Genetic Augmentation of Legume Crops Using Genomic Resources and Genotyping Platforms for Nutritional Food Security
Abstract
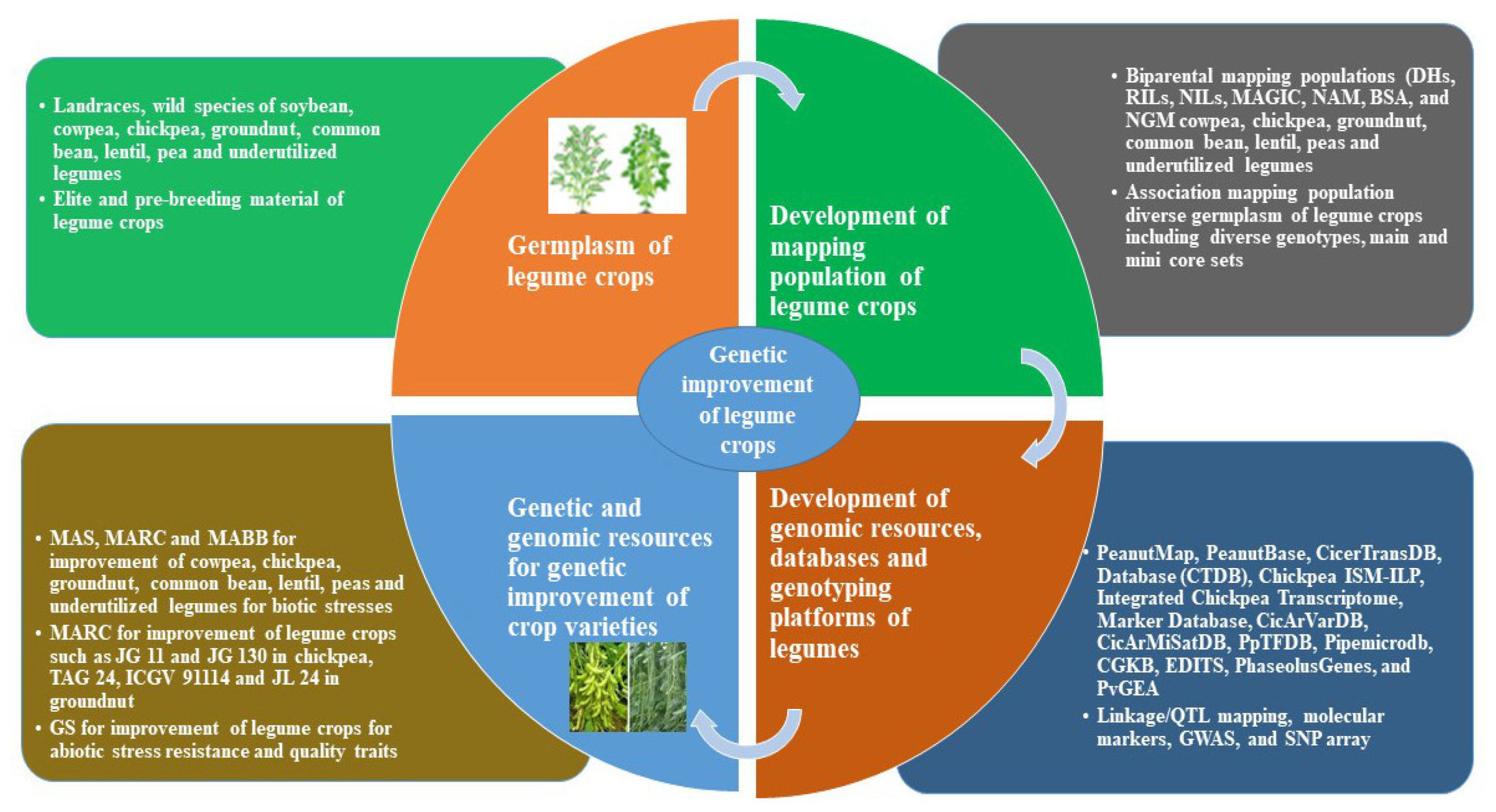
1. Introduction
2. Development of Genomic Resources in Legume Crops
2.1. Linkage and QTL Mapping in Legumes
2.2. Genome-Wide Association Studies
2.3. Databases and Genotyping Platforms
3. Deployment of Genomic Resources and Genotyping Platforms in Legume Breeding
3.1. Cowpea (Vigna unguuiculata)
3.2. Soybean (Glycine max)
3.3. Chickpea (Cicer arietinum)
3.4. Common Bean (Phaseolus vulgaris)
3.5. Groundnut (Arachis hypogaea)
3.6. Pigeonpea (Cajanus cajan)
3.7. Lentil (Lens culinaris)
3.8. Pea (Pisum sativum)
3.9. Underutilized Legume Crops
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bohra, A.; Dubey, A.; Saxena, R.K.; Penmetsa, R.V.; Poornima, K.N.; Kumar, N.; Farmer, A.D.; Srivani, G.; Upadhyaya, H.D.; Gothalwal, R.; et al. Analysis of BAC-end sequences (BESs) and development of BES-SSR markers for genetic mapping and hybrid purity assessment in pigeonpea (Cajanus spp.). BMC Plant Biol. 2011, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.K.; Kudapa, H.; Pazhamala, L.; Chitikineni, A.; Thudi, M.; Bohra, A.; Gaur, P.M.; Janila, P.; Fikre, A.; Kimurto, P.; et al. Translational genomics in agriculture: Some examples in grain legumes. Crit. Rev. iPlant Sci. 2015, 34, 169–194. [Google Scholar] [CrossRef]
- Kumar, V.; Khan, A.W.; Saxena, R.K.; Garg, V.; Varshney, R.K. First generation HapMap in Cajanus spp. reveals untapped variations in parental lines of mapping populations. Plant Biotechnol. J. 2016, 14, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.K.; Pandey, M.K.; Bohra, A.; Singh, V.K.; Thudi, M. Toward the sequence-based breeding in legumes in the post-genome sequencing era. Theor. Appl. Genet. 2018, 132, 797–816. [Google Scholar] [CrossRef]
- UNO. United Nations Organization, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019: Highlights. 2019. Available online: https://population.un.org/wpp/publications/files/wpp2019_highlights.pdf (accessed on 24 April 2022).
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Massawe, F.; Mayes, S.; Cheng, A. Crop diversity: An unexploited treasure trove for food security. Trends Plant Sci. 2016, 21, 365–368. [Google Scholar] [CrossRef]
- Varshney, R.K.; Bohra, A.; Yu, J.; Graner, A.; Zhang, Q.; Sorrells, M.E. Designing future crops: Genomics-assisted breeding comes of age. Trends Plant Sci. 2021, 26, 3010. [Google Scholar] [CrossRef]
- Salgotra, R.K.; Sood, M.; Jasrotia, M. Underutilized Crops and Their Value Addition; Salgotra, R.K., Sood, M., Jasrotia, M., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2021; p. 11788. [Google Scholar] [CrossRef]
- Foyer, C.H.; Lam, H.M.; Nguyen, H.T.; Siddique, K.H.M.; Varshney, R.K.; Colmer, T.D.; Cowling, W.; Bramley, H.; Mori, T.A.; Hodgson, J.M.; et al. Neglecting legumes has compromised human health and sustainable food production. Nat. Plants 2016, 2, 16112. [Google Scholar] [CrossRef]
- Mikic, A.; Peric, V.; Dordevic, V.; Srebric, M.; Mihailovic, V. Anti-nutritional factors in some grain legumes. Biotechnol. Anim. Husb. 2009, 25, 1181–1188. [Google Scholar]
- Gnanasambandam, A.; Paull, J.; Torres, A.; Kaur, S.; Leonforte, T.; Li, H.; Materne, M. Impact of molecular technologies on faba bean (Vicia faba L.) breeding strategies. Agronomy 2012, 2, 132–166. [Google Scholar] [CrossRef]
- Saxena, R.K.; Rathore, A.; Bohra, A.; Yadav, P.; Das, R.R.; Khan, A.W.; Singh, V.K.; Chitikineni, A.; Singh, I.P.; Kumar, C.V.S.; et al. Development and application of high-density Axiom-Cajanus SNP array with 56 K SNPs to understand the genome architecture of released cultivars and founder genotypes. Plant Genome 2018, 11, 180005. [Google Scholar] [CrossRef]
- Thudi, M.; Bohra, A.; Nayak, S.N.; Varghese, N.; Shah, T.M.; Penmetsa, R.V.; Thirunavukkarasu, N.; Gudipati, S.; Gaur, P.M.; Kulwal, P.L.; et al. Novel SSR markers from BAC-end sequences, DArT arrays and a comprehensive genetic map with 1291 marker loci for chickpea (Cicer arietinum L.). PLoS ONE 2011, 6, e27275. [Google Scholar] [CrossRef]
- Afzal, M.; Alghamdi, S.S.; Nawaz, H.; Migdadi, H.H.; Altaf, M.; El-Harty, E.; Al-Fifi, S.A.; Sohaib, M. Genome-wide identification and expression analysis of CC-NB-ARC-LRR (NB-ARC) disease-resistant family members from soybean (Glycine max L.) reveal their response to biotic stress. J. King Saud Univ. 2022, 34, 1758. [Google Scholar] [CrossRef]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphism. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar]
- Williams, J.; Kubelik, A.; Livak, K.; Rafalski, J.; Tingey, S. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990, 18, 6531–6535. [Google Scholar] [CrossRef]
- Tautz, D. Hypervariability of simple sequences as a general source of polymorphic DNA markers. Nucleic Acids Res. 1989, 17, 6463–6471. [Google Scholar] [CrossRef]
- Konieczny, A.; Ausubel, F.M. A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J. 1993, 4, 403–410. [Google Scholar] [CrossRef]
- Paran, I.; Michelmore, R.W. Development of reliable PCR-based markers linked to downy mildew resistance genes in lettuce. Theor. Appl. Genet. 1993, 85, 985–993. [Google Scholar] [CrossRef]
- Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; van de Lee, T.; Hornes, M.; Frijters, A.; Pot, J.; Peleman, J.; Kuiper, M. AFLP: A new technique for DNA fingerprinting. Nucleic Acids Res. 1995, 23, 4407–4414. [Google Scholar] [CrossRef]
- Gupta, P.K.; Roy, J.K.; Prasad, M. Single nucleotide polymorphisms: A new paradigm for molecular marker technology and DNA polymorphism detection with emphasis on their use in plants. Curr. Sci. 2001, 80, 524–535. [Google Scholar]
- Jaccoud, D.; Peng, K.; Feinstein, D.; Kilian, A. Diversity arrays: A solid state technology for sequence information independent genotyping. Nucleic Acids Res. 2001, 29, E25. [Google Scholar] [CrossRef]
- Varshney, R.K.; Mohan, S.M.; Gaur, P.M.; Gangarao, N.V.P.R.; Pandey, M.K.; Bohra, A.; Sawargaonkar, S.L.; Chitikineni, A.; Kimurto, P.K.; Janila, P.; et al. Achievements and prospects of genomics-assisted breeding in three legume crops of the semi-arid tropics. Biotechnol. Adv. 2013, 31, 1120–1134. [Google Scholar] [CrossRef]
- Pandey, M.K.; Roorkiwal, M.; Singh, V.; Ramalingam, A.; Kudapa, H.; Thudi, M.; Chitikineni, A.; Rathore, A.; Varshney, R.K. Emerging genomic tools for legume breeding: Current status and future prospects. Front. Plant Sci. 2016, 7, 455. [Google Scholar] [CrossRef]
- Salgotra, R.K.; Stewart, C.N., Jr. Functional markers for precision plant breeding. Int. J. Mol. Sci. 2020, 21, 4792. [Google Scholar] [CrossRef]
- Salgotra, R.K.; Thompson, M.; Chauhan, B.S. Unravelling the genetic potential of untapped crop wild genetic resources for crop improvement. Conser. Genet. Resour. 2021, 14, 109–124. [Google Scholar] [CrossRef]
- Kumar, J.; Sen Gupta, D.; Baum, M.; Varshney, R.K.; Kumar, S. Genomics-assisted lentil breeding: Current status and future strategies. Legume Sci. 2021, 3, e71. [Google Scholar] [CrossRef]
- Pandey, M.K.; Monyo, E.; Ozias-Akins, P.; Liang, X.; Guimaraes, P.; Nigam, S.N.; Upadhyaya, H.D.; Janila, P.; Zhang, X.; Guo, B.; et al. Advances in Arachis genomics for peanut improvement. Biotechnol. Adv. 2012, 30, 639–651. [Google Scholar] [CrossRef]
