Effects of Supplementation of Branched-Chain Amino Acids to Reduced-Protein Diet on Skeletal Muscle Protein Synthesis and Degradation in the Fed and Fasted States in a Piglet Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
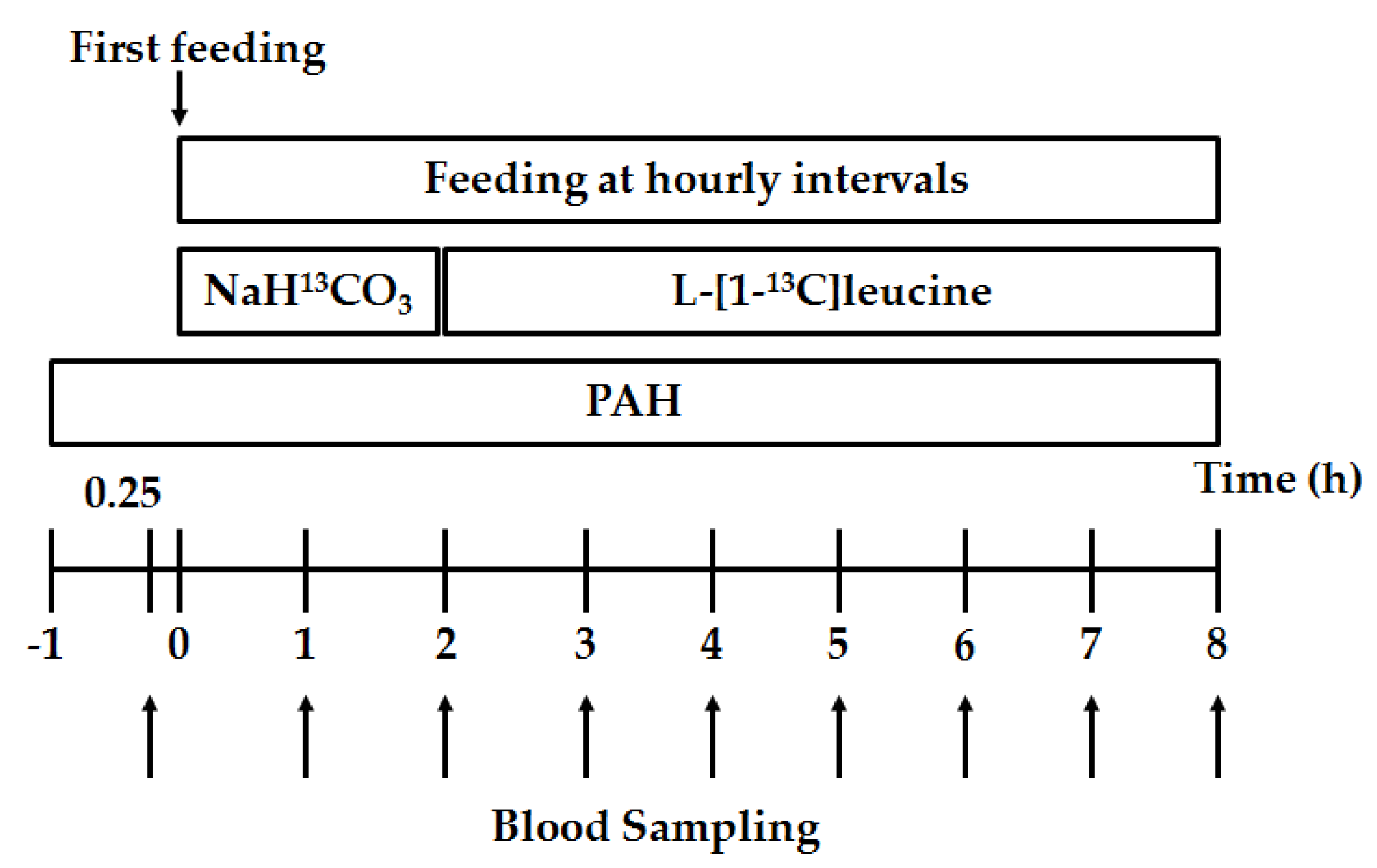
2.2. Tracer Infusion and Blood Sampling Protocols
2.3. Cross-Sectional Area Determination
2.4. Western Blotting
2.5. Plasma Concentrations of Insulin, AA, PAH and KIC
2.6. Analysis of Stable Isotope Tracer Enrichment
2.7. Calculations
2.8. Statistical Analysis
3. Results
3.1. Skeletal Muscle Mass and Cross-Sectional Area of LD Muscle Fibers
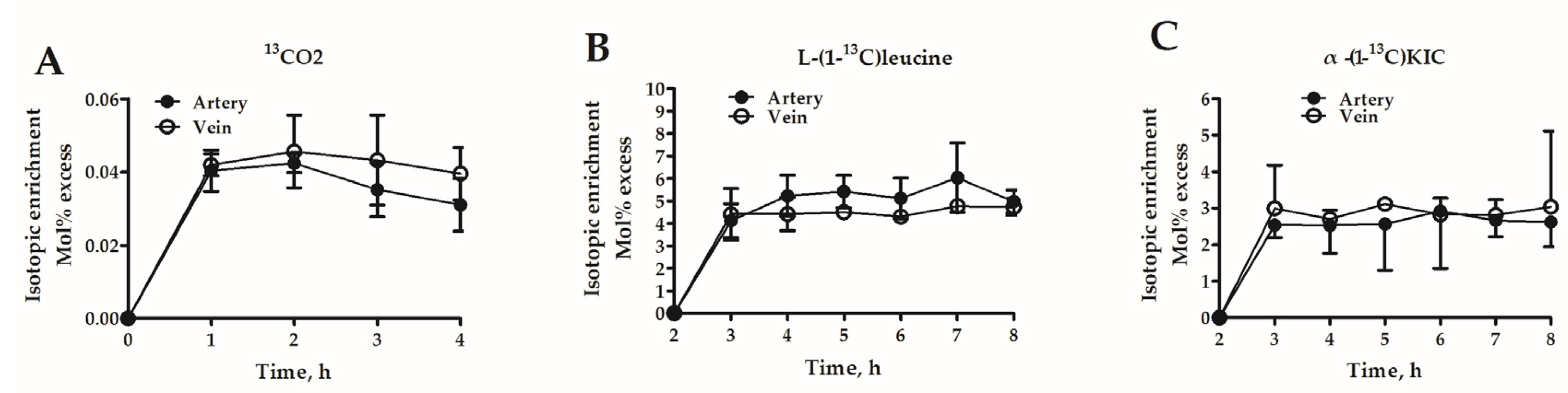
3.2. Plasma Concentrations and Isotopic Enrichment of Leucine and Its Metabolites
3.3. Leucine Kinetics across Hindlimb Muscle
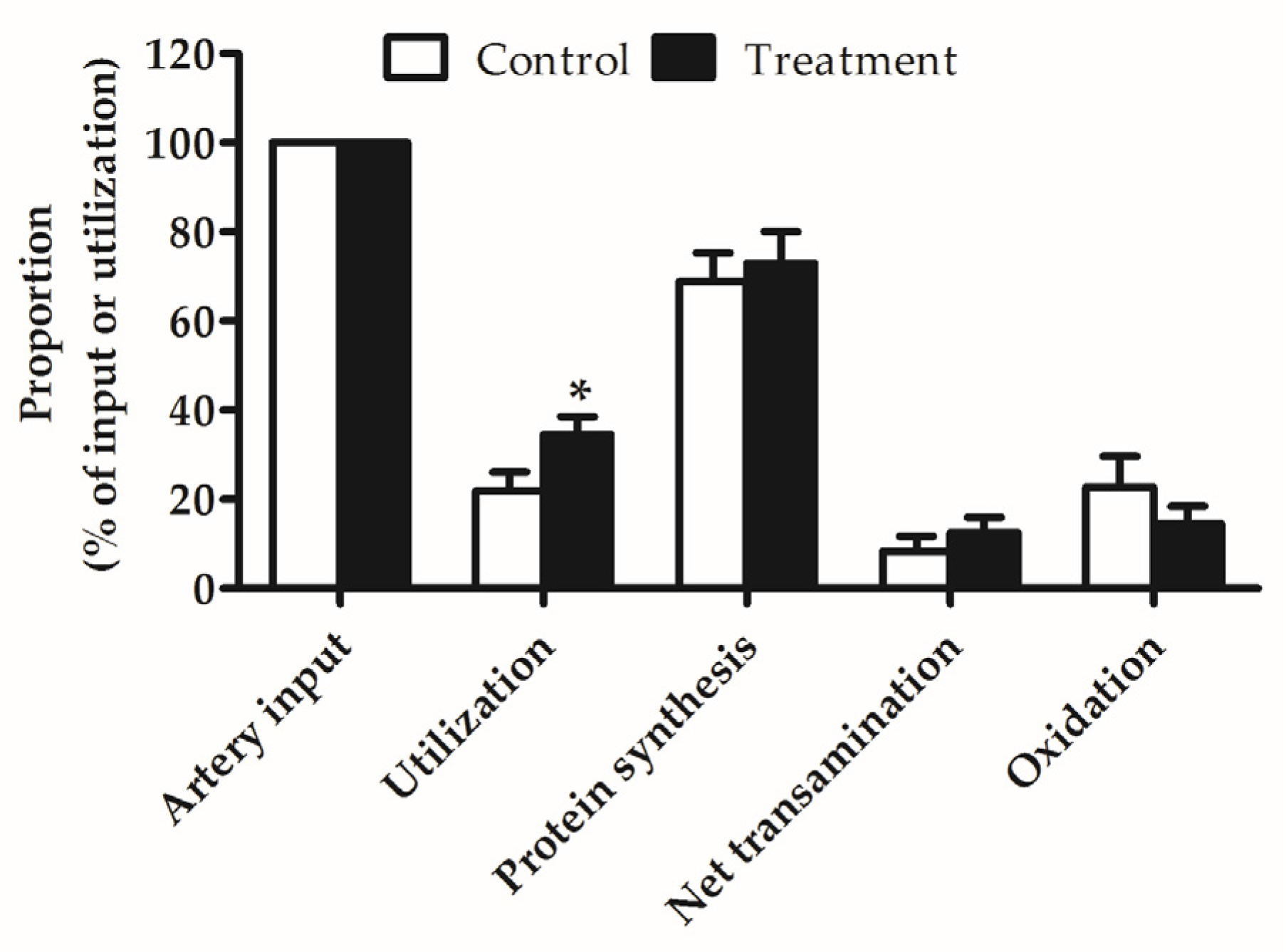
3.4. Leucine Metabolic Fate in Hindlimb Muscle
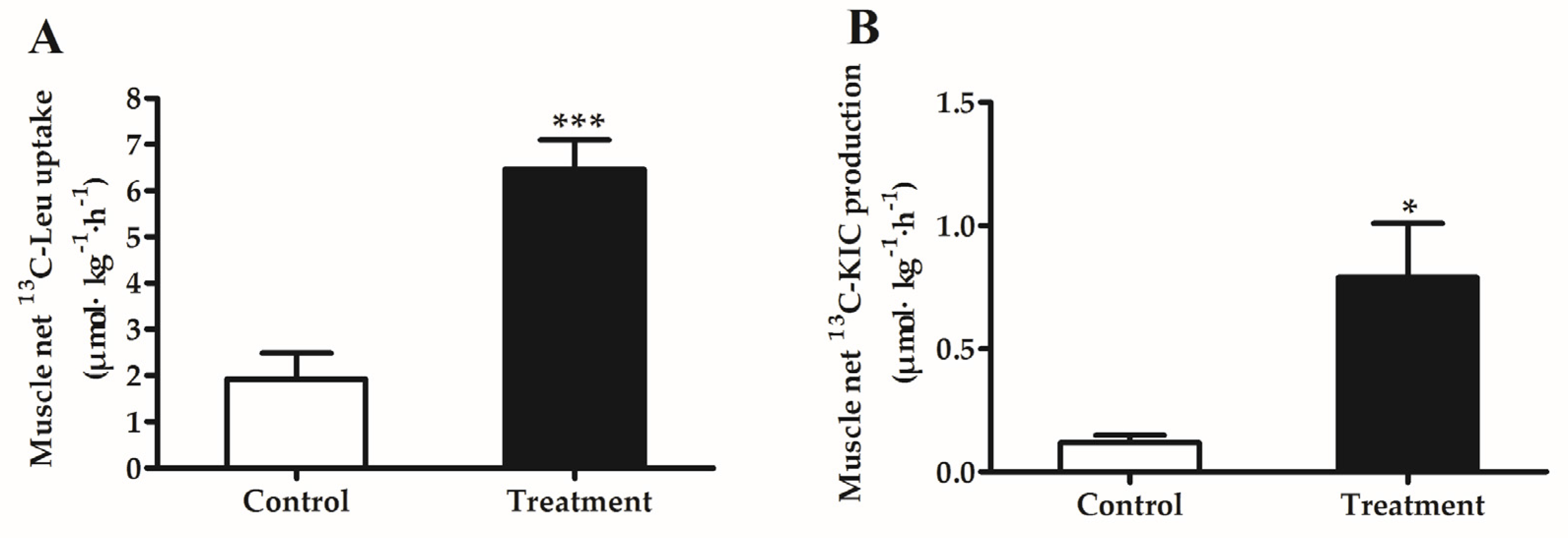
3.5. Muscle Net Uptake of [1-13C]Leucine and Production of [1-13C]KIC
3.6. Correlations
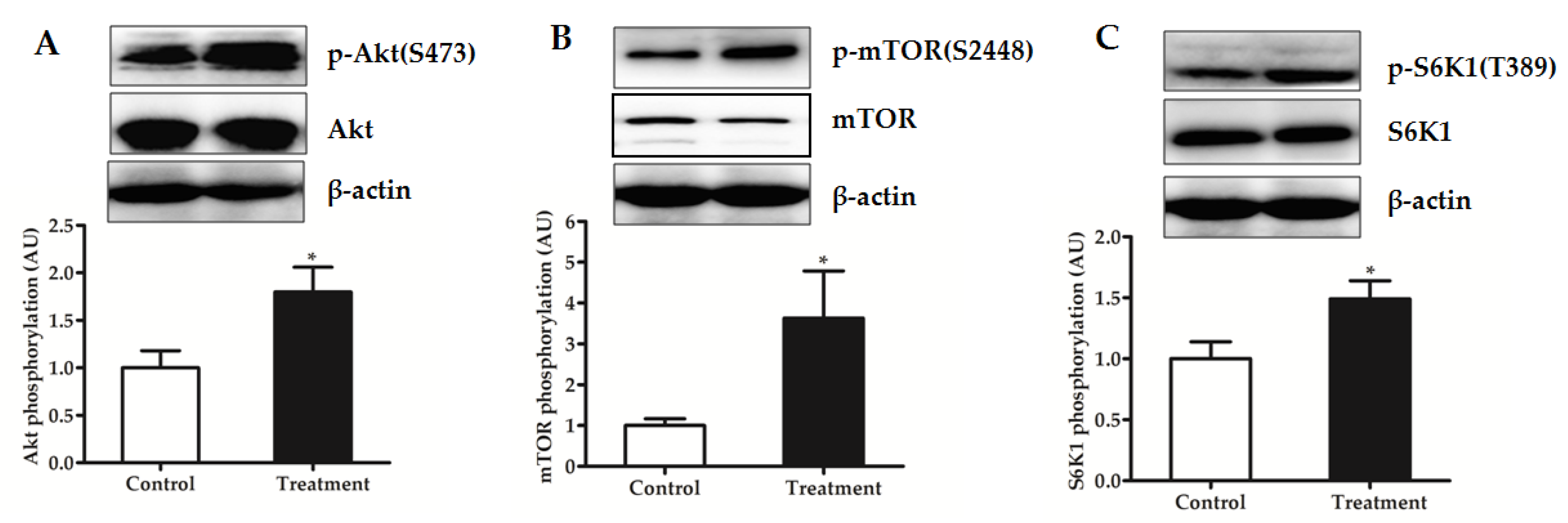
3.7. Protein Translation Initiation and Degradation Signaling of LD Muscle in the Fasted State
4. Discussion
4.1. BCAA Supplementation Increases Skeletal Muscle Mass in Piglets
4.2. BCAA Supplementation Increases Protein Synthesis, Protein Degradation and Conversion of Leucine to KIC in Skeletal Muscle of Piglets in the Fed State
4.3. BCAA Supplementation Promotes Protein Synthesis Signaling and Inhibits Degradation Signaling in Skeletal Muscle of Piglets in the Fasted State
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AA | Amino acids |
| BCAA | Branched-chain AA |
| KIC | α-ketoisocaproate |
| HMB | β-hydroxy-β-methylbutyrate |
| CP | Crude protein |
| SID | Standardized ileal digestible |
| LD | Longissimus dorsi |
| BW | Body weights |
| PAH | p-amino hippurate |
| CSA | Cross-sectional areas |
| IRMS | Isotope ratio mass spectrometry |
| MPE | Mole percent excess |
| mTOR | Mammalian target of rapamycin |
| S6K1 | Ribosomal protein S6 kinase 1 |
| Akt | Protein kinase B |
| LC3 | Microtubule-associated protein 1 light chain 3 |
| NE | Net energy |
Appendix A
Equation (A3) − (Equation (A2) − Equation (A5))
References
- Escobar, J.; Frank, J.W.; Suryawan, A.; Nguyen, H.V.; Kimball, S.R.; Jefferson, L.S.; Davis, T.A. Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E914–E921. [Google Scholar] [CrossRef] [PubMed]
- O'Connor, P.M.; Bush, J.A.; Suryawan, A.; Nguyen, H.V.; Davis, T.A. Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E110–E119. [Google Scholar] [CrossRef] [PubMed]
