | 39 ± 1.5 | 40 ± 1.7 | 32 ± 2.4 |
| Lymphocytes (%) | 45 ± 4.3 | 35 ± 3.2 | 52 ± 1.8 | 52 ± 0.5 | 61 ± 1.2 |
| Eosinophil (%) | 02 ± 0.3 | 02 ± 0.1 | 06 ± 0.2 | 05 ± 0.1 | 03 ± 0.3 |
| Monocytes (%) | 03 ± 0.2 | 03 ± 0.2 | 03 ± 0.02 | 03 ± 0.01 | 04 ± 0.1 |
| RBC (millions/cumm) | 7.3 ± 0.87 | 6.58 ± 2.3 | 6.7 ± 1.8 | 7.9 ± 1.5 | 6.8 ± 0.6 |
| HCT (%) | 50.3 ± 5.3 | 39.5 ± 2.5 | 45.6 ± 1.7 | 47.8 ± 2.1 | 43.2 ± 1.1 |
| MCV (fl) | 68.6 ± 3.3 | 80.6 ± 3.1 | 68.1 ± 1.5 | 60.6 ± 1.1 | 63.4 ± 0.8 |
| MCH (pg) | 19.5 ± 1.2 | 26.5 ± 2.2 | 18.9 ± 1.2 | 17.6 ± 1.4 | 17.8 ± 0.56 |
| MCHC (g/dl) | 28.5 ± 2.1 | 30.5 ± 1.1 | 27.7 ± 2.01 | 29 ± 0.5 | 28.1 ± 1.05 |
| Platelet count (th/µL) | 874 ± 5.3 | 652 ± 4.3 | 745 ± 3.2 | 847 ± 5.3 | 840 ± 6.31 |
| MPV (fl) | 7.1 ± 0.4 | 8.5 ± 1.1 | 7.2 ± 0.5 | 7.6 ± 1.2 | 8.9 ± 0.5 |
| RDW-CV (%) | 15.9 ± 1.2 | 14.5 ± 1.3 | 14.8 ± 0.5 | 16 ± 1.02 | 13.8 ± 0.7 |
| RDW-SD (fl) | 55.8 ± 2.5 | 45.6 ± 1.2 | 47.8 ± 2.1 | 41.5 ± 1.3 | 47.8 ± 2.2 |
| PCT (%) | 0.7 ± 0.2 | 0.6 ± 0.3 | 0.7 ± 0.1 | 0.6 ± 0.2 | 0.8 ± 0.2 |
| PDW-SD (fl) | 15.4 ± 0.3 | 15.6 ± 0.5 | 15.4 ± 0.2 | 15.2 ± 0.1 | 15.9 ± 0.3 |
| Storage Condition | 25 ± 2 °C/60 ± 5% RH | 40 ± 2 °C/75 ± 5% RH | ||||
|---|---|---|---|---|---|---|
| Time (Weeks) | Physical Appearance and Separation | Average Particle Size (nm) | Mean EE% of RLX/NRG ± SD | Physical Appearance and Separation | Average Particle Size (nm) | Mean EE% of RLX/NRG ± SD |
| 0 | Pale yellow free-flowing powder/easily re-dispersible powder | 137.04 | 88.06 ± 1.63 | Pale yellow free-flowing powder/easily re-dispersible powder | 137.04 | 88.06 ± 1.63 |
| 3 | 155.03 | 85.32 ± 0.9 | 167.52 | 80.25 ± 0.74 | ||
| 6 | 165.90 | 82.23 ± 1.25 | 236.49 | 74.76 ± 1.82 | ||
| 9 | 171.22 | 80.05 ± 1.45 | 271.42 | 68.54 ± 1.92 | ||
| 12 | 195.05 | 76.32 ± 1.22 | 325.05 | 55.34 ± 1.47 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhalmi, A.; Amin, S.; Khan, Z.; Beg, S.; Al kamaly, O.; Saleh, A.; Kohli, K. Nanostructured Lipid Carrier-Based Codelivery of Raloxifene and Naringin: Formulation, Optimization, In Vitro, Ex Vivo, In Vivo Assessment, and Acute Toxicity Studies. Pharmaceutics 2022, 14, 1771. https://doi.org/10.3390/pharmaceutics14091771
Alhalmi A, Amin S, Khan Z, Beg S, Al kamaly O, Saleh A, Kohli K. Nanostructured Lipid Carrier-Based Codelivery of Raloxifene and Naringin: Formulation, Optimization, In Vitro, Ex Vivo, In Vivo Assessment, and Acute Toxicity Studies. Pharmaceutics. 2022; 14(9):1771. https://doi.org/10.3390/pharmaceutics14091771
Chicago/Turabian StyleAlhalmi, Abdulsalam, Saima Amin, Zafar Khan, Sarwar Beg, Omkulthom Al kamaly, Asmaa Saleh, and Kanchan Kohli. 2022. "Nanostructured Lipid Carrier-Based Codelivery of Raloxifene and Naringin: Formulation, Optimization, In Vitro, Ex Vivo, In Vivo Assessment, and Acute Toxicity Studies" Pharmaceutics 14, no. 9: 1771. https://doi.org/10.3390/pharmaceutics14091771
APA StyleAlhalmi, A., Amin, S., Khan, Z., Beg, S., Al kamaly, O., Saleh, A., & Kohli, K. (2022). Nanostructured Lipid Carrier-Based Codelivery of Raloxifene and Naringin: Formulation, Optimization, In Vitro, Ex Vivo, In Vivo Assessment, and Acute Toxicity Studies. Pharmaceutics, 14(9), 1771. https://doi.org/10.3390/pharmaceutics14091771