- Thudi, M.; Gaur, P.M.; Krishnamurthy, L.; Mir, R.R.; Kudapa, H. Genomics-assisted breeding for drought tolerance in chickpea. Funct. Plant Biol. 2014, 41, 1178–1190. [Google Scholar] [CrossRef]
- Varshney, R.K. Exciting journey of 10 years from genomes to fields and markets: Some success stories of genomics- assisted breeding in chickpea, pigeonpea and groundnut. Plant Sci. 2016, 242, 98–107. [Google Scholar] [CrossRef]
- Varshney, R.K.; Terauchi, R.; McCouch, S.R. Harvesting the promising fruits of genomics: Applying genome sequencing technologies to crop breeding. PLoS Biol. 2014, 12, e1001883. [Google Scholar] [CrossRef]
- Kumar, J.; Gupta, D.S. Prospects of next generation sequencing in lentil breeding. Mol. Biol. Rep. 2020, 47, 9043–9053. [Google Scholar] [CrossRef] [PubMed]
- Bauchet, G.J.; Bett, K.E.; Cameron, C.T.; Campbell, J.D.; Cannon, E.K.; Cannon, S.B.; Carlson, J.W.; Chan, A.; Cleary, A.; Close, T.J.; et al. The future of legume genetic data resources: Challenges, opportunities, and priorities. Legume Sci. 2019, 1, e16. [Google Scholar] [CrossRef]
- Ma, Y.; Qin, F.; Tran, L.S. Contribution of genomics to gene discovery in plant abiotic stress responses. Mol. Plant 2012, 5, 1176–1178. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, X.; Wang, H.; Xin, H.; Yang, X.; Yan, J.; Li, J.; Tran, L.S.P.; Shinozaki, K.; Shinozaki, K.Y.; et al. Genome- wide analysis of ZmDREB genes and their association with natural variation in drought tolerance at seedling stage of Zea mays L. PLoS Genet. 2013, 9, e1003790. [Google Scholar] [CrossRef]
- Gonçalves-Vidigal, M.C.; Gilio, T.A.S.; Valentini, G.; Vaz-Bisneta, M.; Vidigal Filho, P.S.; Song, Q.; Oblessuc, P.R.; Melotto, M. New Andean source of resistance to anthracnose and angular leaf spot: Fine-mapping of disease-resistance genes in California Dark Red Kidney common bean cultivar. PLoS ONE 2020, 15, e0235215. [Google Scholar] [CrossRef]
- Dinesh, H.B.; Chandappa, L.; Vishwanath, K.P.; Singh, P.; Manjunatha, L.; Ambika, D.S.; Kumar, M.P.K. Genetic analysis and marker assisted backcrossing for transfer of mosaic virus resistance in cowpea [Vigna unguiculata (L.) Walp.]. Legume Res. 2018, 41, 663–668. [Google Scholar] [CrossRef]
- de Almeida, C.P.; de Carvalho Paulino, J.F.; Bonfante, G.F.J.; Perseguini, J.M.K.C.; Santos, I.L.; Gonçalves, J.G.R.; Patrício, F.R.A.; Taniguti, C.H.; Gesteira, G.S.; Garcia, A.A.F.; et al. Angular leaf spot resistance loci associated with different plant growth stages in common bean. Front. Plant Sci. 2021, 13, 647043. [Google Scholar] [CrossRef]
- Varshney, R.K.; Gaur, P.M.; Chamarthi, S.K.; Krishnamurthy, L.; Tripathi, S.; Kashiwagi, J.; Samineni, S.; Singh, V.K.; Thudi, M.; Jaganathan, D. Fast-track introgression of “QTL-hotspot” for root traits and other drought tolerance traits in JG 11, an elite and leading variety of chickpea. Plant Genome 2013, 6, 1–9. [Google Scholar] [CrossRef]
- Bernardo, R.; Charcosset, A. Usefulness of gene information in marker-assisted recurrent selection: A simulation appraisal. Crop Sci. 2006, 46, 614–621. [Google Scholar] [CrossRef]
- Zhao, Y.; Gowda, M.; Liu, W.; Würschum, T.; Maurer, H.P.; Longin, F.H.; Ranc, N.; Reif, J.C. Accuracy of genomic selection in European maize elite breeding populations. Theor. Appl. Genet. 2012, 124, 769–776. [Google Scholar] [CrossRef]
- Roorkiwal, M.; Rathore, A.; Das, R.R.; Singh, M.K.; Srinivasan, S.; Gaur, P.M.; Bharadwaj, C.; Tripathi, S.; Hickey, J.M.; Jannink, J.L.; et al. Towards deploying genomic selection in chickpea breeding. In Proceedings of the Interdrought IV Conference, Perth, Australia, 2–6 September 2013. [Google Scholar]
- Salgotra, R.K.; Zargar, S.M. (Eds.) Rediscovery of Genetic and Genomic Resources for Future Food Security; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Singh, A.K.; Prasanna, B.M. Molecular Mapping in Crop Plants: Development and Characterization of Mapping Populations. In Genetics, Genomics and Breeding of Vegetable Brassicas; Jan, S., Chittaranjan, K., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 1–8. [Google Scholar]
- Grewal, R.K.; Lulsdorf, M.; Croser, J.; Ochatt, S.; Vandenberg, A.; Warkentin, T.D. Doubled-haploid production in chickpea (Cicer arietinum L.): Role of stress treatments. Plant Cell Rep. 2009, 28, 1289–1299. [Google Scholar] [CrossRef]
- Hale, B.; Ferrie, A.M.R.; Chellamma, S.; Samuel, J.P.; Phillips, G.C. Androgenesis-based doubled haploidy: Past, present, and future perspectives. Front. Plant Sci. 2022, 12, 751230. [Google Scholar] [CrossRef]
- Sallam, A.; Martsch, R. Association mapping for frost tolerance using multi-parent advanced generation inter-cross (MAGIC) population in faba bean (Vicia faba L.). Genetica 2015, 143, 501–514. [Google Scholar] [CrossRef]
- Gaur, P.M.; Samineni, S.; Thudi, M.; Tripathi, S.; Sajja, S.B.; Jayalakshmi, V.; Mannur, D.M.; Vijayakumar, A.G.; Ganga Rao, N.V.P.R.; Ojiewo, C.O.; et al. Integrated breeding approaches for improving drought and heat adaptation in chickpea (Cicer arietinum L.). Plant. Breed. 2019, 138, 389–400. [Google Scholar] [CrossRef]
- Huynh, B.L.; Ehlers, J.D.; Huang, B.E.; Muñoz-Amatriaín, M.; Lonardi, S.; Santos, J.R.P.; Ndeve, A.; Batieno, B.J.; Boukar, O.; Cisse, N.; et al. A multi-parent advanced generation inter-cross (MAGIC) population for genetic analysis and improvement of cowpea (Vigna unguiculata L. Walp.). Plant. J. 2018, 93, 1129–1142. [Google Scholar] [CrossRef]
- Shivakumar, M.; Kumawat, G.; Gireesh, C.; Ramesh, S.V.; Husain, S.M. Identification of unique characteristics of deception from facial expression. Curr. Sci. 2018, 114, 901–906. [Google Scholar]
- Arrones, A.; Vilanova, S.; Plazas, M.; Mangino, G.; Pascual, L.; José Díez, M.; Prohens, J.; Gramazio, P. The dawn of the age of multi-parent MAGIC populations in plant breeding: Novel powerful next-generation resources for genetic analysis and selection of recombinant elite. Mater. Biol. 2020, 9, 229. [Google Scholar] [CrossRef]
- Bohra, A.; Pandey, M.K.; Jha, U.C.; Singh, B.; Singh, I.P.; Datta, D.; Chaturvedi, S.K.; Nadarajan, N.; Varshney, R.K. Genomics-assisted breeding in four major pulse crops of developing countries: Present status and prospects. Theor. Appl. Genet. 2014, 127, 1263–1291. [Google Scholar] [CrossRef]
- Dadu, R.H.R.; Bar, I.; Ford, R.; Sambasivam, P.; Croser, J.; Ribalta, F.; Kaur, S.; Sudheesh, S.; Gupta, D. Lens orientalis contributes quantitative trait loci and candidate genes associated with ascochyta blight resistance in lentil. Front. Plant Sci. 2021, 12, 703283. [Google Scholar] [CrossRef]
- Gupta, N.; Zargar, S.M.; Singh, R.; Nazir, M.; Mahajan, R.; Salgotra, R. Marker association study of yield attributing traits in common bean (Phaseolus vulgaris L.). Mol. Biol. Rep. 2020, 12, 1–15. [Google Scholar] [CrossRef]
- Mahajan, R.; Zargar, S.M.; Salgotra, R.K.; Singh, R.; Wani, A.A.; Nazir, M.; Sofi, P.A. Linkage disequilibrium based association mapping of micronutrients in common bean (Phaseolus vulgaris L.): A collection of Jammu & Kashmir, India. 3 Biotech 2017, 7, 295. [Google Scholar] [CrossRef]
- Aguilar-Benitez, D.; Casimiro-Soriguer, I.; Maalouf, F.; Torres, A.M. Linkage mapping and QTL analysis of flowering time in faba bean. Sci. Rep. 2021, 11, 13716. [Google Scholar] [CrossRef]