- Murgas Torrazza, R.; Suryawan, A.; Gazzaneo, M.C.; Orellana, R.A.; Frank, J.W.; Nguyen, H.V.; Fiorotto, M.L.; El-Kadi, S.; Davis, T.A. Leucine supplementation of a low-protein meal increases skeletal muscle and visceral tissue protein synthesis in neonatal pigs by stimulating mTOR-dependent translation initiation. J. Nutr. 2010, 140, 2145–2152. [Google Scholar] [CrossRef] [PubMed]
- Suryawan, A.; Torrazza, R.M.; Gazzaneo, M.C.; Orellana, R.A.; Fiorotto, M.L.; El-Kadi, S.W.; Srivastava, N.; Nguyen, H.V.; Davis, T.A. Enteral leucine supplementation increases protein synthesis in skeletal and cardiac muscles and visceral tissues of neonatal pigs through mTORC1-dependent pathways. Pediatr. Res. 2012, 71, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Yao, K.; Liu, Z.; Gong, M.; Ruan, Z.; Deng, D.; Tan, B.; Liu, Z.; Wu, G. Supplementing L-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 2010, 39, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Manjarín, R.; Columbus, D.A.; Suryawan, A.; Nguyen, H.V.; Hernandez-García, A.D.; Hoang, N.-M.; Fiorotto, M.L.; Davis, T. Leucine supplementation of a chronically restricted protein and energy diet enhances mTOR pathway activation but not muscle protein synthesis in neonatal pigs. Amino Acids 2016, 48, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wei, H.; Cheng, C.; Xiang, Q.; Pang, J.; Peng, J. Supplementation of branched-chain amino acids to a reduced-protein diet improves growth performance in piglets: Involvement of increased feed intake and direct muscle growth-promoting effect. Br. J. Nutr. 2016, 115, 2236–2245. [Google Scholar] [CrossRef] [PubMed]
- Atherton, P.J.; Etheridge, T.; Watt, P.W.; Wilkinson, D.; Selby, A.; Rankin, D.; Smith, K.; Rennie, M.J. Muscle full effect after oral protein: Time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am. J. Clin. Nutr. 2010, 92, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M. Signaling in muscle atrophy and hypertrophy. Physiology 2008, 23, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Burrin, D.G.; Fiorotto, M.L.; Nguyen, H.V. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7-than in 26-day-old pigs. Am. J. Physiol. Endocrinol. Metab. 1996, 270, E802–E809. [Google Scholar]
- Thivierge, M.C.; Bush, J.A.; Suryawan, A.; Nguyen, H.V.; Orellana, R.A.; Burrin, D.G.; Jahoor, F.; Davis, T.A. Whole-body and hindlimb protein breakdown are differentially altered by feeding in neonatal piglets. J. Nutr. 2005, 135, 1430–1437. [Google Scholar] [PubMed]
