1. Introduction
Date palm,
Phoenix dactylifera L., an Islamic religions holy plant, comes under the family Arecaceae [
1]. It is one of the oldest and most economically important fruit tree species in the Arab world. In Saudi Arabia, 320 varieties represented in 23 million trees are grown, with an annual income of USD 500 million [
2,
3]. In addition to its food value and socio-economic importance, date palm provides raw materials for shelter, clothing, fiber, furniture and aesthetics [
4].
Date fruits are the source of various phytochemicals such as flavonoids, sterols, tannins, anthocyanins, carotenoids and procyanidins and also exhibit a variety of nutraceutically important phenolic compounds of high antioxidant value [
5,
6,
7,
8]. These phenolic compounds possess anticancer properties and also have the properties to prevent cardiovascular diseases [
9,
10]. In vitro callus and cell suspension cultures show a great potential to produce commercially useful bioactive compounds with a great antioxidant activity [
11,
12,
13]. Plant growth regulators (PGR) often serve as key factors in the accumulation of bioactive compounds. The type and concentration of auxin and cytokinin affects growth and product formation in callus and plant cell suspension culture [
14,
15,
16]. The medicinal value of these polyphenols is well known, as they have antioxidant properties and are used as raw materials for industries such as food and pharma [
9,
10]. Limited research has been reported with respect to polyphenols production from date palm in vitro cultures [
12,
17,
18,
19].
The objective of the current study is to assess the influence of adding combinations of 2-isopentenyladenine (2-iP) and 2,4-dichlorophenoxyacetic acid (2,4-D) concentrations to the cell suspension culture of date palm with respect to the accumulation of biomass, total phenolic and flavonoid contents, antioxidant activity and polyphenols production (apigenin, caffeic acid, catechin and kaempferol). As per our knowledge, there has been no previous report that has addressed the influence of the studied hormones on polyphenols accumulation in date palm cell suspension culture. The results from this study may provide a new avenue for pharmaceutical industries.
2. Materials and Methods
2.1. Plant Material
The date palm (
Phoenix dactylifera L.) cultivar Shishi was selected for this study because of its abundance and popularity in the Eastern Province of Saudi Arabia, where the experiment was performed. The shoot tip region was excised from 3-year-old offshoots. After the removal of surrounding leaves, the shoot tip was isolated, and the surface was sterilized, sectioned into small pieces and inoculated on the callus initiation medium in accordance with the procedures described by Naik and Al-Khayri [
20].
2.2. Cell Suspension Culture
To induce the cell suspension culture, 0.5 g of the resultant callus of 12 weeks old culture was macerated aseptically using a scalpel and inoculated in 250-mL conical flasks, each containing 50 mL of liquid Murashige and Skoog (MS) medium [
21]. The cell suspension cultures were cultured for 11 weeks under a 16-h photoperiod of 40 µmol/m
2/s light at 25 ± 2 °C and a shaker speed of 150 rpm. The standard culture medium was augmented with 1.5 mg/L 2iP and 10 mg/L naphthaleneacetic acid (NAA) that served as the control for this experiment [
12]. The hormone 2iP (2.5 and 5 mg/L) in combination with 2,4-D (0, 1, 2.5, 5 and 10 mg/L) were evaluated in comparison to the standard culture medium.
2.3. Biomass Determination
To determine the packed cell volume (PCV), 15-mL sterile graduated centrifuge tubes were filled with 10 mL of cell suspension and centrifuged for 5 min at 2000 g. The packed volume of cell mass is recorded as a percentage. To determine the dry weight (DW), cells were collected by filtering through Whatman No. 1 filter paper and then were oven-dried (60 °C) for 24 h. The dried cell mass was stored at 4 °C for 1 month in a vial, and subsequently extraction and analysis were carried out.
