Abstract
Iron limitation in vast water bodies has been linked to decreased algal productivity, despite different iron-acquiring mechanisms, and the presence of ferritin in many algal species that act as an iron internal reservoir. Therefore, iron fertilization has been proposed to increase algal biomass and photosynthesis. This, in turn, will reduce carbon dioxide in the atmosphere and increase oxygen, thereby decreasing global warming, and achieving ecological balance. In addition, algal proliferation will hopefully lead to enhancement in biodiversity, Biological pump, fish productivity and, subsequently marine food industry. Many climate geoengineering experiments in the form of ocean iron fertilization have been conducted globally in order to achieve such a purpose. However, reservations remain as the outcomes are not as promising as were previously expected. As the temporal and spatial scales of iron fertilization experiments are limited, the effects on fish productivity remain speculative. On the other hand, side effects were also recorded. The main purpose of iron fertilization, for carbon dioxide sequestration and global warming mitigation, still remains to be fully realized and verified. Several improvements and future modifications are suggested, and legal issues are discussed in this review.
1. Introduction
Iron is one of the four most ubiquitous elements, as it constitutes about 35% of the mass of the globe. Iron is also an essential element in the body of living organisms, especially in auto-phototrophic organisms such as plants and algae. It is an important cofactor of many enzymes, such as cytochrome oxidase, catalase, peroxidase and nitrogenase [1,2,3]. Those enzymes are involved in three high iron-demanding biochemical processes namely: respiration, photosynthesis and nitrogen fixation [4,5]. It was reported by [2] that 38 iron atoms are required per nitrogenase complex. whereas [1] showed that the electron transport system associated with photosystems requires nearly 22 atoms of iron.
Although iron is abundant, its bioavailability is limited due to its oxidation to insoluble ferric form. Ferrous ions are the most bioavailable iron in biological systems as they are soluble. Unfortunately, they are unstable and can be quickly oxidized into insoluble ferric ions. The most bioavailable forms of iron are found in picomolar/nanomolar concentrations of unchelated iron on surface waters [6,7]. The oxidation rate of ferrous ions is generally dependent on environmental factors such as temperature, oxygen, light and pH. For example, in cold water with a low oxygen level, ferrous ions can be stable and do not undergo oxidation for some time [8]. On the other hand, high temperature causes the rapid oxidation of ferrous ions [9]. Indeed, in warm and well-oxygenated oceans, ferrous ion is quickly oxidized unless the majority of iron is bound to organic ligands [10]. Microorganisms have their own strategies for the use of such bound iron [11]. Other factors can affect iron bioavailability. For example, decreasing pH decreases the bioavailability of iron [12]. Light can also affect iron bioavailability as ferric ions undergo photoreduction [13]. For phytoplankton growth, iron represents an essential element, but is only needed in a small quantity as a micronutrient. Nonetheless, iron is suggested to be the limiting factor for algal growth in the ocean [14]. According to [14], the main sources of iron in the ocean includes upwelling of deep waters, terrestrial export and aeolian dust. Iron can be divided according to size into: particles (>0.45 µm) and dissolved (<0.45µm) and the latter can be further divided into colloidal (0.45–0.02 µm) and soluble iron (<0.02 µm) [15]. Some may be organically linked to ligands [16]. Dissolved, inorganic forms of iron are taken up by marine phytoplankton. Measured, dissolved iron concentrations in some parts of oceans were at ~0.05 nM [17]. Phytoplankton represents the dietary meal for many aquatic animals and can be limiting for their productivity [18]. Phytoplanktons need iron for growth and aquatic animals feed on phytolplankton. Therefore, it can be logically deduced that an increase in iron will increase the productivity of both phytoplankton and aquatic animals. Interestingly, biological recycling of iron could enhance primary productivity of phytoplanktons in iron-limited waters, according to [19]. They reported that large marine animals contribute to the recycling of bioavailable iron by consuming iron-rich prey, for example, antarctic krill, and through defecation they perform natural iron fertilization/enrichment. They suggested that blue whales and, to some extent, fin and humpback whales, contribute to iron recycling, resulting in enhanced primary productivity in iron-limited southern ocean regions. According to [20], low iron availability limits algal growth in as much as 40% of the ocean; these areas are designated as high-nutrient, low-carbon (HNLC). Iron fertilization experiments of HNLC waters aims to induce rapidly growing algal blooms, which capture and sequester carbon from the atmosphere during photosynthesis, thereby lowering global warming. Here, we shed some light on iron requirement by algae and their strategies to deal with iron limitation, while addressing the effect of iron fertilization experiments on both algae and fish productivity.
1.1. Algal-Iron Relationship
According to [16], the calculated algal minimum iron requirements are roughly 3 umol/mol, and for a dividing alga once Fe/C~1–2 umol/mol. Fe:C ratio is estimated to be ~1.5 umol/mol, which is considered the least algal iron requirement; this requirement increases if grown on nitrate instead of ammonium. Nitrogen fixation in cyanobacteria requires even more iron [16]. Therefore, to cope with very low iron concentration in the upper surface water, which is usually in the range of 0.5–1 nM [21], algae either enhance their iron uptake [22] or decrease their iron requirement [16]. Indeed, iron-containing molecules can be replaced in some cases. For example, the cytochrome c-552 and plastocyanin replace each other in some organisms [23]. Similarly, flavodoxin replaces ferredoxin in some cases of cyanobacteria and green algae [24]. Morrissey and Bowler [20] reviewed different algal responses to iron limitation. A modification in the machinery of photosynthesis is a primary response to that limitation [25]. Iron-rich photosystem I, which contains 12 iron atoms, decreases in favor of photosystem II, which has three iron atoms. In addition, the number of phycobilisomes, which are synthesized by iron-containing proteins, decreases. Nickel superoxide dismutase is utilized in place of iron to remove reactive oxygen species [26,27]. Moreover, some genes are associated with iron limitation. For example, the gene isiA is present in several marine Synechococcus species under iron limitation [28]. It is thought to be related to the molecular adaptation to low iron concentrations as it becomes upregulated, leading to the formation of giant PSI-IsiA-chlorophyll–protein–antenna super complexes [28,29]. Iron-starved marine Synechococcus accumulates glycogen granules and decreases chlorophyll, as well as protein content of accessory pigments. Low-iron adapted diatoms, on the other hand, have iron starvation induced proteins (ISIP), a group of proteins that are upregulated under iron limitation [30]. ISIP1 in the diatom Phaeodactylum. tricornutum is associated with the transport of siderophores by endocytosis, leading iron-siderophore complex to the chloroplast, thereby supplying iron for photosynthesis [31]. In another diatom, Thalassiosira pseudonana, iron limitation results in reduced growth [32] and decreased photosynthetic activity [33], as well as increased cell aggregation and silica deposition on the cell wall [34]. Prolonged iron starvation will cause increased oxidative stress and caspase activity, ultimately resulting in programmed cell death [33,35].