- Kahraman, A.; Demirel, U.; Ozden, M.; Muehlbauer, F.J. Mapping of QTLs for leaf area and the association with winter hardiness in fall-sown lentil. Afr. J. Biotechnol. 2010, 9, 8515–8519. [Google Scholar]
- He, Q.; Yang, H.; Xiang, S.; Wang, W.; Xing, G.; Zhao, T.; Gai, J. QTL mapping for the number of branches and pods using wild chromosome segment substitution lines in soybean [Glycine max (L.) Merr.]. Plant Genet. Resour. 2014, 12, 495. [Google Scholar] [CrossRef]
- Abdi, K.; Deokar, A.; Banniza, S.; Tom, W.; Bunyamin, T. QTL mapping of early flowering and resistance to ascochyta blight in chickpea (Cicer arietinum L.). Genome 2016, 9, 413–425. [Google Scholar] [CrossRef]
- Saxena, R.K.; Obala, J.; Sinjushin, A.; Kumar, C.V.S.; Saxena, K.B.; Varshney, R.K. Characterization and mapping of Dt1 locus which co-segregates with CcTFL1 for growth habit in pigeonpea. Theor. Appl. Genet. 2017, 130, 1773–1784. [Google Scholar] [CrossRef]
- Breseghello, F.; Sorrels, M.E. Association analysis as a strategy for improvement of qualitative traits in plants. Crop Sci. 2006, 46, 1323–1330. [Google Scholar] [CrossRef]
- Mahajan, R.; Zargar, S.M.; Singh, R.; Salgotra, R.K.; Sufia, F.; Sonah, H. Population structure analysis and selection of core set among common bean genotypes from Jammu and Kashmir, India. Appl. Biochem. Biotechnol. 2017, 182, 16–28. [Google Scholar] [CrossRef]
- Huang, C.; Nie, X.; Shen, C.; You, C.; Li, W.; Zhao, W.; Zhang, X.; Lin, Z. Population structure and genetic basis of the agronomic traits of upland cotton in China revealed by a genome-wide association study using high-density SNPs. Plant Biotechnol. J. 2017, 15, 1374–1386. [Google Scholar] [CrossRef]
- Rostoks, N.T.; Ramsay, L.; Mackenzie, K.; Cardle, L.; Bhat, P.R.; Roose, M.L.; Svensson, J.T.; Stein, N.; Varshney, R.K.; Marshall, D.F.; et al. Recent history of artificial outcrossing facilitates whole-genome association mapping in elite inbred crop varieties. Proc. Natl. Acad. Sci. USA 2006, 103, 18656–18661. [Google Scholar] [CrossRef]
- Binagwa, P.H.; Traore, S.M.; Egnin, M.; Bernard, G.C.; Ritte, I.; Mortley, D.; Kamfwa, K.; He, G.; Bonsi, C. Genome-wide identification of powdery mildew resistance in common bean (Phaseolus vulgaris L.). Front Genet. 2021, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- Nabi, A.; Lateef, I.; Nisa, Q.; Banoo, A.; Rasool, R.S.; Shah, M.D.; Ahmad, M.; Padder, B.A. Phaseolus vulgaris-Colletotrichum lindemuthianum pathosystem in the post-genomic era: An update. Curr. Microbiol. 2022, 79, 36. [Google Scholar] [CrossRef] [PubMed]
- Perseguini, J.M.; Oblessuc, P.R.; Rosa, J.R.; Gomes, K.A.; Chiorato, A.F.; Carbonell, S.A.; Garcia, A.A.; Vianello, R.P.; Benchimol-Reis, L.L. Genome-wide association studies of anthracnose and angular leaf spot resistance in common bean (Phaseolus vulgaris L.). PLoS ONE 2016, 11, e0150506. [Google Scholar] [CrossRef] [PubMed]
- Ambachew, D.; Blair, M.W. Genome wide association mapping of root traits in the Andean genepool of common bean (Phaseolus vulgaris L.) grown with and without aluminum toxicity. Front. Plant Sci. 2021, 25, 628687. [Google Scholar] [CrossRef]
- Aldemir, S.; Ateş, D.; Temel, H.Y.; Yağmur, B.; Alsaleh, A.; Kahriman, A.; Hakan, O.; Albert, V.; Muhammed Bahattin, T. QTLs for iron concentration in seeds of the cultivated lentil (Lens culinaris Medik.) via genotyping by sequencing. Turk. J. Agric. For. 2017, 41, 243–255. [Google Scholar] [CrossRef]
- Chen, Z.; Ly, V.J.; Ly, V.B.; Buitink, J.; Leprince, O.; Verdier, J. Genome-wide association studies of seed performance traits in response to heat stress in Medicago truncatula uncover miel1 as a regulator of seed germination plasticity. Front. Plant Sci. 2021, 12, 72. [Google Scholar] [CrossRef]
- Kang, Y.; Sakiroglu, M.; Krom, N.; Stanton-Geddes, J.; Wang, M.; Lee, Y.C.; Young, N.D.; Udvardi, M. Genome-wide association of drought-related and biomass traits with HapMap SNPs in Medicago truncatula. Plant Cell Environ. 2015, 38, 1997–2011. [Google Scholar] [CrossRef]
- Kang, Y.; Torres-Jerez, I.; An, Z.; Greve, V.; Huhman, D.; Krom, N.; Udvardi, M. Genome-wide association analysis of salinity responsive traits in Medicago truncatula. Plant Cell Environ. 2019, 42, 1513–1531. [Google Scholar] [CrossRef]
- Sokolkova, A.; Burlyaeva, M.; Valiannikova, T.; Cui, Y.; Vishnyakova, M.; Schafleitner, R.; Lee, C.R.; Ting, C.T.; Nair, R.M.; Nuzhdin, S.; et al. Genome-wide association study in accessions of the mini-core collection of mungbean (Vigna radiata) from the World Vegetable Gene Bank (Taiwan). BMC Plant Biol. 2020, 20, 363. [Google Scholar] [CrossRef]
- Xu, P.; Wu, X.; Muñoz-Amatriaín, M.; Wang, B.; Wu, X.; Hu, Y.; Huynh, B.L.; Close, T.J.; Roberts, P.A.; Zhou, W.; et al. Genomic regions, cellular components and gene regulatory basis underlying pod length variations in cowpea (V. unguiculata L. Walp). Plant Biotechnol. J. 2017, 15, 547–557. [Google Scholar] [CrossRef]
- Hwang, E.U.; Song, Q.; Jia, G.; Specht, J.E.; Hyten, D.L.; Costa, G.L.; Cregan, P.B. A genome wide association study of seed protein and oil content in soybean. BMC Genom. 2014, 15, 1. [Google Scholar] [CrossRef]
- Raggi, L.; Caproni, L.; Carboni, A.; Negri, V. Genome-wide association study reveals candidate genes for flowering time variation in common bean (Phaseolus vulgaris L.). Front. Plant Sci. 2019, 10, 962. [Google Scholar] [CrossRef]
- Cichy, K.A.; Wiesinger, J.A.; Mendoza, F.A. Genetic diversity and genome-wide association analysis of cooking time in dry bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2015, 128, 1555–1567. [Google Scholar] [CrossRef]
- Yan, L.; Hofmann, N.; Li, S.; Ferreira, M.E.; Song, B.; Jiang, G.; Ren, S.; Quigley, C.; Fickus, E.; Cregan, P.; et al. Identification of QTL with large effect on seed weight in a selective population of soybean with genome-wide association and fixation index analyses. BMC Genom. 2017, 18, 529. [Google Scholar] [CrossRef]
- Rajendran, K.; Coyne, C.; Zheng, P.; Saha, G.; Main, D.; Amin, N.; Ma, Y.; Kisha, T.; Bett, K.E.; McGee, R.J.; et al. Genetic diversity and GWAS of agronomic traits using an ICARDA lentil (Lens culinaris Medik.) reference plus collection. Plant Genet. Resour. 2021, 19, 279–288. [Google Scholar] [CrossRef]
- Nkhata, W.; Shimelis, H.; Melis, R.; Chirwa, R.; Mzengeza, T.; Mathew, I.; Shayanowako, A. Genome-wide association analysis of bean fly resistance and agro-morphological traits in common bean. PLoS ONE 2021, 16, e0250729. [Google Scholar] [CrossRef]
- Das, S.; Singh, M.; Srivastava, R.; Bajaj, D.; Saxena, M.S.; Rana, J.C.; Bansal, K.C.; Tyagi, A.K.; Parida, S.K. MQTL-seq delineates functionally relevant candidate gene harbouring a major QTL regulating pod number in chickpea. DNA Res. 2016, 23, 53–65. [Google Scholar] [CrossRef]
- Doddamani, D.; Katta, M.A.; Khan, A.W.; Agarwal, G.; Shah, T.M.; Varshney, R.K. CicArMiSatDB: The chickpea microsatellite database. BMC Bioinformat. 2014, 15, 212. [Google Scholar] [CrossRef]
- Doddamani, D.; Khan, A.W.; Katta, M.A.V.S.K.; Agarwal, G.; Thudi, M.; Ruperao, P.; Edwards, D.; Varshney, R.K. CicArVarDB: SNP and InDel database for advancing genetics research and breeding applications in chickpea. Database 2015, 2015, bav078. [Google Scholar] [CrossRef][Green Version]