- Deutz, N.E.; Wolfe, R.R. Is there a maximal anabolic response to protein intake with a meal? Clin. Nutr. 2013, 32, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nicholatos, J.; Dreier, J.R.; Ricoult, S.J.; Widenmaier, S.B.; Hotamisligil, G.S.; Kwiatkowski, D.J.; Manning, B.D. Coordinated regulation of protein synthesis and degradation by mTORC1. Nature 2014, 513, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Lecker, S.H.; Jagoe, R.T.; Gilbert, A.; Gomes, M.; Baracos, V.; Bailey, J.; Price, S.R.; Mitch, W.E.; Goldberg, A.L. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004, 18, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Ishida, A.; Yamazaki, M.; Abe, H. Leucine suppresses myofibrillar proteolysis by down-regulating ubiquitin–proteasome pathway in chick skeletal muscles. Biochem. Biophys. Res. Commun. 2005, 336, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Yakabe, Y.; Ishida, A.; Yamazaki, M.; Abe, H. Suppression of myofibrillar proteolysis in chick skeletal muscles by α-ketoisocaproate. Amino Acids 2007, 33, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Tischler, M.E.; Desautels, M.; Goldberg, A. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle. J. Biol. Chem. 1982, 257, 1613–1621. [Google Scholar] [PubMed]
- Wessels, A.G.; Kluge, H.; Hirche, F.; Kiowski, A.; Schutkowski, A.; Corrent, E.; Bartelt, J.; König, B.; Stangl, G.I. High leucine diets stimulate cerebral branched-chain amino acid degradation and modify serotonin and ketone body concentrations in a pig model. PLoS ONE 2016, 11, e0150376. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Escobar, J.; Frank, J.W.; Suryawan, A.; Nguyen, H.V.; Van Horn, C.G.; Hutson, S.M.; Davis, T.A. Leucine and α-ketoisocaproic acid, but not norleucine, stimulate skeletal muscle protein synthesis in neonatal pigs. J. Nutr. 2010, 140, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, S.M.; El-Kadi, S.W.; Suryawan, A.; Boutry, C.; Orellana, R.A.; Nguyen, H.V.; Davis, S.R.; Davis, T.A. Protein synthesis in skeletal muscle of neonatal pigs is enhanced by administration of β-hydroxy-β-methylbutyrate. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E91–E99. [Google Scholar] [CrossRef] [PubMed]
- Neve, S.; Aarenstrup, L.; Tornehave, D.; Rahbek-Nielsen, H.; Corydon, T.J.; Roepstorff, P.; Kristiansen, K. Tissue distribution, intracellular localization and proteolytic processing of rat 4-hydroxyphenylpyruvate dioxygenase. Cell Biol. Int. 2003, 27, 611–624. [Google Scholar] [CrossRef]