2.4. Extraction of Cell Suspension Culture
Using a pestle and mortar, a fine powder was made from the dried cell mass. A finely powdered 100 mg sample in a centrifuge tube (15 mL) was used to carry out the cell extraction [
12]. Aqueous methanol (80%,
v/
v, 10 mL) was used as the extraction solvent (for 2 h at 60 °C under a water bath). The extract was centrifuged at 6000 rpm for 20 min, and then a polyvinylidene fluoride (PVDF) membrane filter (Merck Millipore, Cork, Ireland) with a pore size of 0.45 µm was used to filter the supernatant. This filtrate was collected in a round bottom flask (125 mL), and solvent was evaporated under reduced pressure. A total of 1 mL of 80% methanol was used to dissolve dried extracts, and this was filtered through a PVDF 0.45-µm membrane filter, and the filtrate was collected in 2-mL high performance liquid chromatography (HPLC) sample vials.
2.5. Total Phenolic Contents (TPC)
The determination of TPC in date palm cell suspension culture was carried out according to the Modified Folin-Ciocalteu (F-C) method [
22]. A total of 20 µL of methanolic extract was mixed with deionized water (1.58 mL), and then 100 µL of F-C reagent was added. These were mixed thoroughly and kept for 8 min, thereafter adding 20% sodium carbonate (300 µL). This mixture solution was shaken and mixed well, and kept at 20 °C for 2 h to develop the color. The absorbance was measured at 765 nm using an Ultraviolet-visible (UV-Vis) spectrophotometer (Yoke, Shanghai, China). Finally, we recorded the TPC of cell extract expressed as mg gallic acid equivalents (GAE) 100/g DW.
2.6. Total Flavonoid Content (TFC)
The quantification of TFC in the date palm cell suspension culture extracts was based on the modified colorimetric method [
23]. The mixture of 100 µL extract, 10% aluminum chloride (50 µL), 1 M potassium acetate (50 µL) and 1.8 mL deionized water was thoroughly mixed. The mixture was kept at room temperature (30 min) for incubation, and the absorbance was measured at 415 nm using a UV-Vis spectrophotometer (Yoke, China). The quantity of TFC of the date palm cell extract was expressed as mg quercetin equivalents (QE) 100/g DW.
2.7. Evaluation of Radical Scavenging Activity (RSA)