With regard to mechanisms of iron uptake, a review by Behnke and LaRoche [30] showed that algae have different systems for iron uptake including: phytotransferrins [36,37] and siderophores [31], as well as reductive high-affinity iron uptake [38,39,40] and low-affinity iron transporters [41]. Previously, Shaked and Lis [42] reported the existence of two major uptake routes for organically bound iron (FeL): siderophore-mediated iron uptake and the reductive iron uptake pathway. They emphasized that the latter pathway is the prevalent pathway among algae. Reduction operates on both free inorganic iron and organically bound iron, where, in the case of the latter, the dissociation of iron from its ligand is followed by transport into the cell [43]. Another source of iron, but rather overlooked, is aeolian dust [44]. The Atlantic Ocean receives from the Sahara Desert around 0.027 g of aeolian dust/m2/day [45]. The total iron concentration is approximately 3.5% of Saharan dust [46]. Nonetheless, hematite and goethite in the aeolian dust associate with fine (0.3–1 μm) particles, with long travel paths [47].
1.2. Role of Ferritin as Storage Form of Iron in Marine Algae (Iron; Homeostasis)
Marine algae employ different strategies to cope with low iron concentrations. The iron storage protein, ferritin, is strongly regulated by the day/night cycle in the coastal California upwelling region [48]. According to Twining and Baines [49], the cells regulate cellular iron concentrations mostly through downregulation of cross-membrane transporters. However, in the case of an increase in the internal metal concentration well above those needed to sustain maximum growth, storage of iron in the form of ferritin occurs. Ferritin is a protein specialized in iron storage that is widely present in algae, plants, animals and bacteria. It acts as an iron-concentrating protein that supplies iron to the organism under conditions of limited iron supply. It also regulates iron levels [48,49]. According to Marchetti et al. [50] bloom-forming pennate diatoms Pseudo-nitzschia and Fragilariopsis use ferritin to store iron. Interestingly, [30] reported that ferritin is found in all chlorophytes they studied, while it was absent in the haptophytes Emiliana huxelyi and Pavlovales sp. Glutamate residue in ferritin of Pseudo-nitzschia multiseries is an essential factor for the oxidation of iron, thereby changing the function from storage to oxidation [51]. The structural and functional analysis of ferritin revealed that this iron-concentrating protein, from Pseudo-nitzschia multiseries, had a ferroxidase binding-iron activity. It is to be targeted to the chloroplast in order to control the distribution and storage of iron for proper functioning of photosynthesis. Therefore, ferritin is thought to help pennate diatoms survive in low-iron regions.
Ferritin-dependent day/night recycling of iron was investigated, where iron loading was maximum at night [48]. They suggested that ferritin supplies iron for photosystem I and other day iron-binding proteins. At dawn, iron is released from ferritin and becomes available to the iron-containing proteins during the day. The main ferritin function is supposedly the temporal storage of iron in day/night cycle, which is important for repairing and recycling damaged/oxidized iron binding proteins. Ferritin-dependent recycling of intracellular iron also occurs and is related to cell survival to prevent mortality, since high levels of iron are toxic. In the case of cells lacking ferritin, to maintain iron homeostasis, iron uptake is reduced and iron efflux is activated during the day/night cycle [51].
2. Martin’s Hypothesis
Martin’s hypothesis postulates that adding iron to high nitrogen and phosphorus nutrient-rich oceanic regions, either by dust in nature and/or by artificial fertilization, would stimulate phytoplankton blooms, leading to the drawdown of significant amounts of carbon dioxide. Therefore, by adding iron to iron-deficient oceanic regions, phytoplankton blooms will be established, followed by sequestration of carbon dioxide and mass sinking of organic matter [14].
2.1. Iron Fertilization
Based on the amounts of nutrients and chlorophyll, oceans can be divided into regions.
HNLC (high-nutrient, low-chlorophyll), HNHC (high-nutrient, high-chlorophyll), LNLC (low-nutrient, low-chlorophyll), and LNHC (low-nutrient, high-chlorophyll). According to Smetacek and Naqvi [52], some regions of the oceans, namely in the North and Equatorial Pacific and the Antarctic, contain high amounts of nutrients in the upper water (e.g., PO4 > 1 uM), but low biomass (chlorophyll < 0.5 ug/L) and low iron concentrations. Therefore, the subarctic North Pacific, the Equatorial Pacific and the Southern Ocean are regions which are referred to as high nutrient-low chlorophyll (HNLC) regions, as well as regions in Pacific Ocean, the Sub-Pacific Ocean, the Equatorial Ocean, and the Southern Ocean. Phytoplankton do not fully benefit from the nutrients present in these areas, particularly nitrate, due to the deficiency in iron. Most of the iron is bound to organic ligands, making it somewhat less bioavailable [42]. For these reasons, low levels of primary production are observed in areas where iron is low, despite the presence of high phosphate and nitrate levels. Moreover, those HNLC regions are mostly characterized by low dissolved oxygen and high SO2 dispersion. Previously, ref. [42] reviewed the effect of limited iron availability and showed it hinders photosynthesis and growth, thereby limiting primary production, as well as decreasing minerals (CaCO3 and opal) and oceanic CO2 drawdown [53]. There are other reasons for iron being less available for algae. For example, when iron reacts with sulfur under excessive heating at temperatures greater than 600 °C [54], this results in the formation of insoluble black ferrous sulfide, which further reacts with acidic hydrogen sulfide forming hydrogen and pyrite [55]. This condition is typical of volcanic regions of the ocean and it is considered as a reason for failure in achieving the targets of iron fertilization [55].