- Gayali, S.; Acharya, S.; Lande, N.V.; Pandey, A.; Chakraborty, S.; Chakraborty, N. CicerTransDB 1.0: A resource for expression and functional study of chickpea transcription factors. BMC Plant Biol. 2016, 16, 169. [Google Scholar] [CrossRef]
- Sarika, V.A.; Iquebal, M.A.; Rai, A.; Kumar, D. PIPEMicroDB: Microsatellite database and primer generation tool for pigeonpea genome. Database 2013, 2013, bas054. [Google Scholar] [CrossRef] [PubMed]
- Muranaka, S.; Shono, M.; Myoda, T.; Takeuchi, J.; Franco, J.; Nakazawa, Y.; Boukar, O.; Takaji, H. Genetic diversity of physical, nutritional and functional properties of cowpea grain and relationships among the traits. Plant Genet. Resour. 2016, 14, 67–76. [Google Scholar] [CrossRef]
- Grant, D.; Nelson, R.T.; Cannon, S.B.; Shoemaker, R.C. SoyBase, the USDA-ARS soybean genetics and genomics database. Nucl. Acids Res. 2010, 38, D843–D846. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.K.; Agarwal, G.; Kale, V.; Clevenger, J.; Nayak, S.N.; Sriswathi, M.; Chitikineni, A.; Chavarro, C.; Chen, X.; Upadhyaya, H.D.; et al. Development and evaluation of a high density genotyping ‘AxiomArachis’ array with 58K SNPs for accelerating genetics and breeding in groundnut. Sci. Rep. 2017, 7, 40577. [Google Scholar] [CrossRef] [PubMed]
- Roorkiwal, M.; Jain, A.; Kale, S.M.; Doddamani, D.; Chitikineni, A.; Thudi, M.; Varshney, R.K. Development and evaluation of high-density Axiom® Cicer SNP Array for high-resolution genetic mapping and breeding applications in chickpea. Plant Biotechnol. J. 2018, 16, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Munoz, N.; Liu, A.; Kan, L.; Li, M.W.; Lam, H.M. Potential uses of wild germplasms of grain legumes for crop improvement. Int. J. Mol. Sci. 2017, 18, 328. [Google Scholar] [CrossRef]
- Blair, M.W.; Cortés, A.J.; Farmer, A.D.; Huang, W.; Ambachew, D.; Penmetsa, R.V.; Carrasquilla-Garcia, N.; Assefa, T.; Cannon, S.B. Uneven recombination rate and linkage disequilibrium across a reference SNP map for common bean (Phaseolus vulgaris L.). PLoS ONE 2018, 13, e0189597. [Google Scholar] [CrossRef]
- Song, Q.L.; Yan, C.; Quigley, E.; Fickus, H.; Wei, L.; Chen, L.; Dong, F.; Araya, S.; Liu, J.; Hyten, D.; et al. Soybean BARCSoySNP6K: An assay for soybean genetics and breeding research. Plant J. 2020, 104, 800–811. [Google Scholar] [CrossRef]
- Janila, P.; Nigam, S.N.; Pandey, M.K.; Nagesh, P.; Varshney, R. Groundnut improvement: Use of genetic and genomic tools. Front. Plant Sci. 2013, 4, 23. [Google Scholar] [CrossRef]
- Varshney, R.K.; Close, T.J.; Singh, N.K.; Hoisington, D.A.; Cook, D.R. Orphan legume crops enter the genomics era! Curr. Opin. Plant Biol. 2009, 12, 202–210. [Google Scholar] [CrossRef]
- Guimaraes, P.M.; Garsmeur, O.; Proite, K.; Leal-Bertioli, S.C.; Seijo, G.; Chaine, C.; Bertioli, D.J.; D’Hont, A. BAC libraries construction from the ancestral diploid genomes of the allotetraploid cultivated peanut. BMC Plant Biol. 2008, 8, 14. [Google Scholar] [CrossRef]
- Kilian, A. DArT-Based Whole Genome Profiling and Novel Information Technologies in Support System of Modern Breeding of Groundnut. In Proceedings of the 3rd International Conference for Arachis through Genomics and Biotechnology (AAGB), Hyderabad, India, 4–8 November 2008. [Google Scholar]
- Varshney, R.K.; Thudi, M.; May, G.D.; Jackson, S.A. Legume genomics and breeding. Plant Breed. Rev. 2010, 33, 257–304. [Google Scholar]
- Knoll, J.E.; Ramos, M.L.; Zeng, Y.; Holbrook, C.C.; Chow, M.; Chen, S.; Maleki, S.; Bhattacharya, A.; Ozias-Akins, P. TILLING for allergen reduction and improvement of quality traits in peanut (Arachis hypogaea L.). BMC Plant Biol. 2011, 11, 81. [Google Scholar] [CrossRef]
- Arumuganathan, K.; Earle, E.D. Nuclear DNA content of some important plant species. Plant Mol. Biol. Rep. 1991, 9, 208–218. [Google Scholar] [CrossRef]
- Millan, T.; Winter, P.; Jungling, R.; Gil, J.; Rubio, J.; Cho, S.; Cobos, M.J.; Iruela, M.; Rajesh, P.N.; Tekeoglu, M.; et al. A consensus genetic map of chickpea (Cicer arietinum L.) based on 10 mapping populations. Euphytica 2010, 175, 175–189. [Google Scholar] [CrossRef]
- Upadhyaya, H.D.; Thudi, M.; Dronavalli, N.; Gujaria, N.; Singh, S.; Sharma, S.; Varshney, R.K. Genomic tools and germplasm diversity for chickpea improvement. Plant Genet. Resour. 2011, 9, 45–58. [Google Scholar] [CrossRef]
- Varshney, R.K.; Hoisington, D.A.; Upadhyaya, H.D.; Gaur, P.M.; Nigam, S.N.; Saxena, K.; Vadez, V.; Sethy, N.K.; Bhatia, S.; Aruna, R.; et al. Molecular Genetics and Breeding of Grain Legume Crops for the Semi-Arid Tropics. In Genomics-Assisted Crop Improvement, 2. Genomics Applications in Crops; Varshney, R.K., Tuberosa, R., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 207–241. [Google Scholar]
- Jain, A.; Roorkiwal, M.; Kale, S.; Garg, V.; Yadala, R.; Varshney, R.K. InDel markers: An extended marker resource for molecular breeding in chickpea. PLoS ONE 2019, 14, e0213999. [Google Scholar] [CrossRef]
- Varshney, R.K.; Kudapa, H.; Roorkiwal, M.; Mahendar, T.; Pandey, M.K.; Saxena, R.K.; Chamarthi, S.K.; Mohan, S.M.; Mallikarjuna, N.; Upadhyaya, H.; et al. Advances in genetics and molecular breeding of three legume crops of semi-arid tropics using next-generation sequencing and high-throughput genotyping technologies. J. Biosci. 2012, 37, 811–820. [Google Scholar] [CrossRef]
- Argout, X.; Salse, J.; Aury, J.M.; Guiltinan, M.J.; Droc, G.; Gouzy, J.; Allegre, M.; Chaparro, C.; Legavre, T.; Maximova, S.N.; et al. The genome of Theobroma cacao. Nat. Genet. 2011, 43, 101–118. [Google Scholar] [CrossRef]
- Yang, S.Y.; Saxena, R.K.; Kulwal, P.A.; Ash, G.J.; Dubey, A.; Harper, D.I.; Upadhyaya, H.D.; Gothalwal, R.; Kilian, A.; Varshney, R.K. The first genetic map of pigeonpea based on diversity arrays technology (DArT) markers. J. Genet. 2011, 90, 103–109. [Google Scholar] [CrossRef]
- Pedrosa-Harand, A.; Kami, J.; Gepts, P.; Geffroy, V.; Schweizer, D. Cytogenetic mapping of common bean chromosomes reveals a less compartmentalized small-genome plant species. Chrom. Res. 2009, 17, 405–417. [Google Scholar] [CrossRef]
- O’Rourke, J.A.; Iniguez, L.P.; Fu, F.; Bucciarelli, B.; Miller, S.S.; Jackson, S.A.; McClean, P.E.; Li, J.; Dai, X.; Zhao, P.X.; et al. An RNA-Seq based gene expression atlas of the common bean. BMC Genom. 2014, 15, 866. [Google Scholar] [CrossRef]
- Song, Q.; Jia, G.; Hyten, D.L.; Jenkins, J.; Hwang, E.Y.; Schroeder, S.G.; Osorno, J.M.; Schmutz, J.; Jackson, S.A.; McClean, P.E.; et al. SNP assay development for linkage map construction, anchoring whole-genome sequence, and other genetic and genomic applications in common bean. G3 Genes|Genomes|Genet. 2015, 5, 2285–2290. [Google Scholar] [CrossRef]
- Singh, R.J.; Hymowitz, T. The genomic relationship between Glycine max L. Merr. and G. soja Sieb. and Zucc. as revealed by pachytene chromosome analysis. Theor. Appl. Genet. 1988, 76, 705–711. [Google Scholar] [CrossRef]
- Song, Q.; Hyten, D.L.; Jia, G.; Quigley, C.V.; Fickus, E.W.; Nelson, R.L.; Cregan, P.B. Development and evaluation of SoySNP50K, a high-density genotyping array for soybean. PLoS ONE 2013, 8, e54985. [Google Scholar] [CrossRef]
- Jeong, N.; Kim, K.S.; Jeong, S.; Kim, J.Y.; Park, S.K.; Lee, J.S.; Jeong, S.C.; Kang, S.T.; Ha, B.K.; Kim, D.; et al. Korean soybean core collection: Genotypic and phenotypic diversity population structure and genome-wide association study. PLoS ONE 2019, 14, e0224074. [Google Scholar] [CrossRef]