2,2-Diphenyl-1-picrylhydrazyl (DPPH), the stable free radical, has a hydrogen ion-donating ability, and this property is used to evaluate the antioxidant activities of various compounds/cell extracts [
24]. A modified Farag et al. protocol was applied to determine the antioxidant activity [
5]. The original sample was suspended in methanol, and a stock solution was made at value 1 mg/mL. Sample solutions were prepared from this stock solution for various concentrations (2–1000 µg/mL). A standard control, butylated hydroxylanisole (BHA), was prepared similarly to the samples. Extracts of 250 µL were taken from the original sample, and the same solvent was used to make up the 1 mL. To each test, 2 mL of DPPH (0.1 mM in methanol) was added. Methanol (1 mL) and DPPH solution (2 mL) was used to prepare the blank. Incubation condition: dark, room temperature for 30 min. The UV-Vis spectrophotometer reading was kept at 517 nm, and for methanol the absorbance was set to zero and the absorbance was recorded for the blank, standard control and samples. The DPPH disappearance was recorded; from the samples and standard control, the percentage of RSA of the DPPH was calculated based on the following equation:
2.8. Determination of Polyphenols
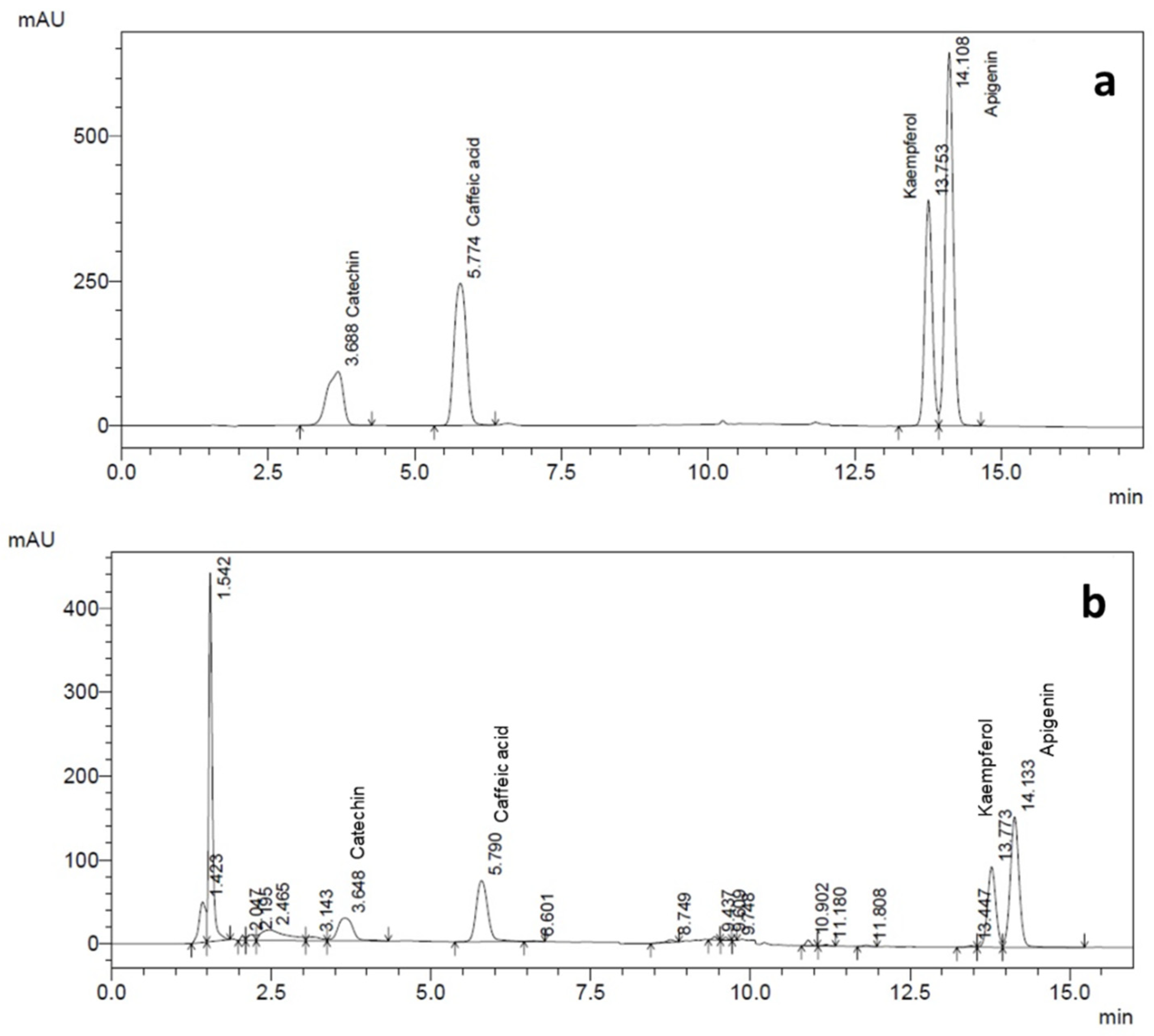
The determination of polyphenols was achieved by using the HPLC unit (Shimadzu Prominence Liquid chromatography, Japan), as per the experimental protocol conducted by Naik and Al-Khayri [
12]. The mobile phases were 0.5% acetic acid in Millipore water (solvent A), methanol (solvent B) and acetonitrile (solvent C). A PVDF membrane filter (0.45 µm) was used to filter the mobile phase, and was then deaerated ultrasonically using a sonicator. The standards, catechin, caffeic acid and kaempferol (Sigma-Aldrich, St. Louis, MO, USA), were dissolved in methanol; apigenin was dissolved in solution containing 1:1 methanol and acetonitrile, and then all the standards were diluted with the mobile phase before injecting in the HPLC system. The concentration ranges of 1, 2, 5, 10, 30 and 40 µg/mL were used to develop a calibration curve. Solutions of standard compounds were chromatographed and served as external standards. When comparing the retention times of the standards, polyphenols of cell extracts were identified (
Figure 1a,b). The quantities of each polyphenol present in the samples were expressed as µg/g.