Biological Pump as a Result of Iron Fertilization
According to De La Rocha and Passow [56], the biological pump is a term that refers to the export of carbon from the upper water as organic matter formed during photosynthesis by algae, which is quickly transported deep down as zooplanktonic grazers feed on those algae and sink [57,58]. By this process, carbon dioxide is sequestered from the atmosphere into organic matter during photosynthesis and transported into the deep ocean by the sinking of organic matter, the migration of zooplankton, or by the downwelling of surface waters rich in dissolved organic matter (Figure 1). Nutrients also come from external sources such as rivers and dust. Physical conditions, phytoplankton types, grazers and biominerals all play important roles in the biological pump [56]. Nutrients needed for phytoplankton growth, such as macronutrients like nitrate, phosphate and silicate, come from different sources, including upwelling from deep ocean into mixed waters, as well as nutrient recycling of nutrients released from decomposing dead marine organisms [59].
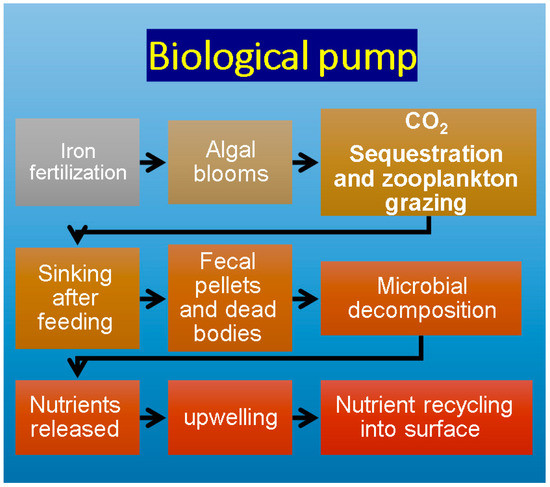
Figure 1.
Schematic sketch of simple biological pump.
2.2. How Is Artificial Iron Fertilization Carried Out
According to [57], artificial fertilization experiments of iron use iron sulphate, which is a relatively soluble agricultural fertilizer, that is dissolved in acidified seawater and pumped into the ocean. However, in warm waters, iron is rapidly transformed into insoluble form. Nevertheless, agents may be added to keep iron soluble for longer. Also, artificial upwelling can be considered where deeper waters are brought near to the surface by pipes that are ~10 m in diameter, with lengths of 100–300 m. According to a UN report, iron fertilization of the ocean is intended to benefit scientific research as it offers a better understanding of nutrient limitation in marine ecosystem. Indeed, those experiments indicated that iron controls primary production in high nutrient—low chlorophyll regions of the ocean. The second intended benefit is carbon sequestration by enhancing the uptake of atmospheric CO2, therefore reducing global warming. However, this remains to be realized as experiments should be repeated several times, and their spatial range should be expanded to the range to thousands of square kilometers, to achieve their targets. The third intended benefit is fishery enhancement, where an increase in primary productivity would supposedly lead to the growth enhancement of fish [57].
2.3. Iron Fertilization Experiments Outcomes
The efficiency of fertilization expressed as the ratio of the carbon export to the iron supplied, was investigated in the Southern Ocean by Blain et al. [60]. They found that this ratio was ten times higher than previous estimates, which suggests that changes in iron supply may have a significant effect on atmospheric carbon dioxide concentrations. According to Savoye et al., [61], the KEOPS cruise examined the ratio of export to primary production and showed that this ratio was lower in iron-supplied, than iron-depleted, conditions. Similar results were obtained for the SOFeX cruise. This is most likely due to nature in the microbial web in both areas, as mesozooplankton grazing was low. At the Fe-supplied region (plateau) there is a low consumption of bacteria by heterotrophic nanoflagellates, whereas at the HNLC site, sequestered carbon was transferred to higher animals, thereby being available for export through mesozooplankton fecal pellets. The HNLC area has greater export efficiency for the KEOPS HNLC area (58%), when compared to the plateau Fe-supplied area (28%), with regard to the fate of the fertilized iron in relation to carbon dioxide sequestered. In the early 1990s, experiments in HNLC regions induced algal growth dominated by diatoms [53]. In some of the experiments, overall increase in photosynthetic chlorophyll content and photosynthetic activity were most noticeable in warm, shallow, well-mixed water. Shift of the plankton composition, from small cyanobacteria, to medium haptophytes to large diatoms, was noticed. Indeed, diatoms dominated phytoplanktons community in all but one experiment (SEEDS II) after iron addition, but dominant species varied among locations [57].
2.4. Experiment near the Gulf Area
During the southwest monsoon [62], Fe-rich dust from the Arabian and Syrian deserts were obstructed by high Omani Mountains. Therefore, dust could not reach the southern Omani coast, where many volcanoes are active within the perimeter in Yemen, Saudi Arabia and Iran. According to Kim et al. [55], oxygen poor regions form due to the oxidation of volcanic sulfur compounds to sulfates, thereby consuming dissolved oxygen in waters. Moffit et al. [63] studied Arabian Sea iron biogeochemistry. They showed that this sea is a productive water body with seasonal upwelling and mixing, high content of surface nutrients and widespread algal blooms, as well as being an intense oxygen minimum zone, which is a major sink for nitrate in the ocean. As a result, iron is highly enriched in the Eastern Arabian Sea, mostly in the form of ferrous ions, and this is largely associated with the oxygen minimum zone. In contrast, iron concentration is much lower in the Western Arabian Sea and largely separated from the Fe-rich oxygen minimum zone by large water boundary. Primary production during the southwest monsoon was found to be strongly limited by iron.
2.5. Why Are Diatoms the Most Dominant Algal Group to Iron Fertilization Experiments?
According to Smetacek and Naqvi [52], diatoms dominated phytoplankton communities during most of the iron fertilization experiments. This may be attributed to different factors including: their lower mortality rates because of their large surface; and volume ratio, thereby outcompeting other planktons in resources exploitation. They also accumulate biomass to better protect themselves against pathogens and grazers. A broad range of diatom species has the ability to take up iron in different experiments. With regard to the impact of grazing, it has yet to be firmly established whether krill grazing enhances carbon export or not.
