Can Chest Ultrasound Replace Chest X-ray in Thoracic Surgery?
Abstract
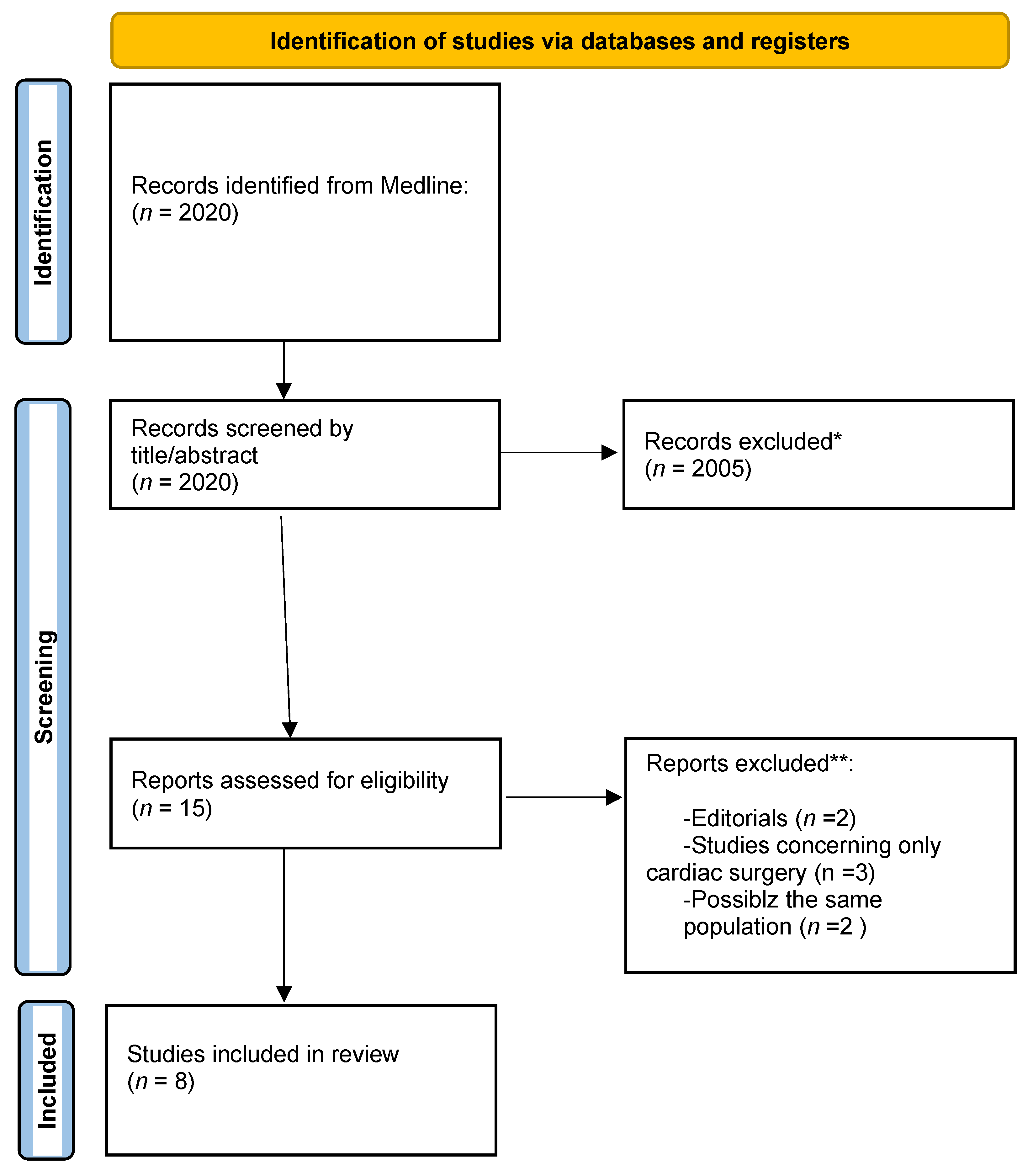
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- (I)
- the following article types: reviews, letters, laboratory research, and animal experiments;
- (II)
- if the language was not English;
- (III)
- studies including only patients after cardiac surgery;
- (IV)
- studies from the same institution retrospectively examining the same population.