2.9. Statistical Analysis
The data were collected in three replications per treatment, and an analysis of variance (ANOVA) was applied to the collected data using SPSS (Statistics version 22.0; IBM corp. New York, NY, USA). Based on Duncan’s multiple range test (DMRT) at p ≤ 0.05, the mean values were separated.
3. Results
3.1. Biomass Accumulation
For any cell suspension culture system, the evaluation of biomass accumulation is the most important feature. In the present experiment, the results showed that there was an increase in biomass when the culture was grown in medium treated with 2.5 and 5 mg/L 2iP. However, the 1 mg/L 2,4-D + 5 mg/L 2iP hormone combination in cell suspension culture produced an optimum PCV and DW accumulation followed by 5 mg/L 2,4-D + 2.5 mg/L 2iP, when compared to the control and other hormonal combinations tested (
Figure 2 and
Figure 3). The hormone combination 10 mg/L 2,4-D + 5 mg/L 2iP culture showed the least accumulation of biomass. The result indicating higher concentrations of the auxin and cytokinin combination resulted in a reduction of the biomass accumulation.
3.2. Total Phenolic Content, Flavonoids and Radical Scavenging Activity (RSA)
The cell suspension culture grown in the medium fortified with 2.5 and 5 mg/L 2iP showed that there was an elevation in the content of the total phenolic content, flavonoids and RSA when compared to the control. On the other hand, the hormone 5 mg/L 2,4-D + 2.5 mg/L 2iP supplemented culture yielded the maximum accumulation of total phenolic content, flavonoids and RSA (90.65 %) over the control experiment, and also over other tested hormones and their combinations (
Table 1). In the auxin and cytokinin combination, the culture medium treated with 2.5 mg/L 2,4-D + 2.5 mg/L 2iP induced a significantly lower concentration of total phenolic content, flavonoids and RSA (49.29%) when compared to the control and other hormone concentrations. This indicates that the hormones with low and equal concentrations are not favoring the accumulation of the total phenolic content, flavonoids and RSA. The cell suspension culture medium supplemented with a higher concentration of auxin/cytokinin (10 mg/L 2,4-D + 5 mg/L 2iP) also accumulated the least concentration of the total phenolic content, flavonoids and RSA. The cell suspension cultures with the other hormone combinations are inconsistent with the accumulation of the total phenolic content, flavonoids and RSA value. However, the hormone-treated date palm cell suspension culture showed a strong positive correlation between the total phenolic content, flavonoids and RSA.
3.3. Polyphenol Content
The culture medium augmented with 2.5 and 5 mg/L 2iP showed the same level of polyphenol production when compared to the control. From the different hormones and combinations tested, the 5 mg/L 2,4-D + 2.5 mg/L 2iP supplemented cell suspension culture resulted in a significantly higher content of caffeic acid-37.1 µg/g DW production when compared to the control culture (
Table 2). It also showed the highest accumulation of catechin (30.6 µg/g DW). With respect to the other polyphenols (kaempferol and apigenin), the control culture performed well compared to the other tested hormones in cultures (
Table 2). The cell suspension culture medium fortified with a higher concentration, that is to say the 10 mg/L 2,4-D + 5 mg/L 2iP hormone, accumulated the overall lowest concentration of polyphenols when compared to the control and other hormone concentrations. The culture medium treated with 2.5 mg/L 2,4-D + 2.5 mg/L 2iP also showed a significantly lower synthesis of polyphenols.
4. Discussion
Plant growth regulators are the key elements in the suspension/cell culture medium and are essential for cell proliferation and differentiation. The concentration and type of auxin or the ratio of auxin/cytokinins significantly influence both cell growth and the in vitro synthesis of polyphenol compounds [
25]. Praveen and Murthy reported an altered cell biomass accumulation in
Withania somnifera (L.) Dunal when the culture media were augmented with auxins at different concentrations [
26]. They recorded the optimum accumulation of biomass in the medium treated with 2 mg/L 2,4-D. However, the maximum biomass accumulation was also noted with the culture medium in combination with 2 mg/L 2,4-D + 0.5 mg/L kinetin (KN). In the
Fagonia indica (L.) adventitious root culture, NAA resulted in the highest accumulation of biomass [
27].