2.6. Reservations on Iron Fertilization Experiments
Many reservations were put forward about the iron fertilization experiments. Those reservations include: the fate of algal biomass; the ratio of iron added to carbon sequestered; and the negative impacts of fertilization. Also, these experiments are unpopular as it is considered to be meddling with nature, with unpredictable outcomes [53,57]. Some of the reservations include [57]:
- (1)
- Nutrient depletion and co-limitation of both iron and silicate [57]. Colimitation, which means that limiting concentrations of one metal may also affect the requirement for another metal and this can subsequently lead to decreased phytoplankton growth. Silica will become depleted, as well as nitrate, after addition of iron as diatoms growth increases accompanied by silica consumptio. Moreover, co-limitation increases oxidative e stress in diatoms, and as a consequence more Mn is required for superoxide dismutase. On the other hand, low-Fe increases the Cu requirements as it replaces iron in some proteins [64];
- (2)
- The iron dispersed may become, in part, adsorbed onto sinking particles without benefiting the phytoplankton. Therefore, some iron can partly be used by phytoplanktons, but the rest is buried;
- (3)
- The experiments are of short duration, limited range, and the amount of nutrient added may not be good enough for CO2 export;
- (4)
- Any fertilization-enhanced biomass will decrease oxygen levels in the sub-surface ocean. Also, this would affect the release of CO2 to subsurface seawater during decomposition of planktons and reduce pH (acidification) and carbonate ion concentration [57];
- (5)
- Ocean acidification, where global warming with increased carbon dioxide leads to higher concentrations of dissolved CO2 in surface marine waters as grazers feed on blooms of phytoplanktons and respire, resulting in ocean acidification [12]. An increase in ocean acidification in deep water may result from large-scale fertilization as this will lead to an increase in CO2 sequestration at depth. Consequently, this will change the depth at which carbonate biominerals, thereby limiting their supply to deep-ocean organisms that build shells and structures, like sea coral. One important aspect is greenhouse gas emissions. The ocean is an important source of N2O, but the evolution of this gas can increase due to iron fertilization. If fertilization takes place in warm waters low in oxygen, N2O yield will be large. Decomposition of sinking biomass can produce persistent greenhouse gases, nitrous oxide and methane, with much higher global warming potentials than CO2 [57];
- (6)
- Induction of toxic algal blooms such as that of the diatom Pseudo-nizchia or dinoflagellates:
- (a)
- Pseudo-nitzschia spp. produces the neurotoxin domoic acid, which binds iron with a low affinity, but sufficient enough to facilitate iron uptake [65]. The ability to monopolize iron availability via a species specific phytosiderophore could thus explain the dominance of Pseudo-nitzschia in blooms. The fact that domoic acid is neurotoxin adds to the side effects of iron fertilization in promoting toxic algal blooms. Trick et al. [66] demonstrated that the sparse oceanic Pseudo-nitzschia community at the high-nitrate, low-chlorophyll ocean station PAPA (50° N, 145° W), produced approximately 200 pg domoic acid per litre. They reported that in response to iron addition, domoic acid changes phytoplankton community structure in favor of Pseudo-nitzschia, and that oceanic Pseudo-nitzschia are toxic. This further makes large-scale iron fertilizations questionable with regard to benefits and sustainability.
- (b)
- Dinoflagellates overgrowth as a result of iron fertilization. Indeed, community composition of microzooplankton (dinoflagellates and ciliates) of the naturally iron-fertilized Kerguelen area (Southern Ocean) region was characterized. This region has a complex mesoscale circulation resulting in a patchy distribution of phytoplankton blooms. Ninety-seven morphospecies of dinoflagellates and 41 ciliates were identified, in addition to 202 Alveolata-related operational taxonomical units. Diatom-consuming dinoflagellates were the most enhanced taxa in the blooms. A clear difference in the phytoplankton and microzooplankton community structures between the iron-fertilized and HNLC regions was observed. Dinoflagellates and ciliates role as phytoplankton consumers and as prey for mesozooplankton was evaluated. Dinoflagellates were most likely the major phytoplankton grazers, and a potential food source for copepods. Some of the dinoflagellates that were found were Gyrodinium spp., Gymnodinium spp., Amphidinium spp. [67].
With regard to the artificial iron fertilization experiment, EIFEX was conducted within a stationary eddy adjacent to the Antarctic Polar Front in 2004, and induced a massive bloom. Acantharia, dinoflagellates and ciliates together contributed >90% of protozooplankton biomass in the upper water. Total protozooplankton biomass increased slightly. Smaller, less-defended taxas, such as athecate dinoflagellates, declined, whereas large, spiny and armored taxa, particularly thecate dinoflagellates, increased. This is attributed to the selective heavy grazing pressure by copepods [68].
2.7. Improvement to Be Made to the Experiments
According to Yoon et al. [58], in order to maximize the effectiveness of iron fertilization experiments, several factors must be taken into considerations, including:
- (1)
- Fertilization must take place in the center of an eddy where grazing is low and silicates are high;
- (2)
- Duration of the experiment would be favorable if it was over a minimum of ~40 days, with repeated iron discharges of ~2000 kg each within ~10–15 days in region 300 km2 and a ~2 nM concentration;
- (3)
- Tracking of iron fertilized both physically and biogeochemically;
- (4)
- Using neutral sediment traps;
- (5)
- Monitoring of hazardous gases (e.g., N2O, DMS; and halogenated volatile organic compounds
Another reason iron fertilization is not paying off is due to the formation of iron sulfides, as a result of reaction with volcanic sulfur and ash undersea. All of the previous iron fertilization experiments were conducted at or near volcanic sulfur-compounds-enriched HNLC regions [55]. The UN report [57] showed that when iron fertilization was performed in high nutrient regions over a limited area range (40–300 square kilometers), complete achievement s for geoengineering or fishery enhancement objectives, is not reached and experiments need to involve areas of around 10,000 square kilometers.
3. Fish Productivity
The most notable experiment for enhancing fish productivity through iron fertilization was one carried out by the Haida Salmon Restoration Corporation (HSRC) in 2012, for enhancement of the salmon fish population, which is one of the most important marine food industry products. In this experiment, ocean iron fertilization was conducted for the purpose of salmon population restoration to benefit the village of Old Massett, on the islands of Haida Gwaii, British Columbia [69]. According to [69], fertilization with iron sulphate and oxide occurred in summer 2012, which induced phytoplankton bloom detected by satellite images. Comparisons with samples from the same region in the years 2000–2011 showed that phytoplankton and microzooplankton abundance indices were the lowest ever recorded in autumn of 2012, while crustacean were higher than previously recorded in the autumn. This suggests that the iron-induced bloom may have caused an increase in zooplankton that grazed heavily on the phytoplankton and microzooplankton in autumn 2012. The results are controversial with regard to salmon productivity.