- Ettrup, K.S.; Glud, A.N.; Orlowski, D.; Fitting, L.M.; Meier, K.; Soerensen, J.C.; Bjarkam, C.R.; Alstrup, A.K.O. Basic surgical techniques in the Göttingen minipig: Intubation, bladder catheterization, femoral vessel catheterization, and transcardial perfusion. J. Vis. Exp. 2011, 52, 2652. [Google Scholar]
- Council, N.R. Nutrient Requirements of Swine, 11th ed.; The National Academies Press: Washington, DC, USA, 2012; p. 400. [Google Scholar]
- Council, N.R. Nutrient Requirements of Swine, 10th ed.; The National Academies Press: Washington, DC, USA, 1998; p. 212. [Google Scholar]
- Fang, Z.F.; Luo, J.; Qi, Z.L.; Huang, F.R.; Zhao, S.J.; Liu, M.Y.; Jiang, S.W.; Peng, J. Effects of 2-hydroxy-4-methylthiobutyrate on portal plasma flow and net portal appearance of amino acids in piglets. Amino Acids 2009, 36, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.; Henry, J.; Reeds, P.J.; Yu, H.; Jahoor, F.; Burrin, D.G. Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets. J. Nutr. 1998, 128, 606–614. [Google Scholar] [PubMed]
- Riedijk, M.A.; Stoll, B.; Chacko, S.; Schierbeek, H.; Sunehag, A.L.; van Goudoever, J.B.; Burrin, D.G. Methionine transmethylation and transsulfuration in the piglet gastrointestinal tract. Proc. Natl. Acad. Sci. USA 2007, 104, 3408–3413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Luo, J.; Yu, B.; Zheng, P.; Huang, Z.; Mao, X.; He, J.; Yu, J.; Chen, J.; Chen, D. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat Sci. 2015, 102, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Li, T.; Li, Z.; Dang, T.; He, P. Determination of melatonin and its metabolites in biological fluids and eggs using high-performance liquid chromatography with fluorescence and quadrupole-orbitrap high-resolution mass spectrometry. Food Anal. Methods 2016, 9, 1142–1149. [Google Scholar] [CrossRef]
- Olson, K.C.; Chen, G.; Lynch, C.J. Quantification of branched-chain keto acids in tissue by ultra fast liquid chromatography-mass spectrometry. Anal. Biochem. 2013, 439, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Bush, J.A.; Burrin, D.G.; Suryawan, A.; O'Connor, P.M.; Nguyen, H.V.; Reeds, P.J.; Steele, N.C.; Van Goudoever, J.B.; Davis, T.A. Somatotropin-induced protein anabolism in hindquarters and portal-drained viscera of growing pigs. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E302–E312. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-M.; Wei, H.-W. The effects of dietary lysine deficiency on muscle protein turnover in postweanling pigs. Asian-Australas. J. Anim. Sci. 2005, 18, 1326–1335. [Google Scholar] [CrossRef]