Hormone concentration is the major factor in secondary product accumulation such as phenolics and flavonoids [
14]. Corroborating our results with a number of previous research suggested that there was a tight association between the observed antioxidant activities and the phenolic compounds produced by in vitro cultures [
18,
28]. In the current investigation, RSA levels were found to be at a maximum in the cell suspension culture of date palm, which indicates that the commercial-scale utilization of date palm cell suspension cultures for the production of nutraceuticals is promising. The MS medium augmented with 27.1 μM 2,4-D favored the optimum accumulation of total phenols in the callus culture of
Cnidium officinale Makino. [
29]. Similarly to our findings, the
Garcinia brasiliensis Mart. callus culture containing 2,4-D and 2iP produced a significantly higher content of total flavonoids [
30].
Plant hormones are chemical compounds and a group of key signal molecules that are actively involved in the synthesis of plant secondary metabolites and also in regulating development and plant growth [
31]. Plant hormones play a crucial role in plant tissue and cell suspension cultures, not only in cell growth, morphogenesis and plant regeneration but also in metabolite accumulation [
29,
30]. In the cell suspension culture of
Panax quinquefolium L., where several growth regulators such as BA, KN, NAA and 2,4-D augmented the MS medium, the optimum accumulation of ginsenosides was observed in 1.0 mg/L 2, 4-D and 0.25 mg/L KN fortified medium [
32]. In another study, 4.5 and 6.8 µM 2,4-D concentrations played an important role in the production of optimum levels of phthalide and 3-butylidenephthalide, respectively, in the callus culture of
C. officinale Makino [
30]. The above discussion indicates that different auxin/cytokinin combinations had key effects on various plant species and affected the synthesis of bioactive compounds at different levels.
5. Conclusions
In the cell suspension culture system, auxin/cytokinin with various concentrations and combinations induces biomass and polyphenols at different levels with respect to the selected plant species. The results obtained from the present investigation indicated that 1 mg/L 2,4-D + 5 mg/L 2iP-supplemented date palm cell suspension culture favored the accumulation of biomass. Cell suspension cultures treated with 5 mg/L 2,4-D and 2.5 mg/L 2iP induced significantly higher levels of phenolics, flavonoids and radical scavenging activity (90.65%) and also produced the highest content of caffeic acid (37.1 µg/g DW). However, a higher concentration of 2,4-D + 2iP in date palm cell suspension culture is not suitable for the production of biomass and polyphenols. The valuable information obtained in this study is encouraging for the large-scale production of nutraceutically important polyphenols, which are the basic materials for various pharma and food industries.
Author Contributions
Conceptualization, J.M.A.-K.; Data curation, P.M.N.; Formal analysis, J.M.A.-K.; Funding acquisition, J.M.A.-K.; Investigation, P.M.N.; Methodology, P.M.N.; Project administration, J.M.A.-K.; Supervision, J.M.A.-K.; Writing—original draft preparation, P.M.N.; Writing—review & editing, J.M.A.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported through the Annual Funding track by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Project No. AN000506).
Data Availability Statement
All the related data are presented in the manuscript.