With regard to artificial iron fertilization, Galbraith et al. [70] suggested that in iron-poor oceanic regions, low iron content is insufficient to support many fish species throughout their life cycles. Therefore, relatively limited fishing occurs in high nitrate low chlorophyll (HNLC) regions. This emphasizes the importance of iron fertilization.
4. Legal Aspects
According to [71] (https://www.whoi.edu/oceanus/feature/dumping-iron-and-trading-carbon/, accessed 16 May 2022), iron fertilization will most likely take place outside the borders of any country’s 200-mile exclusive economic zone; therefore, international law will be concerned with any issues regarding future conflicts. The 1972 London Convention regulates effective control of marine pollution and the disposal of waste at sea for its 82 participants. Nonetheless, this was updated by the 1996 London Protocol, where all dumping is prohibited, except for a few specified wastes. Iron fertilization is not strictly dumping because disposal of the iron is not the project’s expressed purpose. However, fertilization may qualify as dumping if the treaty nations decide that such projects contravene the treaties’ aims.
In the 2008 London Convention and London Protocol meeting, a resolution prohibiting ocean fertilization until specific guidance is developed was proposed, but with an exception for small-scale scientific research. [72]. An assessment framework was developed in 2010 for scientific research, on a case-by-case basis, and demands preliminary investigations before any OIF [73]. In 2013, the LC/LP introduced an amendment that regards ocean iron fertilization as marine geoengineering, but allowing it for scientific research within the boundaries set within the assessment framework [74]. In any case, international treaties rely on the individual nations that sign the treaties to implement them, but not obliging nations that did not sign the treaty.
In the United States, the London Convention is implemented by the Ocean Dumping Act, which governs ocean dumping of material transported from the United States; transported inside U.S. waters from outside the U.S.; and by U.S. agencies and U.S.-flagged vessels or aircraft. Permits can be issued for dumping only after careful evaluation of the need for the dumping, potential dumping sites, and the potential environmental impacts. Exemptions are found in The Ocean Dumping Act for placement of certain materials in the ocean for a purpose other than disposal (https://www.whoi.edu/oceanus/feature/dumping-iron-and-trading-carbon/, accessed 16 May 2022) [72].
5. Future Improvements
Interestingly, [44] proposed the use of crystalline Fe-oxides, produced by chemosynthetic iron-oxidizing bacteria, as an iron source for iron fertilization of the ocean; fine powder is produced from dried oxides, which could be dispersed. Iron replete conditions will stimulate the biological pump to partially mitigate global warming. The most recent proposal is ocean fertilization by pyrogenic aerosol iron [75], which reviewed the latest laboratory experiments and indicated that iron solubility of pyrogenic aerosol is higher than lithogenic aerosol, and represents a readily soluble supply of iron that is more effective in enhancing marine productivity than lithogenic sources. Fe-containing particles are emitted by pyrogenic sources [76], which include burning biomass, coal combustion, ships’ emissions and the metal smelting industry. The solubility of iron in aerosols is enhanced by photochemical processing during atmospheric travel. Overall, the iron fertilization hypothesis is still under examination, with different proposals being put forward regarding the origin and form of iron used in fertilization, as well as different supply methods. The hypothesis remains to be tested and verified in both the short and long term.
Funding
This research was supported by the Annual grant number 33, funded by Deanship of Scientific research, Vice Presidency of Graduate studies and Scientific Researc, King Faisal University, AlHufuf, Al-Ahsa, Kingdom of Saudi Arabia, Post code: 31982. PO box: 400.
Acknowledgments
The author expresses her deepest gratitude for the Deanship of Scientific Research, King Faisal University, Al Hufuf, Al Ahsa, Kingdom of Saudi Arabia, PO box: 400, Post code: 31982, for financial support annual research grant number 33.
Conflicts of Interest
The author declares no conflict of interest.
References
- Raven, J.A.; Evans, M.C.W.; Korb, R.E. The role of trace metals in photosynthetic electron transport in O2-evolving organisms. Photosyn. Res. 1999, 60, 111–150. [Google Scholar] [CrossRef]
- Whittaker, S.; Bidle, K.D.; Kustka, A.B.; Falkowski, P.G. Quantification of nitrogenase in Trichodesmium IMS 101: Implications for iron limitation of nitrogen fixation in the ocean. Environ. Microbiol. Rep. 2011, 3, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, A.; Maldonado, M.T. Iron. In The Physiology of Microalgae; Borowitzka, M.A., Beardall, J., Raven, J.A., Eds.; Springer: Dordrecht, The Netherland, 2016; pp. 233–279. [Google Scholar]
- Weinberg, E.D. Cellular regulation of iron assimilation. Quart. Rev. Biol. 1989, 64, 261–290. [Google Scholar] [CrossRef] [PubMed]
- Geider, R.J.; La Roche, J. The role of iron in phytoplankton photosynthesis, and the potential for iron-limitation of primary productivity in the sea. Photosyn. Res. 1994, 39, 275–301. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.S.; Gordon, R.M.; Coale, K.H. What controls dissolved iron concentrations in the world ocean? Mar. Chem. 1997, 57, 137–161. [Google Scholar] [CrossRef]