- Wang, J.; Chu, S.; Zhang, H.; Zhu, Y.; Cheng, H.; Yu, D. Development and application of a novel genome-wide SNP array reveals domestication history in soybean. Sci. Rep. 2016, 6, 20728. [Google Scholar] [CrossRef]
- Kole, C.; Muthamilarasan, M.; Henry, R.; Edwards, D.; Sharma, R.; Abberton, M.; Batley, J.; Bentley, A.; Blakeney, M.; Bryant, J.; et al. Application of genomics-assisted breeding for generation of climate resilient crops: Progress and prospects. Front. Plant Sci. 2015, 6, 563. [Google Scholar] [CrossRef]
- Chandra, A.K.; Kumar, A.; Bharati, A.; Joshi, R.; Agrawal, A.; Kumar, S. Microbial-assisted and genomic-assisted breeding: A two-way approach for the improvement of nutritional quality traits in agricultural crops. 3 Biotech 2020, 10, 2. [Google Scholar] [CrossRef]
- Dikshit, H.; Singh, A.; Singh, D.; Aski, M.; Jain, N.; Hegde, V.S.; Basandrai, A.; Basandrai, D.; Sharma, T. Tagging and mapping of SSR marker for rust resistance gene in lentil (Lens culinaris Medikus subsp. culinaris). Indian J. Exp. Biol. 2016, 54, 394–399. [Google Scholar]
- Polanco, C.; Saenz de Miera, L.E.; Gonzalez, A.I.; Garcia, P.; Fratini, R.; Vaquero, F.; Vences, F.J.; Pérez de la Vega, M. Construction of a highdensity interspecific (Lens culinaris x L. odemensis) genetic map based on functional markers for mapping morphological and agronomical traits, and QTLs affecting resistance to ascochyta in lentil. PLoS ONE 2019, 14, e0214409. [Google Scholar]
- Singh, G.; Singh, I.; Taggar, G.K.; Rani, U.; Sharma, P.; Gupta, M.; Singh, S. Introgression of productivity enhancing traits, resistance to pod borer and Phytopthora stem blight from Cajanus scarabaeoides to cultivated pigeonpea. Physiol. Mol. Biol. Plants 2020, 26, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Fredua-Agyeman, R.; Hwang, S.F.; Chang, K.F.; Conner, R.L.; McLaren, D.L.; Strelkov, S.E. Mapping QTL associated with partial resistance to Aphanomyces root rot in pea (Pisum sativum L.) using a 13.2 K SNP array and SSR markers. Theor. Appl. Genet. 2021, 134, 2965–2990. [Google Scholar] [CrossRef] [PubMed]
- Meuwissen, T. Genomic selection: Marker assisted selection on a genome wide scale. J. Anim. Breed. Genet. 2007, 124, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Goddard, M.E.; Hayes, B.J. Genomic selection. J. Anim. Breed. Genet. 2007, 124, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, X.; Fu, J.; Wang, H.; Wang, J.; Huang, C.; Prasanna, B.M.; Olsen, M.S.; Wang, G.; Zhang, A. Enhancing genetic gain through genomic selection: From livestock to plants. Plant Comm. 2020, 1, 100005. [Google Scholar] [CrossRef] [PubMed]
- Goddard, M. Genomic selection: Prediction of accuracy and maximisation of long term response. Genetica 2009, 136, 245–257. [Google Scholar] [CrossRef]
- Crossa, J.; Perez-Rodriguez, P.; Cuevas, J.; Montesinos-Lopez, O.; Jarquin, D.; de los Campos, G.; Burgueño, J.; Juan, M.; Camacho, G.; Pérez-Elizalde, S.; et al. Genomic selection in plant breeding: Methods, models, and perspectives. Trends Plant Sci. 2017, 22, 961–975. [Google Scholar] [CrossRef]
- Langridge, P.; Reynolds, M.P. Genomic tools to assist breeding for drought tolerance. Curr. Opin. Biotechnol. 2015, 32, 130–135. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Hu, Z.; Xu, C. Genomic selection methods for crop improvement: Current status and prospects. Crop J. 2008, 6, 330–340. [Google Scholar] [CrossRef]
- Jain, A.; Roorkiwal, M.; Pandey, M.K.; Varshney, R.K. Current Status and Prospects of Genomic Selection in Legumes. In Genomic Selection for Crop Improvement: New Molecular Breeding Strategies for Crop. Improvement; Springer: Berlin/Heidelberg, Germany, 2017; pp. 131–147. ISBN 978-3-319-63170-7. [Google Scholar]
- Batieno, B.J.; Danquah, E.; Tignegre, J.B.; Huynh, B.L.; Drabo, I.; Close, T.; Ofori, K.; Roberts, P.; Ouedraogo, T.J. Application of marker-assisted backcrossing to improve cowpea (Vigna unguiculata L. Walp) for drought tolerance. J. Plant. Breed. Crop Sci. 2016, 8, 273–286. [Google Scholar]
- Schneider, K.A.; Rosales-Serna, R.; Ibarra-Perez, F.; Cazares-Enriquez, B.; Acosta-Gallegos, J.A.; Ramirez-Vallejo, P.; Wassimi, N.; Kelly, J.D. Improving common bean performance under drought stress. Crop Sci. 1997, 37, 43–50. [Google Scholar] [CrossRef]
- Zargar, S.M.; Nazir, M.; Gupta, M.; Farhat, S.; Mahajan, R.; Salgotra, R.K.; Mir, R.A. Molecular marker assisted approaches (MMAA) for enhancing low water stress tolerance in common bean: An update. Mol. Plant Breed. 2014, 5, 1–12. [Google Scholar] [CrossRef][Green Version]
- Diaz, M.L.; Ricaurte, J.; Tovar, E.; Cajiao, C.E.; Teran, H.; Grajales, M.; Polanıa, J.; Rao, I.; Beebe, S.; Raatz, B. QTL analyses for tolerance to abiotic stresses in a common bean (Phaseolus vulgaris L.) population. PLoS ONE 2018, 13, e0202342. [Google Scholar] [CrossRef]
- Vasconcellos, R.C.; Oraguzie, O.B.; Soler, A.; Arkwazee, H.; Myers, J.R.; Ferreira, J.J.; Song, Q.; McClean, P.; Miklas, P.N. Meta-QTL for resistance to white mold in common bean. PLoS ONE 2017, 12, e0171685. [Google Scholar] [CrossRef]
- Garzon, L.N.; Ligarreto, G.A.; Blair, M.W. Molecular marker-assisted backcrossing of anthracnose resistance into andean climbing beans (Phaseolus vulgaris L.). Crop Sci. 2008, 48, 562–570. [Google Scholar] [CrossRef]
- Blair, M.W.; Muñoz, C.; Buendía, H.F.; Flower, J.; Bueno, J.M.; Cardona, C. Genetic mapping of microsatellite markers around the arcelin bruchid resistance locus in common bean. Theor. Appl. Genet. 2010, 121, 393–402. [Google Scholar] [CrossRef][Green Version]
- Singh, D.; Singh, C.K.; Tomar, R.S.S.; Sharma, S.; Karwa, S.; Pal, M.; Singh, V.; Sanwal, S.K.; Sharma, P.C. Genetics and molecular mapping for salinity stress tolerance at seedling stage in lentil (Lens culinaris Medik). Crop Sci. 2020, 60, 1254–1266. [Google Scholar] [CrossRef]
- Tayeh, N.; Aluome, C.; Falque, M.; Jacquin, F.; Klein, A.; Chauveau, A.; Bérard, A.; Houtin, H.; Rond, A.; Kreplak, J.; et al. Development of two major resources for pea genomics: The GenoPea 13.2K SNP Array and a high-density, high-resolution consensus genetic map. Plant J. 2015, 84, 1257–1273. [Google Scholar] [CrossRef]
- Katoch, V.; Sharma, S.; Pathania, S.; Banayal, D.; Sharma, S.; Rathour, R. Molecular mapping of pea powdery mildew resistance gene er2 to pea linkage group III. Mol. Breed. 2010, 25, 229–237. [Google Scholar] [CrossRef]
- Cobos, M.J.; Satovic, Z.; Rubiales, D.; Fondevilla, S. Er3 gene, conferring resistance to powdery mildew in pea, is located in pea LGIV. Euphytica 2018, 214, 203. [Google Scholar] [CrossRef]
- Leonforte, A.; Forster, J.; Redden, R.; Nicolas, M.; Salisbury, P. Sources of high tolerance to salinity in pea (Pisum sativum L.). Euphytica 2013, 189, 203–216. [Google Scholar] [CrossRef]
- Kim, M.; Hyten, D.L.; Niblack, T.L.; Diers, B.W. Stacking resistance alleles from wild and domestic soybean sources improves soybean cyst nematode resistance. Crop Sci. 2011, 51, 934–943. [Google Scholar] [CrossRef]
- Arahana, V.S.; Graef, G.L.; Specht, J.E.; Steadman, J.R.; Eskridge, K.M. Identification of QTLs for resistance to in soybean. Crop Sci. 2001, 41, 180–188. [Google Scholar] [CrossRef]