Acknowledgments
The authors are thankful to the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia for funding this research project (This work was supported through the Annual Funding track by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia; Project No. AN000506).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al-Khayri, J.M.; Ibraheem, Y. In vitro selection of abiotic stress tolerant date palm (Phoenix dactylifera L.). Emir. J. Food Agric. 2014, 26, 921–933. [Google Scholar] [CrossRef] [Green Version]
- Trabzuni, D.M.; Ahmed, S.E.B.; Abu-Tarboush, H.M. Chemical composition, minerals and antioxidants of the heart of date palm from three Saudi cultivars. Food Nutr. Sci. 2014, 5, 1374–1382. [Google Scholar] [CrossRef] [Green Version]
- Aleid, S.M.; Al-Khayri, J.M.; Al-Bahrany, A.M. Date palm status and perspective in Saudi Arabia. In Date Palm Genetic Resources and Utilization, Vol 2: Asia and Europe; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 49–95. [Google Scholar]
- Al-Khayri, J.M.; Naik, P.M.; Jain, S.M.; Johnson, D.V. Advances in date palm (Phoenix dactylifera L.) Breeding. In Advances in Plant Breeding Strategies: Fruits; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 727–771. [Google Scholar]
- Farag, M.A.; Handoussa, H.; Fekry, M.I.; Wessjohann, L.A. Metabolite profiling in 18 Saudi date palm fruit cultivars and their antioxidant potential via UPLC-qTOF-MS and multivariate data analyses. Food Funct. 2016, 7, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Echegaray, N.; Pateiro, M.; Gullón, B.; Amarowicz, R.; Misihairabgwi, J.M.; Lorenzo, J.M. Phoenix dactylifera products in human health–A review. Trends Food Sci. Technol. 2020, 105, 238–250. [Google Scholar] [CrossRef]
- Najm, O.A.; Addnan, F.H.; Mohd-Manzor, N.F.; Elkadi, M.A.; Abdullah, W.O.; Ismail, A.; Mansur, F.A.F. Identification of Phytochemicals of Phoenix dactylifera L. Cv Ajwa with UHPLC-ESI-QTOF-MS/MS. Int. J. Fruit Sci. 2021, 21, 848–867. [Google Scholar] [CrossRef]
- Pasha, A.Z.; Bukhari, S.A.; Enshasy, H.A.E.; Adawi, H.E.; Obaid, S.A. Compositional analysis and physicochemical evaluation of date palm (Phoenix dactylifera L.) mucilage for medicinal purposes. Saudi J. Biol. Sci. 2022, 29, 774–780. [Google Scholar] [CrossRef]
- Vayalil, P.K. Date fruits (Phoenix dactylifera Linn): An emerging medicinal food. Crit. Rev. Food Sci. Nutr. 2012, 52, 249–271. [Google Scholar] [CrossRef]
- Khattak, M.N.K.; Shanableh, A.; Hussain, M.I.; Khan, A.A.; Abdulwahab, M.; Radeef, W.; Samreen, M.H. Anticancer activities of selected Emirati Date (Phoenix dactylifera L.) varieties pits in human triple negative breast cancer MDA-MB-231 cells. Saudi J. Biol. Sci. 2020, 27, 3390–3396. [Google Scholar] [CrossRef]
- Ali, A.M.A.; El-Nour, M.E.M.; Yagi, S.M. Total phenolic and flavonoid contents and antioxidant activity of ginger (Zingiber officinale Rosc.) rhizome, callus and callus treated with some elicitors. J. Genet. Eng. Biotechnol. 2018, 16, 677–682. [Google Scholar] [CrossRef]
- Naik, P.M.; Al-Khayri, J.M. Cell suspension culture as a means to produce polyphenols from date palm (Phoenix dactylifera L.). Cienc. Agrotecnol. 2018, 42, 464–473. [Google Scholar] [CrossRef] [Green Version]
- Sanyal, R.; Nandi, S.; Pandey, S.; Chatterjee, U.; Mishra, T.; Datta, S.; Prasanth, D.A.; Anand, U.; Mane, A.B.; Kant, N.; et al. Biotechnology for propagation and secondary metabolite production in Bacopa monnieri. Appl. Microbiol. Biotechnol. 2022, 106, 1837–1854. [Google Scholar] [CrossRef] [PubMed]
- Bienaimé, C.; Melin, A.; Bensaddek, L.; Attoumbré, J.; Nava-Saucedo, E.; Baltora-Rosset, S. Effects of plant growth regulators on cell growth and alkaloids production by cell cultures of Lycopodiella inundata. Plant Cell Tissue Organ Cult. 2015, 123, 523–533. [Google Scholar] [CrossRef]