- Morel, F.M.; Kustka, A.B.; Shaked, Y. The role of unchelated Fe in the iron nutrition of phytoplankton. Limnol. Oceanogr. 2008, 53, 400–404. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, L.J.; Breitbarth, E.; Boyd, P.W.; Hunter, K.A. Influence of ocean warming and acidification on trace metal biogeochemistry. Mar. Ecol. Prog. Ser. 2012, 470, 191–205. [Google Scholar] [CrossRef] [Green Version]
- Samperio-Ramos, G.; Santana Casiano, J.M.; González Dávila, M. Effect of ocean warming and acidification on the Fe (II) oxidation rate in oligotrophic and eutrophic natural waters. Biogeochemistry 2016, 128, 19–34. [Google Scholar] [CrossRef]
- Gledhill, M.; Buck, K.N. The organic complexation of iron in the marine environment: A review. Front. Microbiol. 2012, 3, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Kenshi, K. Biogeochemistry of iron in seawater. Chikyu. Ac. Jp 2001, III, 93–102. [Google Scholar]
- Shi, D.; Xu, Y.; Hopkinson, B.M.; Morel, F.M.M. Effect of ocean acidification on iron availability to marine phytoplankton. Science 2010, 327, 676–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, A.; Theisen, R.M. Iron (III)-siderophore coordination chemistry: Reactivity of marine siderophores. Coord. Chem. Rev. 2010, 254, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.H.; Coale, K.H.; Johnson, K.S.; Fitzwater, S.E.; Gordon, R.M.; Tanner, S.J.; Hunter, C.N.; Elrod, V.A.; Nowicki, J.L.; Coley, T.L.; et al. Testing the iron hypothesis in ecosystems of the equatorial Pacific Ocean. Nature 2010, 371, 123–129. [Google Scholar] [CrossRef]
- Wu, J.; Boyle, E.; Sunda, W.; Wen, L. Soluble and colloidal iron in the oligotrophic North Atlantic and North Pacific. Science 2001, 293, 847–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morel, F.M.; Rueter, J.; Price, N. Iron nutrition of phytoplankton and its possible importance in the ecology of ocean regions with high nutrient and low biomass. Oceanography 1991, 4, 1991. [Google Scholar] [CrossRef]
- Martin, R.M.; Gordon, S.; Fitzwater, W.W. Broenkow, VERTEX: Phytoplankton/iron studies in the Gulf of Alaska. Deep-Sea Res. 1994, 36, 649–680. [Google Scholar] [CrossRef]
- Miller, C.B.; Frost, B.W.; Booth, B.; Wheeler, P.A.; Landry, M.R.; Welschmeyer, N. Ecological processes in the subarctic Pacific: Iron-limitation cannot be the whole story. Oceanography 1991, 4, 71–78. [Google Scholar] [CrossRef] [Green Version]
- El Semary, N.A. Algae and Fishes: Benefits and Hazards. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Ratnarajah, L.; Melbourne-Thomas, J.; Marzloff, M.P.; Lannuzel, D.; Meiners, K.M.; Chever, F.; Nicol, S.; Bowie, A.R. A preliminary model of iron fertilization by Baleen whales and Antarctic krill in the Southern Ocean: Sensitivity of primary productivity estimates to parameter uncertainty. Ecol. Model. 2016, 320, 203–212. [Google Scholar] [CrossRef]
- Morrissey, J.; Bowler, C. Iron utilization in marine cyanobacteria and eukaryotic algae. Front. Microbiol. 2012, 3, 43. [Google Scholar] [CrossRef] [Green Version]
- Misumi, K.; Lindsay, K.; Moore, J.K.; Doney, S.C.; Bryan, F.O.; Tsumune, D.; Yoshida, Y. The iron budget in ocean surface waters in the 20th and 21st centuries: Projections by the community earth system model version 1. Biogeosciences 2014, 11, 33–55. [Google Scholar] [CrossRef] [Green Version]
- Hudson, R.J.M.; Morel, F.M. Iron transport in marine phytoplankton: Kinetics of cellular and medium coordination reactions. Limnol. Oceanogr. 1990, 35, 1002–1020. [Google Scholar] [CrossRef]
- Wood, P.M. Interchangeable copper and iron proteins in algal photosynthesis. Eur. Biochem. 1978, 87, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Zumft, W.G.; Spiller, H. Characterization of a flavodoxin from the green alga Chlorella. Biochem. Biophys. Res. Comm. 1971, 45, 112–118. [Google Scholar] [CrossRef]
- Barber, J.; Nield, J.; Duncan, J.; Bibby, T.S. Accessory Chlorophyll Proteins in Cyanobacterial Photosystem I; Golbeck, J.H., Ed.; Springer: Dordrecht, The Netherland, 2006; pp. 99–117. [Google Scholar]
- Palenik, B.; Brahamsha, B.; Larimer, F.W.; Land, M.; Hauser, L.; Chain, P.; Lamerdin, J.; Regala, W.; Allen, E.E.; McCarren, J.; et al. The genome of a motile marine Synechococcus. Nature 2003, 424, 1037–1042. [Google Scholar] [CrossRef] [Green Version]
- Rocap, G.; Larimer, F.W.; Lamerdin, J.; Malfatti, S.; Chain, P.; Ahlgren, N.A.; Arellano, A.; Coleman, M.; Hauser, L.; Hess, W.R.; et al. Genome divergence in two Prochlorococcus ecotypes reflects oceanic niche differentiation. Nature 2003, 424, 1042–1047. [Google Scholar] [CrossRef]
- Bibby, T.S.; Zhang, Y.; Chen, M. Biogeography of photosynthetic light-harvesting genes in marine phytoplankton. PLoS ONE 2009, 4, e4601. [Google Scholar] [CrossRef] [Green Version]
- Boekema, E.J.; Hifney, A.; Yakushevska, A.E.; Piotrowski, M.; Keegstra, W.; Berry, S.; Michel, K.P.; Pistorius, E.K.; Kruip, J. A giant chlorophyll-protein complex induced by iron deficiency in cyanobacteria. Nature 2001, 412, 745–748. [Google Scholar] [CrossRef] [Green Version]
- Behnke, L.; Laroche, J. Iron uptake proteins in algae and the role of Iron Starvation-Induced Proteins (ISIPs). Eur. J. Phycol. 2020, 55, 339–360. [Google Scholar] [CrossRef]
- Kazamia, E.; Sutak, R.; Paz-Yepes, J.; Dorrell, R.G.; Vieira, F.R.J.; Mach, J.; Morrissey, J.; Leon, S.; Lam, F.; Pelletier, E.; et al. Endocytosis-mediated siderophore uptake as a strategy for Fe acquisition in diatoms. Sci. Adv. 2018, 4, eaar4536. [Google Scholar] [CrossRef] [Green Version]
- Sunda, W.; Huntsman, S.A. Iron uptake and growth limitation in oceanic and coastal phytoplankton. Mar. Chem. 1995, 50, 189–206. [Google Scholar] [CrossRef] [Green Version]