- Mulvaney, D.R.; Merkel, R.A.; Bergen, W.G. Skeletal muscle protein turnover in young male pigs. J. Nutr. 1985, 115, 105–1064. [Google Scholar]
- Lefaucheur, L.; Ecolan, P.; Barzic, Y.-M.; Marion, J.; Le Dividich, J. Early postnatal food intake alters myofiber maturation in pig skeletal muscle. J. Nutr. 2003, 133, 140–147. [Google Scholar] [PubMed]
- Davies, A. A comparison of tissue development in Pietrain and Large White pigs from birth to 64 kg live weight 2. Growth changes in muscle distribution. Anim. Prod. 1974, 19, 377–387. [Google Scholar] [CrossRef]
- Davis, T.A.; Fiorotto, M.L.; Burrin, D.G.; Reeds, P.J.; Nguyen, H.V.; Beckett, P.R.; Vann, R.C.; O’Connor, P.M.J. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E880–E890. [Google Scholar] [CrossRef] [PubMed]
- Shavlakadze, T.; Grounds, M. Of bears, frogs, meat, mice and men: Complexity of factors affecting skeletal muscle mass and fat. Bioessays 2006, 28, 994–1009. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.J.; Esser, K.A. Anabolic and catabolic pathways regulating skeletal muscle mass. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Thivierge, M.C.; Bush, J.A.; Suryawan, A.; Nguyen, H.V.; Orellana, R.A.; Burrin, D.G.; Jahoor, F.; Davis, T.A. Positive net movements of amino acids in the hindlimb after overnight food deprivation contribute to sustaining the elevated anabolism of neonatal pigs. J. Appl. Physiol. 2008, 105, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Pacy, P.; Dworzak, F.; Ford, G.; Halliday, D. Influence of fasting on leucine and muscle protein metabolism across the human forearm determined using l-[1-13C, 15N] leucine as the tracer. Clin. Sci. 1987, 73, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Odessey, R.; Goldberg, A.L. Oxidation of leucine by rat skeletal muscle. Am. J. Physiol. 1972, 223, 1376–1383. [Google Scholar] [PubMed]
- Chen, L.; Li, P.; Wang, J.; Li, X.; Gao, H.; Yin, Y.; Hou, Y.; Wu, G. Catabolism of nutritionally essential amino acids in developing porcine enterocytes. Amino Acids 2009, 37, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Knabe, D.A.; Kim, S.W.; Lynch, C.J.; Hutson, S.M.; Wu, G. Lactating porcine mammary tissue catabolizes branched-chain amino acids for glutamine and aspartate synthesis. J. Nutr. 2009, 139, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Hossain, T.; Hill, D.; Phillips, B.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.; Breen, L.; Phillips, S. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. (Lond.) 2013, 591, 2911–2923. [Google Scholar] [CrossRef] [PubMed]