- Dar, S.A.; Nawchoo, I.A.; Tyub, S.; Kamili, A.N. Effect of plant growth regulators on in vitro induction and maintenance of callus from leaf and root explants of Atropa acuminata Royal ex Lindl. Biotechnol. Rep. 2021, 32, e00688. [Google Scholar] [CrossRef]
- Ahmadpoor, F.; Zare, N.; Asghari, R.; Sheikhzadeh, P. Sterilization protocols and the effect of plant growth regulators on callus induction and secondary metabolites production in in vitro cultures Melia azedarach L. AMB Express 2022, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Taha, H.S.; Abdel-El-Kawy, A.M.; Abd-El-Kareem, F.M.; El-Shabrawi, H.M. Implement of DMSO for enhancement and production of phenolic and peroxides compounds in suspension cultures of Egyptian date palm (Zaghlool and Samany) cultivars. J. Biotechnol. Biochem. 2010, 1, 1–10. [Google Scholar]
- Naik, P.M.; Al-Khayri, J.M. Influence of culture parameters on phenolics, flavonoids and antioxidant activity in cell culture extracts of date palm (Phoenix dactylifera L.). Erwerbs-Obstbau 2020, 62, 181–188. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Naik, P.M. Elicitor-induced production of biomass and pharmaceutical phenolic compounds in cell suspension culture of date palm (Phoenix dactylifera L.). Molecules 2020, 25, 4669. [Google Scholar] [CrossRef]
- Naik, P.M.; Al-Khayri, J.M. Somatic embryogenesis of date palm (Phoenix dactylifera L.) through cell suspension culture. In Protocols for In Vitro Cultures and Secondary Metabolite Analysis of Aromatic and Medicinal Plants, 2nd ed.; Jain, S.M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2016; Volume 1391, pp. 357–366. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 37, 144–158. [Google Scholar]
- Thiruvengadam, M.; Praveen, N.; John, K.M.M.; Yang, Y.S.; Kim, S.H.; Chung, I.M. Establishment of Momordica charantia hairy root cultures for the production of phenolic compounds and determination of their biological activities. Plant Cell Tissue Organ Cult. 2014, 118, 545–557. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Murthy, H.N.; Lee, E.J.; Paek, K.Y. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Praveen, N.; Murthy, H.N. Establishment of cell suspension cultures of Withania somnifera for the production of withanolide A. Bioresour. Technol. 2010, 101, 6735–6739. [Google Scholar]
- Khan, T.; Abbasi, B.H.; Khan, M.A.; Azeem, M. Production of biomass and useful compounds through elicitation in adventitious root cultures of Fagonia indica. Ind. Crop Prod. 2017, 108, 451–457. [Google Scholar] [CrossRef]
- Al Khateeb, W.; Hussein, E.; Qouta, L.; Alu’datt, M.; Al-Shara, B.; Abu-Zaiton, A. In vitro propagation and characterization of phenolic content along with antioxidant and antimicrobial activities of Cichorium pumilum Jacq. Plant Cell Tissue Organ Cult. 2012, 110, 103–110. [Google Scholar] [CrossRef]
- Adil, M.; Ren, X.; Kang, D.I.; Thi, L.T.; Jeong, B.R. Effect of explant type and plant growth regulators on callus induction, growth and secondary metabolites production in Cnidium officinale Makino. Mol. Biol. Rep. 2018, 45, 1919–1927. [Google Scholar] [CrossRef]
- Teixeira, M.G.; Carvalho, M.; Leite, M.A.; Barbosa, S.; dos Santos filho, P.R.; Santos, B.R. Effect of salicylic acid, 2,4-D and 2i-P on the production of secondary metabolites in Garcinia brasiliensis Mart. callus. Braz. Arch. Biol. Technol. 2019, 62, e19170303. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Gao, W.Y.; Wang, J.; Li, J.; Wang, Q. Production of biomass and bioactive compounds from cell suspension cultures of Panax quinquefolium L. and Glycyrrhiza uralensis Fisch. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Paek, K.Y., Murthy, H.N., Zhong, J.J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 143–164. [Google Scholar]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).