- Bidle, K.D.; Bender, S.J. Iron starvation and culture age activate metacaspases and programmed cell death in the marine diatom Thalassiosira pseudonana. Eukaryot. Cell 2007, 7, 223–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mock, T.; Samanta, M.P.; Iverson, V.; Berthiaume, C.; Robison, M.; Holtermann, K.; Durkin, C.; BonDurant, S.S.; Richmond, K.; Rodesch, M.; et al. Wholegenome expression profiling of the marine diatom Thalassiosira pseudonana identifies genes involved in silicon bioprocesses. Proc. Natl. Acad. Sci. USA 2008, 105, 1579–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thamatrakoln, K.; Korenovska, O.; Niheu, A.K.; Bidle, K.D. Whole-genome expression analysis reveals a role for death-related genes in stress acclimation of the diatom Thalassiosira pseudonana. Environ. Microbiol. 2011, 14, 67–81. [Google Scholar] [CrossRef]
- Morrissey, J.; Sutak, R.; Paz-Yepes, J.; Tanaka, A.; Moustafa, A.; Veluchamy, A.; Thomas, Y.; Botebol, H.; Bouget, F.Y.; McQuaid, J.B.; et al. A novel protein, ubiquitous in marine phytoplankton, concentrates iron at the cell surface and facilitates uptake. Curr. Biol. 2015, 25, 364–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQuaid, J.B.; Kustka, A.B.; Oborník, M.; Horák, A.; McCrow, J.P.; Karas, B.J.; Zheng, H.; Kindeberg, T.; Andersson, A.J.; Barbeau, K.A.; et al. Carbonate-sensitive phytotransferrin controls high-affinity iron uptake in diatoms. Nature 2018, 555, 534–537. [Google Scholar] [CrossRef]
- Maldonado, M.T.; Price, N.M. Utilization of iron bound to strong organic ligands by plankton communities in the subarctic Pacific Ocean. Deep-Sea Res. Part II Top. Stud. Oceanogr. 1999, 46, 2447–2473. [Google Scholar] [CrossRef]
- Maldonado, M.T.; Price, N.M. Reduction and transport of organically bound iron by Thalassiosira oceanica (Bacillariophyceae). J. Phycol. 2001, 37, 298–309. [Google Scholar] [CrossRef]
- Terzulli, A.; Kosman, D.J. Analysis of the highaffinity iron uptake system at the Chlamydomonas reinhardtii plasma membrane. Euk. Cell 2010, 9, 815–826. [Google Scholar] [CrossRef] [Green Version]
- Allen, A.E.; Laroche, J.; Maheswari, U.; Lommer, M.; Schauer, N.; Lopez, P.J.; Finazzi, G.; Fernie, A.R.; Bowler, C. Whole-cell response of the pennate diatom Phaeodactylum tricornutum to iron starvation. Proc. Natl. Acad. Sci. USA 2008, 105, 10438–10443. [Google Scholar] [CrossRef] [Green Version]
- Shaked, Y.; Lis, H. Disassembling iron availability to phytoplankton. Front. Microbiol. 2012, 3, 123. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, A.; Guerinot, M. Metal transport. In The Plant Plasma Membrane; Murphy, A.S., Schulz, B., W. Peer, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 303–330. [Google Scholar]
- Emerson, D. Biogenic iron dust: A novel approach to ocean iron fertilization as a means of large scale removal of carbon dioxide from the atmosphere. Hypothesis Theory Artic. Front. Mar. Sci. 2019, 7, 2019. [Google Scholar] [CrossRef]
- Anderson, R.F.; Cheng, H.; Edwards, R.L.; Fleisher, M.Q.; Hayes, C.T.; Huang, K.-F.; Kadko, D.; Lam, P.J.; Landing, W.M.; Lao, Y.; et al. How well can we quantify dust deposition to the ocean? Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150285. [Google Scholar] [CrossRef] [Green Version]
- Jickells, T.D. Global iron connections between desert dust, ocean biogeochemistry, and climate. Science 2005, 308, 67–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, B.A.; Prospero, J.M.; Mackie, D.; Gaiero, D.; Hesse, P.P.; Balkanski, Y. Global Connections between Aeolian Dust, Climate and Ocean Biogeoche-mistry at the Present Day and at the Last Glacial Maximum. Earth-Sci. Rev. 2010, 99, 61–97. [Google Scholar] [CrossRef]
- Botebol, H.; Lesuisse, E.; Šuták, R.; Six, C.; Lozano, J.C.; Schatt, P.; Vergé, V.; Kirilovsky, A.; Morrissey, J.; Léger, T.; et al. Central role for ferritin in the day/night regulation of iron homeostasis in marine phytoplankton. Proc. Natl. Acad. Sci. USA 2015, 112, 14652–14657. [Google Scholar] [CrossRef] [Green Version]
- Twining, B.S.; Baines, S. The Trace Metal Composition of Marine Phytoplankton. Annu. Rev. Mar. Sci. 2013, 5, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, A.; Parker, M.S.; Moccia, L.P.; Lin, E.O.; Arrieta, A.L.; Ribalet, F.; Murphy, M.E.P.; Maldonado, M.T.; Armbrust, E.V. Ferritin is used for iron storage in bloom-forming marine pennate diatoms. Nature 2009, 457, 467–470. [Google Scholar] [CrossRef]
- Pfaffen, S.; Bradley, J.M.; Abdulqadir, R.; Firme, M.R.; Moore, G.R.; Brun, N.E.L.; Murphy, M.E.P. A diatom ferritin optimized for iron oxidation but not iron storage. J. Biol. Chem. 2015, 290, 28416–28427. [Google Scholar] [CrossRef] [Green Version]
- Smetacek, Y.V.; Naqvi, S.W.A. The next generation of iron fertilization experiments in the Southern Ocean. Phil. Trans. R. Soc. A 2008. [Google Scholar] [CrossRef]
- Boyd, P.W.; Jickells, T.; Law, C.S.; Blain, S.; Boyle, E.A.; Buesseler, K.O.; Coale, K.H.; Cullen, J.J.; De Baar, H.J.W.; Follows, M.; et al. Mesoscale iron enrichment experiments 1993–2005: Synthesis and future directions. Science 2007, 315, 612–617. [Google Scholar] [CrossRef] [Green Version]
- Langmann, B. On the Role of Climate Forcing by Volcanic Sulphate and Volcanic Ash. Adv. Meteorol 2014, 340123. [Google Scholar] [CrossRef]
- Kim, T.; Hong, G.; Kim, D.; Baskaran, M. Iron Fertilization with Enhanced Phytoplankton Productivity under Minimal Sulfur Compounds and Grazing Control Analysis in HNLC Region. Amer. J. Clim. Chang. 2019, 8, 14–39. [Google Scholar] [CrossRef] [Green Version]
- De La Rocha, C.L.; Passow, U. 8.4—The Biological Pump. In Treatise on Geochemistry, 2nd ed.; Turekian, H.D.H.K., Ed.; Elsevier: Oxford, UK, 2014. [Google Scholar]
- UN Report; Ocean Fertilization, Scientific Summary for Policy Makers; UN: Washington, DC, USA, 2010.