- Sekulić, A.; Hudson, C.C.; Homme, J.L.; Yin, P.; Otterness, D.M.; Karnitz, L.M.; Abraham, R.T. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000, 60, 3504–3513. [Google Scholar] [PubMed]
- Zhang, S.; Ren, M.; Zeng, X.; He, P.; Ma, X.; Qiao, S. Leucine stimulates ASCT2 amino acid transporter expression in porcine jejunal epithelial cell line (IPEC-J2) through PI3K/Akt/mTOR and ERK signaling pathways. Amino Acids 2014, 46, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Atherton, P.; Smith, K.; Rennie, M.J. Human muscle protein synthesis and breakdown during and after exercise. J. Appl. Physiol. 2009, 106, 2026–2039. [Google Scholar] [CrossRef] [PubMed]
- Louard, R.J.; Barrett, E.J.; Gelfand, R.A. Overnight branched-chain amino acid infusion causes sustained suppression of muscle proteolysis. Metabolism 1995, 44, 424–429. [Google Scholar] [CrossRef]
- Maki, T.; Yamamoto, D.; Nakanishi, S.; Iida, K.; Iguchi, G.; Takahashi, Y.; Kaji, H.; Chihara, K.; Okimura, Y. Branched-chain amino acids reduce hindlimb suspension-induced muscle atrophy and protein levels of atrogin-1 and MuRF1 in rats. Nutr. Res. 2012, 32, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Ito, Y.; Nishizawa, N.; Nagasawa, T. Regulation of muscle protein degradation, not synthesis, by dietary leucine in rats fed a protein-deficient diet. Amino Acids 2009, 37, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Baptista, I.L.; Leal, M.L.; Artioli, G.G.; Aoki, M.S.; Fiamoncini, J.; Turri, A.O.; Curi, R.; Miyabara, E.H.; Moriscot, A.S. Leucine attenuates skeletal muscle wasting via inhibition of ubiquitin ligases. Muscle Nerve 2010, 41, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Boutry, C.; El-Kadi, S.W.; Suryawan, A.; Wheatley, S.M.; Orellana, R.A.; Kimball, S.R.; Nguyen, H.V.; Davis, T.A. Leucine pulses enhance skeletal muscle protein synthesis during continuous feeding in neonatal pigs. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E620–E631. [Google Scholar] [CrossRef] [PubMed]

| Items | Control | Treatment |
|---|---|---|
| Ingredient (%) | ||
| Corn | 70.09 | 70.09 |
| Soybean meal | 10.70 | 10.40 |
| Whey powder | 5.00 | 5.00 |
| Fish meal | 4.00 | 4.00 |
| Concentrated soybean protein | 5.00 | 5.00 |
| Soybean oil | 0.40 | 0.50 |
| l-Lys·HCl | 0.48 | 0.48 |
| DL-Met | 0.23 | 0.23 |
| l-Thr | 0.22 | 0.23 |
| l-Trp | 0.06 | 0.06 |
| l-Ile | - | 0.17 |
| l-Leu | - | 0.24 |
| l-Val | - | 0.16 |
| l-Ala | 0.42 | - |
| Dicalcium phosphate | 1.30 | 1.30 |
| Limestone | 0.60 | 0.60 |
| Salt | 0.30 | 0.30 |
| Bentonite | 0.20 | 0.24 |
| Premix * | 1.00 | 1.00 |