2.3. Quality Assessment
3. Results
3.1. Characteristics and Qualities of the Included Studies
3.2. Postoperative Evaluation of PTX
3.3. Postoperative Evaluation of Pleural Effusion (PE)
3.4. Evaluation of Other Chest Pathologies
3.5. Detection of Pulmonary Oedema
3.6. Random Findings in Performing CUS
3.7. Overcoming Challenges in Performing CUS
3.8. Reduction in CXR by Performing CUS
4. Discussion
4.1. Limitations of This Meta-Analysis
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- MacDuff, A.; Arnold, A.; Harvey, J.; BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010, 65 (Suppl. 2), ii18–ii31. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.H.; Strange, C.; Heffner, J.E.; Light, R.; Kirby, T.J.; Klein, J.; Luketich, J.D.; Panacek, E.A.; Sahn, S.A.; Group, A.P.C. Management of spontaneous pneumothorax: An American College of Chest Physicians Delphi consensus statement. Chest 2001, 119, 590–602. [Google Scholar] [CrossRef]
- Schnell, J.; Beer, M.; Eggeling, S.; Gesierich, W.; Gottlieb, J.; Herth, F.J.F.; Hofmann, H.S.; Jany, B.; Kreuter, M.; Ley-Zaporozhan, J.; et al. Management of Spontaneous Pneumothorax and Post-Interventional Pneumothorax: German S3 Guideline. Respiration 2019, 97, 370–402. [Google Scholar] [CrossRef]
- Alrajab, S.; Youssef, A.M.; Akkus, N.I.; Caldito, G. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: Review of the literature and meta-analysis. Crit. Care 2013, 17, R208. [Google Scholar] [CrossRef]
- Wilkerson, R.G.; Stone, M.B. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad. Emerg. Med. 2010, 17, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Reeb, J.; Falcoz, P.E.; Olland, A.; Massard, G. Are daily routine chest radiographs necessary after pulmonary surgery in adult patients? Interact. Cardiovasc. Thorac. Surg. 2013, 17, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Goudie, E.; Bah, I.; Khereba, M.; Ferraro, P.; Duranceau, A.; Martin, J.; Thiffault, V.; Liberman, M. Prospective trial evaluating sonography after thoracic surgery in postoperative care and decision making. Eur. J. Cardiothorac. Surg. 2012, 41, 1025–1030. [Google Scholar] [CrossRef][Green Version]
- Chiappetta, M.; Meacci, E.; Cesario, A.; Smargiassi, A.; Inchingolo, R.; Petracca Ciavarella, L.; Lopatriello, S.; Contegiacomo, A.; Congedo, M.T.; Margaritora, S. Postoperative chest ultrasound findings and effectiveness after thoracic surgery: A pilot study. Ultrasound Med. Biol. 2018, 44, 1960–1967. [Google Scholar] [CrossRef]
- Touw, H.R.; Parlevliet, K.L.; Beerepoot, M.; Schober, P.; Vonk, A.; Twisk, J.W.; Elbers, P.W.; Boer, C.; Tuinman, P.R. Lung ultrasound compared with chest X-ray in diagnosing postoperative pulmonary complications following cardiothoracic surgery: A prospective observational study. Anaesthesia 2018, 73, 946–954. [Google Scholar] [CrossRef]
- Canty, D.; Ford, J.; Heiberg, J.; Brennan, A.; Royse, C.; El-Ansary, D.; Royse, A. Point-of-care diagnosis of perioperative lung pathology with lung ultrasound in cardiothoracic surgery—Comparison with clinical examination and chest X-ray. J. Cardiothorac. Vasc. Anesth. 2017, 31, S44–S45. [Google Scholar] [CrossRef]