- Bachman, M.S.; Tamulonis, J.P.; Nickell, C.D.; Bent, A.F. Molecular markers linked to brown stem rot resistance genes, Rbs1 and Rbs2, in soybean. Crop Sci. 2001, 41, 527–535. [Google Scholar] [CrossRef]
- Yang, W.; Weaver, D.; Nielsen, B.; Qiu, J.; Salamini, F. Molecular mapping of a new gene for resistance to frogeye leaf spot of soya bean in ‘Peking’. Plant Breed. 2001, 120, 73–78. [Google Scholar] [CrossRef]
- Ramalingam, J.; Alagarasan, G.; Savitha, P.; Lydia, K.; Pothiraj, G.; Vijayakumar, E.; Sudhagar, R.; Singh, A.; Vedna, K.; Vanniarajan, C. Improved host-plant resistance to Phytophthora rot and powdery mildew in soybean (Glycine max (L.) Merr.). Sci. Rep. 2020, 10, 13928. [Google Scholar] [CrossRef]
- Wang, D.G.; Lin, Z.; Kai, L.; Ying, M.; Wang, L.Q.; Yang, Y.Q.; Yang, Y.H.; Zhi, H.J. Marker-assisted pyramiding of soybean resistance genes RSC4, RSC8, and RSC14Q to soybean mosaic virus. J. Integr. Agric. 2017, 16, 2413–2420. [Google Scholar] [CrossRef]
- Kumar, V.; Rani, A.; Rawal, R. Deployment of gene specific marker in development of kunitz trypsin inhibitor free soybean genotypes. Indian J. Exp. Biol. 2013, 51, 1125–1129. [Google Scholar]
- Kumar, V.; Rani, A.; Rawal, R.; Mourya, V. Marker assisted accelerated introgression of null allele of kunitz trypsin inhibitor in soybean. Breed. Sci. 2015, 65, 447–452. [Google Scholar] [CrossRef]
- Maranna, S.; Verma, K.; Talukdar, A.; Lal, S.K.; Kumar, A.; Mukherjee, K. Introgression of null allele of kunitz trypsin inhibitor through marker-assisted backcross breeding in soybean (Glycine max L. Merr.). BMC Genet. 2016, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Rawal, R.; Kumar, V.; Rani, A.; Gokhale, S.M. Genetic elimination of off-flavour generating lipoxygenase-2 gene of soybean through marker assisted backcrossing and its effect on seed longevity. Plant Breed. Biotech. 2020, 8, 163–173. [Google Scholar] [CrossRef]
- Varshney, R.K.; Pandey, M.K.; Janila, P.; Nigam, S.N.; Sudini, H.; Gowda, M.V.; Sriswathi, M.; Radhakrishnan, T.; Manohar, S.S.; Nagesh, P. Marker-assisted introgression of a QTL region to improve rust resistance in three elite and popular varieties of peanut (Arachis hypogaea L.). Theor. Appl. Genet. 2014, 127, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Gill, R.; Clevenger, J.; Timper, P.; Holbrook, C.C.; Ozias-Akins, P. Identification of rare recombinants leads to tightly linked markers for nematode resistance in peanut. Peanut Sci. 2016, 43, 88–93. [Google Scholar] [CrossRef][Green Version]
- Chu, Y.; Wu, C.L.; Holbrook, C.C.; Tillman, B.L.; Person, G.; Ozias-Akins, P. Marker-assisted selection to pyramid nematode resistance and the high oleic trait in peanut. Plant Genome 2011, 4, 110–117. [Google Scholar] [CrossRef]
- Janila, P.; Pandey, M.K.; Manohar, S.S.; Variath, M.T.; Nallathambi, P.; Nadaf, H.L.; Sudini, H.; Varshney, R.K. Foliar fungal disease-resistant introgression lines of groundnut (Arachis hypogaea L.) record higher pod and haulm yield in multilocation testing. Plant Breed. 2016, 135, 355–366. [Google Scholar] [CrossRef]
- Shasidhar, Y.; Variath, M.T.; Vishwakarma, M.K.; Manohar, S.S.; Gangurde, S.S.; Sriswathi, M.; Sudini, H.K.; Dobariya, K.L.; Bera, S.K.; Radhakrishnan, T.; et al. Improvement of three Indian popular groundnut varieties for foliar disease resistance and high oleic acid using SSR markers and SNP array in marker-assisted backcrossing. Crop. J. 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Varshney, R.K.; Mohan, S.M.; Gaur, P.M.; Chamarthi, S.K.; Singh, V.K.; Srinivasan, S.; Swapna, N.; Sharma, M.; Pande, S.; Singh, S.; et al. Marker-assisted backcrossing to introgress resistance to fusarium wilt race 1 and ascochyta blight in C 214, an elite cultivar of chickpea. Plant Genome 2014, 7, 35. [Google Scholar] [CrossRef]
- Boukar, O.; Fatokun, C.A.; Huynh, B.L.; Roberts, P.A.; Close, T.J. Genomic tools in cowpea breeding programs: Status and perspectives. Front. Plant Sci. 2016, 7, 757. [Google Scholar] [CrossRef]
- Souleymane, A.; Aken’Ova, M.; Fatokun, C.; Alabi, O. Screening for resistance to cowpea aphid (Aphis craccivora koch) in wild and cultivated cowpea (Vigna unguiculata L. Walp.) accessions. Int. J. Sci. Environ. Technol. 2013, 2, 611–621. [Google Scholar]
- Kongjaimun, A.; Kaga, A.; Tomooka, N.; Somta, P.; Shimizu, T.; Shu, Y.; Isemura, T.; Vaughan, D.A.; Srinives, P. An SSR-based linkage map of yardlong bean (Vigna unguiculata (L.) Walp. subsp. unguiculata Sesquipedalis group) and QTL analysis of pod length. Genome 2012, 55, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Kongjaimun, A.; Somta, P.; Tomooka, N.; Kaga, A.; Vaughan, D.A.; Srinives, P. QTL mapping of pod tenderness and total soluble solid in yardlong bean [Vigna unguiculata (L.) Walp. subsp. unguiculata cv.-gr. sesquipedalis]. Euphytica 2013, 189, 217–223. [Google Scholar] [CrossRef]
- Andargie, M.; Knudsen, J.T.; Pasquet, R.S.; Gowda, B.S.; Muluvi, G.M.; Timko, M.P. Mapping of quantitative trait loci for floral scent compounds in cowpea (Vigna unguiculata L.). Plant Breed. 2014, 133, 92–100. [Google Scholar] [CrossRef]
- Ravelombola, W.; Shi, A.; Huynh, B.L. Loci discovery, network-guided approach, and genomic prediction for drought tolerance index in a multi-parent advanced generation intercross (MAGIC) cowpea population. Hort. Res. 2021, 8, 24. [Google Scholar] [CrossRef]
- Paudel, D.; Dareus, R.; Rosenwald, J.; Muñoz-Amatriaín, M.; Rios, E.F. Genome-wide association study reveals candidate genes for flowering time in cowpea (Vigna unguiculata [L.] Walp.). Front. Genet. 2021, 12, 667038. [Google Scholar] [CrossRef]
- Wu, X.; Cortés, A.J.; Blair, M.W. Genetic differentiation of grain, fodder and pod vegetable type cowpeas (Vigna unguiculata L.) identified through single nucleotide polymorphisms from genotyping-by-sequencing. Mol. Hort. 2022, 8. [Google Scholar] [CrossRef]
- Zhang, D.; Cheng, H.; Wang, H.; Zhang, H.Y.; Liu, C.Y.; Yu, D.Y. Identification of genomic regions determining flower and pod numbers development in soybean (Glycine max L.). J. Genet. Genom. 2010, 37, 545–556. [Google Scholar] [CrossRef]
- Kerem, Z.; Lev-Yadun, S.; Gopher, A.; Weinberg, P.; Abbo, S. Chickpea domestication in the neolithic levant through the nutritional perspective. J. Archaeol. Sci. 2010, 34, 1289–1293. [Google Scholar] [CrossRef]
- Warkentin, T.; Banniza, S.; Vandenberg, A. CDC frontier kabuli chickpea. Can. J. Plant Sci. 2005, 85, 909–910. [Google Scholar] [CrossRef]
- Moreno, M.T.; Cubero, J. Variation in Cicer arietinum L. Euphytica 1978, 27, 465–485. [Google Scholar] [CrossRef]
- Jain, M.; Misra, G.; Patel, R.K.; Priya, P.; Jhanwar, S.; Khan, A.W.; Shah, N.; Singh, V.K.; Garg, R.; Jeena, G. A draft genome sequence of the pulse crop chickpea (Cicer arietinum L.). Plant J. 2013, 74, 715–729. [Google Scholar] [CrossRef]
- Bajaj, D.; Das, S.; Upadhyaya, H.D.; Ranjan, R.; Badoni, S.; Kumar, V.; Tripathi, S.; Gowda, C.L.; Sharma, S.; Singh, S. A genome-wide combinatorial strategy dissects complex genetic architecture of seed coat color in chickpec. Front. Plant Sci. 2015, 6, 979. [Google Scholar] [CrossRef]
- Upadhyaya, H.D.; Bajaj, D.; Das, S.; Saxena, M.S.; Badoni, S.; Kumar, V.; Tripathi, S.; Gowda, C.; Sharma, S.; Tyagi, A.K. A genome-scale integrated approach aids in genetic dissection of complex flowering time trait in chickpea. Plant Mol. Biol. 2015, 89, 403–420. [Google Scholar] [CrossRef]