| Analyzed composition (%) | ||
| CP | 16.7 | 16.7 |
| Ether extract | 8.48 | 8.95 |
| Crude Fiber | 2.34 | 2.54 |
| Lys | 1.32 | 1.34 |
| Met + Cys | 0.88 | 0.88 |
| Thr | 0.87 | 0.92 |
| Trp | 0.21 | 0.22 |
| Ile | 0.60 | 0.81 |
| Leu | 1.45 | 1.83 |
| Val | 0.76 | 0.95 |
| His | 0.74 | 0.77 |
| Phe | 0.42 | 0.44 |
| Arg | 1.02 | 1.02 |
| Calculated composition † | ||
| NE, MJ/kg | 10.38 | 10.38 |
| SID (Met + Cys):Lys | 0.55 | 0.55 |
| SID Thr:Lys | 0.59 | 0.59 |
| SID Trp:Lys | 0.16 | 0.16 |
| SID Ile:Lys | 0.47 | 0.61 |
| SID Leu:Lys | 1.07 | 1.27 |
| SID Val:Lys | 0.55 | 0.68 |
| Items | Control | Treatment | Pooled SEM | p Value |
|---|---|---|---|---|
| Muscle mass in the forequarter (g) | ||||
| Trapezius | 25.0 | 29.2 | 5.5 | 0.469 |
| Supraspinatus | 78.3 | 105.8 | 9.0 | 0.012 |
| Infraspinatus | 55.0 | 57.5 | 6.4 | 0.705 |
| Teres major | 23.3 | 27.5 | 2.0 | 0.065 |
| Deltoids | 14.2 | 16.7 | 1.3 | 0.092 |
| Subscapularis | 22.5 | 26.7 | 2.4 | 0.111 |
| Tricepsbrachii | 148.3 | 170.8 | 13.0 | 0.115 |
| Tensor fasciae antebrachii | 10.8 | 11.7 | 2.3 | 0.721 |
| Biceps | 36.7 | 42.5 | 3.7 | 0.150 |
| Brachii | 23.3 | 25.0 | 1.7 | 0.341 |
| Muscle mass in the midquarter (g) | ||||
| Latissimus dorsi | 90.0 | 99.2 | 10.6 | 0.407 |
| Pectoralis profundus | 109.0 | 116.6 | 6.8 | 0.272 |
| Longissimus dorsi | 401.7 | 517.5 | 43.6 | 0.024 |
| Psoas major | 54.2 | 61.7 | 4.1 | 0.097 |
| Muscle mass in the hindquarter (g) | ||||
| Glutaeus superficialis | 120.8 | 150.0 | 9.0 | 0.009 |
| Gluteus medius | 25.8 | 34.2 | 3.4 | 0.033 |
| Biceps femoris | 245.0 | 335.0 | 33.4 | 0.023 |
| Semitendinosus | 64.2 | 85.8 | 7.3 | 0.014 |
| Semembranosus | 202.5 | 239.2 | 12.4 | 0.014 |
| Tensor fascia latae | 36.7 | 44.2 | 3.7 | 0.068 |
| Gracilis | 43.3 | 60.8 | 4.1 | 0.002 |
| Adductor | 67.5 | 80.8 | 6.2 | 0.058 |
| Quadriceps femoris | 187.5 | 240.8 | 14.2 | 0.004 |
| Relative total muscle mass (%) * | 20.07 | 21.24 | 0.44 | 0.025 |
| Cross-sectional areas (μm2) | ||||
| Longissimus dorsi | 784 | 1086 | 95 | 0.010 |
| Items | Control | Treatment | Pooled SEM | p Value |
|---|---|---|---|---|
| Arterial leucine | ||||
| Concentration, μmol/L | 135.80 | 187.81 | 21.63 | 0.037 |
| [1-13C]leucine, MPE | 4.68 | 6.41 | 1.05 | 0.130 |
| Venous leucine | ||||
| Concentration, μmol/L | 124.67 | 154.30 | 18.82 | 0.146 |
| [1-13C]leucine, MPE | 3.94 | 4.97 | 0.61 | 0.121 |
| Arterial KIC | ||||
| Concentration, μmol/L | 46.84 | 64.88 | 4.77 | 0.004 |
| [1-13C]KIC, MPE | 1.43 | 2.10 | 0.53 | 0.238 |
| Venous KIC | ||||