- Vezzani, A.; Manca, T.; Brusasco, C.; Santori, G.; Valentino, M.; Nicolini, F.; Molardi, A.; Gherli, T.; Corradi, F. Diagnostic value of chest ultrasound after cardiac surgery: A comparison with chest X-ray and auscultation. J. Cardiothorac. Vasc. Anesth. 2014, 28, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Alsaddique, A.; Royse, A.G.; Royse, C.F.; Mobeirek, A.; El Shaer, F.; AlBackr, H.; Fouda, M.; Canty, D.J. Repeated Monitoring with Transthoracic Echocardiography and Lung Ultrasound After Cardiac Surgery: Feasibility and Impact on Diagnosis. J. Cardiothorac. Vasc. Anesth. 2016, 30, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Patella, M.; Saporito, A.; Puligheddu, C.; Mongelli, F.; La Regina, D.; Pini, R.; Inderbitzi, R.; Cafarotti, S. Lung Ultrasound to Detect Residual Pneumothorax after Chest Drain Removal in Lung Resections. Ann. Thorac. Surg. 2018, 105, 1537–1542. [Google Scholar] [CrossRef]
- Galetin, T.; Defosse, J.; Schieren, M.; Marks, B.; Lopez-Pastorini, A.; Koryllos, A.; Kosse, N.; Wappler, F.; Stoelben, E. Sensitivity of chest ultrasound for postoperative pneumothorax in comparison to chest X-ray after lung resecting surgery. Eur. J. Cardiothorac. Surg. 2020, 57, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Galetin, T.; Schieren, M.; Marks, B.; Defosse, J.; Stoelben, E. Sensitivity of lung ultrasound for the detection of pneumothorax one day after pulmonary resection—a prospective observational study. Eur. Surg. 2021, 53, 23–28. [Google Scholar] [CrossRef]
- Dzian, A.; Malik, M.; Hamada, L.; Skalicanova, M.; Zelenak, K.; Stevik, M.; Grendar, M. Lung ultrasound could reduce X-ray after major lung resection. Bratisl. Lek. Listy 2021, 122, 871–875. [Google Scholar] [CrossRef]
- Malik, M.; Dzian, A.; Skalicanova, M.; Hamada, L.; Zelenak, K.; Grendar, M. Chest Ultrasound Can Reduce the Use of Roentgenograms in Postoperative Care After Thoracic Surgery. Ann. Thorac. Surg. 2021, 112, 897–904. [Google Scholar] [CrossRef]
- Winkler, M.H.; Touw, H.R.; van de Ven, P.M.; Twisk, J.; Tuinman, P.R. Diagnostic Accuracy of Chest Radiograph, and When Concomitantly Studied Lung Ultrasound, in Critically Ill Patients with Respiratory Symptoms: A Systematic Review and Meta-Analysis. Crit. Care Med. 2018, 46, e707–e714. [Google Scholar] [CrossRef]
- Bhakhri, K.; Coonar, A. Editorial of current practise in the use of chest ultrasound in thoracic surgery. J. Thorac. Dis. 2019, 11, 5706–5707. [Google Scholar] [CrossRef]
- Nooitgedacht, J.; Haaksma, M.; Touw, H.R.W.; Tuinman, P.R. Perioperative care with an ultrasound device is as Michael Jordan with Scotty Pippen: At its best! J. Thorac. Dis. 2018, 10, 6436–6441. [Google Scholar] [CrossRef]
- Blaivas, M.; Lyon, M.; Duggal, S. A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad. Emerg. Med. 2005, 12, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Testa, A.; Sher, S.; Pignataro, G.; La Sala, M.; Silveri, N.G. Occult traumatic pneumothorax: Diagnostic accuracy of lung ultrasonography in the emergency department. Chest 2008, 133, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Knudtson, J.L.; Dort, J.M.; Helmer, S.D.; Smith, R.S. Surgeon-performed ultrasound for pneumothorax in the trauma suite. J. Trauma 2004, 56, 527–530. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Meziere, G.A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: The BLUE protocol. Chest 2008, 134, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.A. BLUE-protocol and FALLS-protocol: Two applications of lung ultrasound in the critically ill. Chest 2015, 147, 1659–1670. [Google Scholar] [CrossRef]