- Yoon, J.-E.; Yoo, K.-C.; Macdonald, A.M.; Yoon, H.-I.; Park, K.-T.; Yang, E.J.; Kim, H.-C.; Lee, J.I.; Lee, M.K.; Jung, J.; et al. Reviews and syntheses: Ocean iron fertilization experiments—Past, present, and future looking to a future Korean Iron Fertilization Experiment in the Southern Ocean (KIFES) project. Biogeosciences 2018, 15, 5847–5889. [Google Scholar] [CrossRef] [Green Version]
- Sarmiento, J.L.; Gruber, N. Ocean Biogeochemical Dynamics; Princeton University Press: Princeton, NJ, USA, 2006. [Google Scholar]
- Blain, S.; Quéguiner, B.; Armand, L.; Belviso, S.; Bombled, B.; Bopp, L.; Bowie, A.; Brunet, C.; Brussaard, C.; Carlotti, F.; et al. Effect of natural iron fertilization on carbon sequestration in the Southern Ocean. Nature 2007, 446, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Savoye, T.W.; Trull, S.H.M.; Jacquet, J.; Navez, F.D. 234Th-based export fluxes during a natural iron fertilization experiment in the Southern Ocean (KEOPS) Deep Sea Research Part II: Trop. Stud. Oceanogr. 2008, 55, 841–855. [Google Scholar] [CrossRef]
- Naqvi, S.W.A.; Moffett, W.; Gauns, M.U.; Narvekar, P.V.; Pratihary, A.K.; Naik, H.; Shenoy, D.M.; Jayakumar, D.A. The Arabian Sea as a High-Nutrient, Low-Chlorophyll Region during the Late Southwest Monsoon. Biogeosciences 2010, 7, 2091–2100. [Google Scholar] [CrossRef] [Green Version]
- Mofftt, J.; Vedamati, J.; Goepfert, T.; Pratihary, A.; Gauns, M.; Naqvi, S.W.A. Biogeochemistry of iron in the Arabian Sea. Limnol. Oceanogr. 2015, 60, 10132. [Google Scholar] [CrossRef]
- Peers, G.; Price, N.M. Copper-containing plastocyanin used for electron transport by an oceanic diatom. Nature 2006, 441, 341–344. [Google Scholar] [CrossRef]
- Rue, E.; Bruland, K. Domoic acid binds iron and copper: A possible role for the toxin produced by the marine diatom Pseudo-nitzschia. Mar. Chem. 2001, 76, 127–134. [Google Scholar] [CrossRef]
- Trick, C.G.; Bill, B.D.; Cochlan, W.P.; Wells, M.L.; Trainer, V.L.; Pickell, L.D. Iron enrichment stimulates toxic diatom production in high-nitrate, low-chlorophyll areas. Proc. Natl. Acad. Sci. USA 2010, 107, 5887–5892. [Google Scholar] [CrossRef] [Green Version]
- Christaki, U.; Georges, C.; Genitsaris, S.; Monchy, S. Microzooplankton community associated with phytoplankton blooms in the naturally iron-fertilized Kerguelen area (Southern Ocean). FEMS Microbiol. Ecol. 2015, 91, fiv068. [Google Scholar] [CrossRef] [PubMed]
- Assmy, P.; Jansen, S.; Fuchs, N.; Kragefsky, S.; Latasa, M.; Steigenberger, S.; Herndl, G.J.; Webb, A.; Breitbarth, E.; Berg, G.M.; et al. Thick-shelled, grazer-protected diatoms decouple ocean carbon and silicon cycles in the iron-limited Antarctic Circumpolar Current. Proc. Natl. Acad. Sci. USA 2013, 110, 20633–20638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batten, S.D.; James, F.; Gower, R. Did the iron fertilization near Haida Gwaii in 2012 affect the pelagic lower trophic level ecosystem? J. Plank. Res. 2014, 36, 925–932. [Google Scholar] [CrossRef] [Green Version]
- Galbraith, E.D.; Le Mézo, P.; Solanes Hernandez, G.; Bianchi, D.; Kroodsma, D. Growth Limitation of Marine Fish by low Iron availability in the open Ocean. Front. Mar. Sci. 2019, 6, 509. Available online: https://www.frontiersin.org/article/10.3389/fmars.2019.00509 (accessed on 16 May 2022). [CrossRef]
- Oceanus. Available online: https://www.whoi.edu/oceanus/feature/dumping-iron-and-trading-carbon (accessed on 16 May 2022).
- IMO. Resolution LC-LP.1. Regulation of Ocean Fertilization, LC 30/16, Annex 6; IMO: London, UK, 2008. [Google Scholar]
- IMO. Resolution LC-LP.2. Assessment Framework for Scientific Research Involving Ocean Fertilization, LC 32/15, Annex 6; IMO: London, UK, 2010. [Google Scholar]
- IMO. Resolution LP.4 (8). Amendment to the London Protocol to Regulate the Placement of Matter for Ocean Fertilization and Other Marine Geoengineering Activities, LP.8, LC 35/15, Annex 4, Annex 5; IMO: London, UK, 2013. [Google Scholar]
- Ito, A.; Ye, Y.; Baldo, C.; Shi, Z. Ocean fertilization by pyrogenic aerosol iron. NPJ Clim. Atmos. Sci. 2021, 4, 30. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).