| Concentration, μmol/L | 47.86 | 72.42 | 6.28 | 0.003 |
| [1-13C]KIC, MPE | 1.58 | 2.46 | 0.54 | 0.133 |
| Arterial CO2 | ||||
| Concentration, mmol/L | 18.64 | 18.62 | 1.07 | 0.988 |
| 13CO2, MPE | 0.0290 | 0.0333 | 0.0056 | 0.453 |
| Venous CO2 | ||||
| Concentration, mmol/L | 22.98 | 20.70 | 1.47 | 0.153 |
| 13CO2, MPE | 0.0259 | 0.0329 | 0.0060 | 0.271 |
| Items | Control | Treatment | Pooled SEM | p Value |
|---|---|---|---|---|
| Arterial input | 176.52 | 329.01 | 53.28 | 0.017 |
| Net uptake | 14.90 | 57.35 | 10.92 | 0.003 |
| Utilization | 38.60 | 106.26 | 14.57 | 0.001 |
| Net transamination | 2.44 | 14.38 | 5.21 | 0.045 |
| Oxidation | 8.96 | 17.06 | 7.47 | 0.304 |
| Protein synthesis | 27.21 | 74.82 | 10.92 | 0.001 |
| Protein degradation | 23.71 | 48.91 | 11.21 | 0.048 |
| Protein deposition | 3.50 | 25.91 | 8.51 | 0.025 |
| 13C-Leucine Uptake | 13C-KIC Production | Protein Synthesis | Protein Degradation | Protein Deposition | |
|---|---|---|---|---|---|
| 13C-leucine uptake | 1.00 | 0.70 * | 0.86 ** | 0.73 * | 0.43 |
| 13C-KIC production | 1.00 | 0.64 * | 0.55 | 0.31 | |
| Protein synthesis | 1.00 | 0.83 ** | 0.54 | ||
| Protein degradation | 1.00 | −0.02 | |||
| Protein deposition | 1.00 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Wei, H.; He, P.; Zhao, S.; Xiang, Q.; Pang, J.; Peng, J. Effects of Supplementation of Branched-Chain Amino Acids to Reduced-Protein Diet on Skeletal Muscle Protein Synthesis and Degradation in the Fed and Fasted States in a Piglet Model. Nutrients 2017, 9, 17. https://doi.org/10.3390/nu9010017
Zheng L, Wei H, He P, Zhao S, Xiang Q, Pang J, Peng J. Effects of Supplementation of Branched-Chain Amino Acids to Reduced-Protein Diet on Skeletal Muscle Protein Synthesis and Degradation in the Fed and Fasted States in a Piglet Model. Nutrients. 2017; 9(1):17. https://doi.org/10.3390/nu9010017
Chicago/Turabian StyleZheng, Liufeng, Hongkui Wei, Pingli He, Shengjun Zhao, Quanhang Xiang, Jiaman Pang, and Jian Peng. 2017. "Effects of Supplementation of Branched-Chain Amino Acids to Reduced-Protein Diet on Skeletal Muscle Protein Synthesis and Degradation in the Fed and Fasted States in a Piglet Model" Nutrients 9, no. 1: 17. https://doi.org/10.3390/nu9010017
APA StyleZheng, L., Wei, H., He, P., Zhao, S., Xiang, Q., Pang, J., & Peng, J. (2017). Effects of Supplementation of Branched-Chain Amino Acids to Reduced-Protein Diet on Skeletal Muscle Protein Synthesis and Degradation in the Fed and Fasted States in a Piglet Model. Nutrients, 9(1), 17. https://doi.org/10.3390/nu9010017