- Galetin, T.; Merres, J.; Schieren, M.; Marks, B.; Haffke, Y.; Defosse, J.; Wappler, F.; Koryllos, A.; Stoelben, E. Most patient conditions do not a priori debilitate the sensitivity of thoracic ultrasound in thoracic surgery-a prospective comparative study. J. Cardiothorac. Surg. 2021, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Maskell, N.A.; Butland, R.J.; Group Pleural Diseases; Comittee Standards of Care; Society British Thoracic. BTS guidelines for the investigation of a unilateral pleural effusion in adults. Thorax 2003, 58 (Suppl. 2), ii8–ii17. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, K.M.; Bahner, D.P. Teaching bedside sonography using peer mentoring: A prospective randomized trial. J. Ultrasound Med. 2012, 31, 455–459. [Google Scholar] [CrossRef]
- House, J.B.; Choe, C.H.; Wourman, H.L.; Berg, K.M.; Fischer, J.P.; Santen, S.A. Efficient and Effective Use of Peer Teaching for Medical Student Simulation. West. J. Emerg. Med. 2017, 18, 137–141. [Google Scholar] [CrossRef]
| Study | Patient Source | Total Number of Patients | Number of Lobectomies | Identified PTX with CXR vs. CUS | Identified PE with CXR vs. CUS | Key Results | NOS-Score |
|---|---|---|---|---|---|---|---|
| Goudie, 2011 | Canada | 120 | 36 | 157 vs. 29 | 148 vs. 118 | -PE sensitivity: 83%, specificity: 59% -PTX: sensitivity: 21%, specificity: 95% -adequate method to evaluate PE, uncertain for PTX -postop. CUS may reduce CRX if previously PTX is ruled out -CUS has not have high enough accuracy to replace CXRs. -Limitation: -CUS only in sitting position -lung point not always searched | 7 |
| Patella, 2017 | Switzerland | 50 | 33 | 15 vs. 24 | -CUS for PTX: -71% positive predictive value -100% negative predictive value -86% CRX saved | 7 | |
| Chiapetta, 2018 | Italy | 24 | 6 | 0 vs. 11 | 0 vs. 5 | -CUS exhaustive in -67% cases of open surgery -85% cases of VATS -CXR needed only in 20.8% due to massive subcutaneous emphysema | 8 |
| Malik, 2020 | Slovakia | 297 | 45 | 69 vs. 51 | 169 vs. 117 | -CUS sensitivity and specificiity for -for PTX up to 59.4% and 94.8% -for PE up to 60.9% and 91.3% -61.6% CXR saved -Non-physiologic finding -> other imaging modality | 7 |
| Dzian, 2021 | Slovakia | 48 | -CUS sensitivity for -PTX up to 58.5% -PE up to 86.2% -2 PTX missed from CRX, all other mismatch clinical irrelevant -CUS could reduce CRX -BLUE protocol | 6 | |||
| Galetin, 2019 | Germany | 123 | 44 | 44 vs. 26 | -CUS sensitivity and specificity for large PTX 100% and 82% -No clinically relevant PTX missed. -Agreement between CUS and routine-based therapeutic decisions ≧ 97% | 8 | |
| Galetin, 2021 | Germany | 68 | 31 | 23 vs. 18 | -CUS sensitivity and specificity for PTX 81% and 81–100% | 8 | |
| Touw, 2019 | Netherlands | 177 | 0 | 7 vs. 2 | 51 vs. 60 | -CUS detected more clinically-relevant postoperative pulmonary complications and earlier than CXR -BLUE protocol | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grapatsas, K.; Leivaditis, V.; Ehle, B.; Papaporfyriou, A. Can Chest Ultrasound Replace Chest X-ray in Thoracic Surgery? Tomography 2022, 8, 2083-2092. https://doi.org/10.3390/tomography8040175
Grapatsas K, Leivaditis V, Ehle B, Papaporfyriou A. Can Chest Ultrasound Replace Chest X-ray in Thoracic Surgery? Tomography. 2022; 8(4):2083-2092. https://doi.org/10.3390/tomography8040175
Chicago/Turabian StyleGrapatsas, Konstantinos, Vasileios Leivaditis, Benjamin Ehle, and Anastasia Papaporfyriou. 2022. "Can Chest Ultrasound Replace Chest X-ray in Thoracic Surgery?" Tomography 8, no. 4: 2083-2092. https://doi.org/10.3390/tomography8040175
APA StyleGrapatsas, K., Leivaditis, V., Ehle, B., & Papaporfyriou, A. (2022). Can Chest Ultrasound Replace Chest X-ray in Thoracic Surgery? Tomography, 8(4), 2083-2092. https://doi.org/10.3390/tomography8040175