- Srivastava, R.; Singh, M.; Bajaj, D.; Parida, S.K. A high-resolution InDel (insertion–deletion) markers-anchored consensus genetic map identifies major QTLs governing pod number and seed yield in chickpea. Front. Plant Sci. 2016, 7, 1362. [Google Scholar] [CrossRef]
- Gupta, C.; Gupta, M.; Gupta, S.; Salgotra, R.K. Screening of common bean (Phaseolus vulgaris L.) germplasm against Colletotrichum lindemuthianum inciting bean anthracnose. Res. J. Biotech. 2022, 17, 13–18. [Google Scholar] [CrossRef]
- Assefa, E. Application of biotechnological tools for common bean (Phaseolus vulgaris L.) improvement. Zenodo 2020, 1–24. [Google Scholar] [CrossRef]
- Vlasova, A.; Capella-Gutiérrez, S.; Rendón-Anaya, M.; Hernández-Oñate, M.; Minoche, A.E.; Erb, I.; Camara, F.; Prieto-Barja, P.; Corvelo, A.; Sanseverino, W.; et al. Genome and transcriptome analysis of the Mesoamerican common bean and the role of gene duplications in establishing tissue and temporal specialization of genes. Genome Biol. 2016, 17, 32. [Google Scholar] [CrossRef]
- Thudi, M.; Palakurthi, R.; Schnable, J.C.; Chitikineni, A.; Dreisigacker, S.; Mace, E.; Srivastava, R.K.; Satyavathi, C.T.; Odeny, D.; Tiwari, V.K.; et al. Genomic resources in plant breeding for sustainable agriculture. J. Plant Physiol. 2021, 257, 153351. [Google Scholar] [CrossRef]
- Blair, M.W.; Astudillo, C.; Rengifo, J.; Beebe, S.E.; Graham, R. QTL analyses for seed iron and zinc concentrations in an intra-genepool population of andean common beans (Phaseolus vulgaris L.). Theor. Appl. Genet. 2011, 122, 511–521. [Google Scholar] [CrossRef]
- Miklas, P.N.; Kelly, J.D.; Singh, S.P. Registration of anthracnose resistant pinto bean germplasm line USPT-ANT-1. Crop Sci. 2003, 43, 1889–1990. [Google Scholar] [CrossRef]
- Miklas, P.N.; Kelly, J.D.; Beebe, S.E.; Blair, M.W. Common bean breeding for resistance against biotic and abiotic stresses: From classical to MAS breeding. Euphytica 2006, 147, 105–131. [Google Scholar] [CrossRef]
- Obala, J.; Mukankusi, C.; Rubaihayo, P.R.; Gibson, P.; Edema, R. Improvement of resistance to Fusarium root rot through gene pyramiding in common bean. Afr. Crop Sci. J. 2012, 20, 1–13. [Google Scholar]
- Nzungize, J.; Gepts, P.; Buruchara, R.A.; Male, A.; Ragama, P.; Busogoro, J.P.; Baudoin, J.P. Introgression of pythium root rot resistance gene into Rwandan susceptible common bean cultivars. Afr. J. Plant Sci. 2011, 5, 193–200. [Google Scholar]
- Uwera, A.; Rusagara, J.; Msolla, S.; Musoni, A.; Assefa, T. Molecular marker-assisted backcrossing of anthracnose resistance genes into common beans (Phaseolus vulgaris L.) varieties. Am. J. Plant Sci. 2021, 12, 771–781. [Google Scholar] [CrossRef]
- Keller, B.; Ariza-Suarez, D.; de la Hoz, J.; Aparicio, J.S.; Portilla-Benavides, A.E.; Buendia, H.F.; Mayor, V.M.; Studer, B.; Raatz, B. Genomic prediction of agronomic traits in common bean (Phaseolus vulgaris L.) under environmental stress. Front. Plant Sci. 2020, 11, 1. [Google Scholar] [CrossRef]
- Pandey, M.K.; Pandey, A.K.; Kumar, R.; Nwosu, C.V.; Guo, B.; Wright, G.C.; Bhat, R.S.; Chen, X.; Bera, S.K.; Yuan, M.; et al. Translational genomics for achieving higher genetic gains in groundnut. Theor. Appl. Genet. 2020, 133, 1679–1702. [Google Scholar] [CrossRef]
- Akohoue, F.; Achigan-Dako, E.G.; Sneller, C.; Van Deynze, A.; and Sibiya, J. Genetic diversity, SNP-trait associations and genomic selection accuracy in a west African collection of Kersting’s groundnut [Macrotyloma geocarpum(Harms) Maréchal & Baudet]. PLoS ONE 2020, 15, e0234769. [Google Scholar] [CrossRef]
- Pazhamala, L.; Saxena, R.K.; Singh, V.K.; Sameerkumar, C.V.; Kumar, V.; Sinha, P.; Patel, K.; Obala, J.; Kaoneka, S.R.; Tongoona, P.; et al. Genomics-assisted breeding for boosting crop improvement in pigeonpea (Cajanus cajan). Front. Plant Sci. 2015, 17, 50. [Google Scholar] [CrossRef]
- Saxena, R.K.; Hake, A.; Hingane, A.J.; Kumar, C.V.S.; Bohra, A.; Sonnappa, M.; Rathore, A.; Kumar, A.V.; Mishra, A.; Tikle, A.N.; et al. Translational pigeonpea genomics consortium for accelerating genetic gains in pigeonpea (Cajanus cajan L.). Agronomy 2020, 10, 1289. [Google Scholar] [CrossRef]
- Bohra, A.; Mir, R.R.; Jha, R.; Maurya, A.K.; Varshney, R.K. Advances in Genomics and Molecular Breeding for Legume Improvement. In Advancement in Crop Improvement Techniques; Narendra, T., Renu, T., Nishat, P., Shabnam, K.S., Eds.; Woodhead Publishing: Kidlington, UK, 2020; pp. 129–139. [Google Scholar] [CrossRef]
- Tullu, A.; Bett, K.; Banniza, S.; Vail, S.; Vandenberg, A. Widening the genetic base of cultivated lentil through hybridization of Lens culinaris “Eston” and L. ervoides accession IG72815. Can. J. Plant Sci. 2013, 93, 1037–1047. [Google Scholar] [CrossRef]
- Matthew, R.S.; Jennifer, D.; Muhammad, J.; Shimna, S.; Sara, B.; John, F.W.; Kaur, S. Molecular breeding for ascochyta blight resistance in lentil: Current progress and future directions. Front. Plant Sci. 2017, 8, 1136. [Google Scholar]
- Sharpe, A.G.; Ramsay, L.; Sanderson, L.A.; Fedoruk, M.J.; Clarke, W.E.; Li, R.; Kagale, S.; Vijayan, P.; Vandenberg, A.; Bett, K.E. Ancient orphan crop joins modern era: Gene-based SNP discovery and mapping in lentil. BMC Genom. 2013, 14, 192. [Google Scholar] [CrossRef]
- Duran, Y.; Fratini, R.; Garcia, P.; Pérez de la Vega, M. An intersubspecific genetic map of Lens. Theor. Appl. Genet. 2004, 108, 1265–1273. [Google Scholar] [CrossRef]
- Hamwieh, A.; Udupa, S.; Choumane, W.; Sarker, A.; Dreyer, F.; Jung, C.; Baum, M. A genetic linkage map of Lens sp. based on microsatellite and AFLP markers and the localization of fusarium vascular wilt resistance. Theor. Appl. Genet. 2005, 110, 669–677. [Google Scholar] [CrossRef]
- Aldemir, S.B.; Sever, T.; Ates, D.; Yagmur, B.; Kaya, H.B.; Temel, H.Y.; Kahriman, A.; Ozkan, H.; Tanyolac, M.B. QTL Mapping of Genes Controlling Fe Uptake in Lentil (Lens culinaris L.) Seed Using Recombinant Inbred Lines. In Proceedings of the Plant and Animal Genome Conference XXII P3360, San Diego, CA, USA, 8 April 2014. [Google Scholar]
- Boutet, G.; Alves Carvalho, S.; Falque, M.; Peterlongo, P.; Lhuillier, E.; Bouchez, O.; Lavaud, C.; Pilet-Nayel, M.L.; Riviere, N.; Baranger, A. SNP discovery and genetic mapping using genotyping by sequencing of whole genome genomic DNA from a pea RIL population. BMC Genom. 2016, 17, 121. [Google Scholar] [CrossRef]
- Haggard, J.E. Characterization of physiological resistance to white mold and search for molecular markers linked to resistance via advanced backcross QTL analysis in an interspecific cross between Phaseolus coccineus and P. vulgaris. HortSci 2007, 41, 973. [Google Scholar] [CrossRef]
- Fondevilla, S.; Satovic, Z.; Rubiales, D.; Moreno, M.T.; Torres, A.M. Mapping of quantitative trait loci for resistance to Mycosphaerella pinodes in Pisum sativum subsp. syriacum. Mol. Breed. 2008, 21, 439–454. [Google Scholar] [CrossRef]
- Devi, J.; Mishra, G.P.; Sagar, V.; Kaswan, V.; Dubey, R.K.; Singh, P.M.; Sharma, S.K.; Behera, T.K. Gene-based resistance to Erysiphe species causing powdery mildew disease in peas (Pisum sativum L.). Genes 2022, 13, 316. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Nazzicari, N.; Pecetti, L.; Romani, M.; Russi, L. Pea genomic selection for Italian environments. BMC Genom. 2019, 20, 603. [Google Scholar] [CrossRef]
- Baldermann, S.; Blagojevic, L.; Frede, K.; Klopsch, R.; Neugart, S.; Neumann, A.; Ngwene, B.; Norkeweit, J.; Schröter, D.; Schröter, A.; et al. Are neglected plants the food for the future? Crit. Rev. Plant Sci. 2016, 35, 106–119. [Google Scholar] [CrossRef]
- Popoola, J.; Ojuederie, O.; Omonhinmin, C.; Adegbite, A. Neglected and underutilized legume crops: Improvement and future prospects. In Recent Advances in Grain Crops Research; Shah, F., Khan, Z., Iqbal, A., Turan, M., Olgun, M., Eds.; IntechOpen: Rijeka, Croatia, 2019; pp. 1–23. [Google Scholar]
- Rathi, D.; Chakraborty, S.; Chakraborty, N. Grasspea, a critical recruit among neglected and underutilized legumes, for tapping genomic resources. Curr. Plant Biol. 2021, 26, 100200. [Google Scholar] [CrossRef]
- Plewinski, P.; Książkiewicz, M.; Rychel-Bielska, S.; Rudy, E.; Wolko, B. Candidate domestication-related genes revealed by expression quantitative trait loci mapping of narrow-leafed lupin (Lupinus angustifolius L.). Int. J. Mol. Sci. 2019, 20, 5670. [Google Scholar] [CrossRef]
- Reddy, V.R.; Das, S.; Dikshit, H.K.; Mishra, G.P.; Aski, M.S.; Singh, A.; Tripathi, K.; Pandey, R.; Bansal, R.; Pal Singh, M.; et al. Genetic dissection of phosphorous uptake and utilization efficiency traits using GWAS in mungbean. Agronomy 2021, 11, 1401. [Google Scholar] [CrossRef]
- Rychel, S.; Książkiewicz, M. Development of gene-based molecular markers tagging low alkaloid pauper locus in white lupin (Lupinus albus L.). J. Appl. Genet. 2019, 60, 269–281. [Google Scholar] [CrossRef]
- Somta, P.; Jomsangawong, A.; Yundaeng, C.; Yuan, X.; Chen, J.; Tomooka, N.; Chen, X. Genetic dissection of Azuki bean weevil (Callosobruchus chinensis L.) resistance in moth bean (Vigna aconitifolia [Jaqc.] Maréchal). Genes 2018, 9, 555. [Google Scholar] [CrossRef]
| Crop | Genomic Resources | References | |
|---|---|---|---|
| Groundnut (Arachis hypogaea) | Species | Arachis hypogaea (Tetraploid) Arachis duranensis and Arachis ipaensis (Diploid) | [94] |
| Genome size | 2890 Mbp (Tetraploid) 1260 Mbp (Diploid) | [95] | |
| Genetic maps | Diploid (AA)-3, Diploid (BB)-2, Tetraploid-13 maps, and one reference consensus map | [29] | |
| BAC libraries | ca. 5.3×–Diploid (BB); ca. 7.4×–diploid (AA) | [96] | |
| DArT clones | ca. 15,000 | [97,98] | |
| SNPs array | 2000 SNPs, 58 K Affymetrix Axiom | [29,89] | |
| TILLING population | 3400 mutant M2 lines | [99] | |
| Database | PeanutMap, PeanutBase | [82] | |
| Chickpea (Cicer arietinum) | Species | Diploid | [100] |
| Genome size | 740 Mbp | [95] | |
| Genetic maps | 24 (15 inter-specific & 9 intra-specific) | [101,102,103] | |
| BAC libraries | 10× | [14] | |
| DArT clones | 5397 | [14] | |
| SNPs array | >9000 50 K Affymetrix Axiom | [90] | |
| InDel markers | 231,658 InDels | [104] | |
| Physical maps | BAC/BIBAC-based, BAC-based | [24] | |
| Database | CicerTransDB, Chickpea ISM-ILP, Marker Database, Integrated Chickpea Transcriptome, Database (CTDB), CicArVarDB, CicArMiSatDB | [83,84,85] | |
| Number of genes | 28,269 | [24] | |
| Number of ESTs | 46,064 | [24] | |
| Pigeonpea (Cajanus cajan) | Species | Diploid | [105] |
| Genome size | 833.07 Mbp | [95] | |
| Genetic maps | Reference genetic map, six intra-specific maps, one consensus map and DArT based maternal and paternal maps | [106,107] | |
| BAC libraries | 11× | [1] | |
| DArT clones | 15,360 | [99,107] | |
| SNPs array | >10,000 50 K Affymetrix Axiom | [13] | |
| TILLING population | ca.5000 mutant lines | [99] | |
| Database | PpTFDB, Pipemicrodb | [86] | |
| Number of genes | 48,680 | [105] | |
| Number of ESTs | 25,640 | [105] | |
| Cowpea (Vigna unguiculata) | Species | Diploid | [100] |
| Genome size | 613 Mbp | [95] | |
| Database | CGKB, EDITS | [87] | |
| SNP array | 51 K Illumina Infinium | [91] | |
| Common bean (Phaseolus vulgaris) | Species | Diploid | [108] |
| Genome size | 578 Mbp | [95] | |
| Database | PhaseolusGenes, PvGEA | [109] | |
| SNP array | 768 K Illumina Goldengate assay 6 K Illumina Infinium BeadChip | [92,110] | |
| Soybean (Glycine max) | Species | Diploid | [111] |
| Genome size | 950 Mbp | [95] | |
| Database | SoyBase, SoyGD | [88] | |
| SNP array | 50 K Illumina Infinium BeadChip 6 K, Illumina Infinium 180 K Affymetrix Axiom 355 K Affymetrix Axiom | [93,112,113,114] | |
| Crop | Molecular Breeding Approaches | Trait(s) Improved | Reference |
|---|---|---|---|
| Cowpea | MABC | Mosaic virus (CpMV) resistant | [38] |
| MABC | Root-knot nematode Resistance | [129] | |
| Cowpea KASP genotyping platform | Background selection | [129] | |
| Common bean | QTL mapping (RIL population) | Improved drought adaptation | [130,131,132] |
| Fine-mapping | Resistance against angular leaf spot | [37,39] | |
| Meta-QTL | Resistance against white mold | [133] | |
| MABC | Anthracnose resistance | [134] | |
| QTL mapping | Bruchid and virus resistance | [135] | |
| Lentil | QTL mapping | Ascochyta blight resistance | [118] |
| QTL mapping | Rust resistance | [117] | |
| QTL mapping | Salt tolerance | [136] | |
| Pea | QTL mapping with 13.2 K SNP array | Resistance against Aphanomyces root rot | [120] |
| 13.2K SNP array | days to flowering and 1000-seed weight | [137] | |
| QTL mapping | Resistance against powdery mildew | [138,139] | |
| QTL mapping | Salt tolerance | [140] | |
| Soybean | MAS and MABC | Several soybean cyst nematodes and multiple disease-resistant genotypes | [141] |
| QTL mapping | Resistance to leaf rust | [142] | |
| QTL mapping | Black pod-of-staff | [143] | |
| QTL mapping | Resistance to stain frogeye | [144] | |
| MABB | Powdery mildew diseases resistance | [145] | |
| MABC | Soybean mosaic virus (SMV) resistance | [146] | |
| MABC | Free kunitz trypsin inhibitor | [147,148,149] | |
| MABC | Eliminate lipoxygenase-2, | [150] | |
| Groundnut | MABC | Introgression lines showing higher yield and increased rust resistance | [151] |
| MABC | Resistance to nematode | [152,153] | |
| MABC SSR markers and SNP array | Enhanced oleic acid | [154,155] | |
| Chickpea | MABC | Resistance to fusarium wilt | [24,156] |
| MABC | Resistance to ascochyta blight | [156] | |
| MABC | Drought tolerance | [40] | |
| MABC | Eliminate lipoxygenase-2, | [150] | |
| 3000 DArT-Seq markers | Breeding values of traits 100 grain weight and seed yield per plant | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgotra, R.K.; Stewart, C.N., Jr. Genetic Augmentation of Legume Crops Using Genomic Resources and Genotyping Platforms for Nutritional Food Security. Plants 2022, 11, 1866. https://doi.org/10.3390/plants11141866
Salgotra RK, Stewart CN Jr. Genetic Augmentation of Legume Crops Using Genomic Resources and Genotyping Platforms for Nutritional Food Security. Plants. 2022; 11(14):1866. https://doi.org/10.3390/plants11141866
Chicago/Turabian StyleSalgotra, Romesh K., and Charles Neal Stewart, Jr. 2022. "Genetic Augmentation of Legume Crops Using Genomic Resources and Genotyping Platforms for Nutritional Food Security" Plants 11, no. 14: 1866. https://doi.org/10.3390/plants11141866
APA StyleSalgotra, R. K., & Stewart, C. N., Jr. (2022). Genetic Augmentation of Legume Crops Using Genomic Resources and Genotyping Platforms for Nutritional Food Security. Plants, 11(14), 1866. https://doi.org/10.3390/plants11141866







