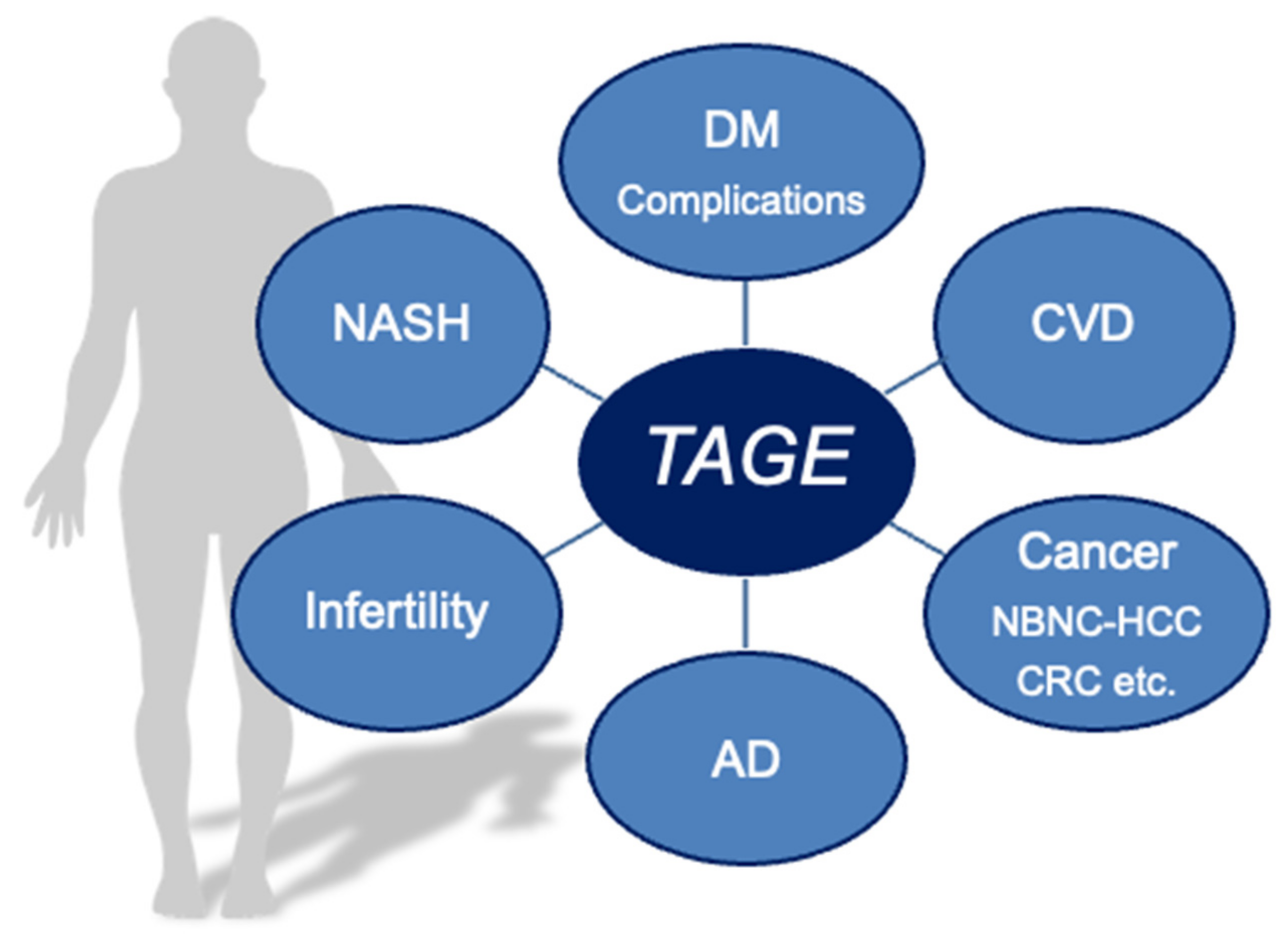
Effects of Toxic AGEs (TAGE) on Human Health
Abstract
:1. Introduction
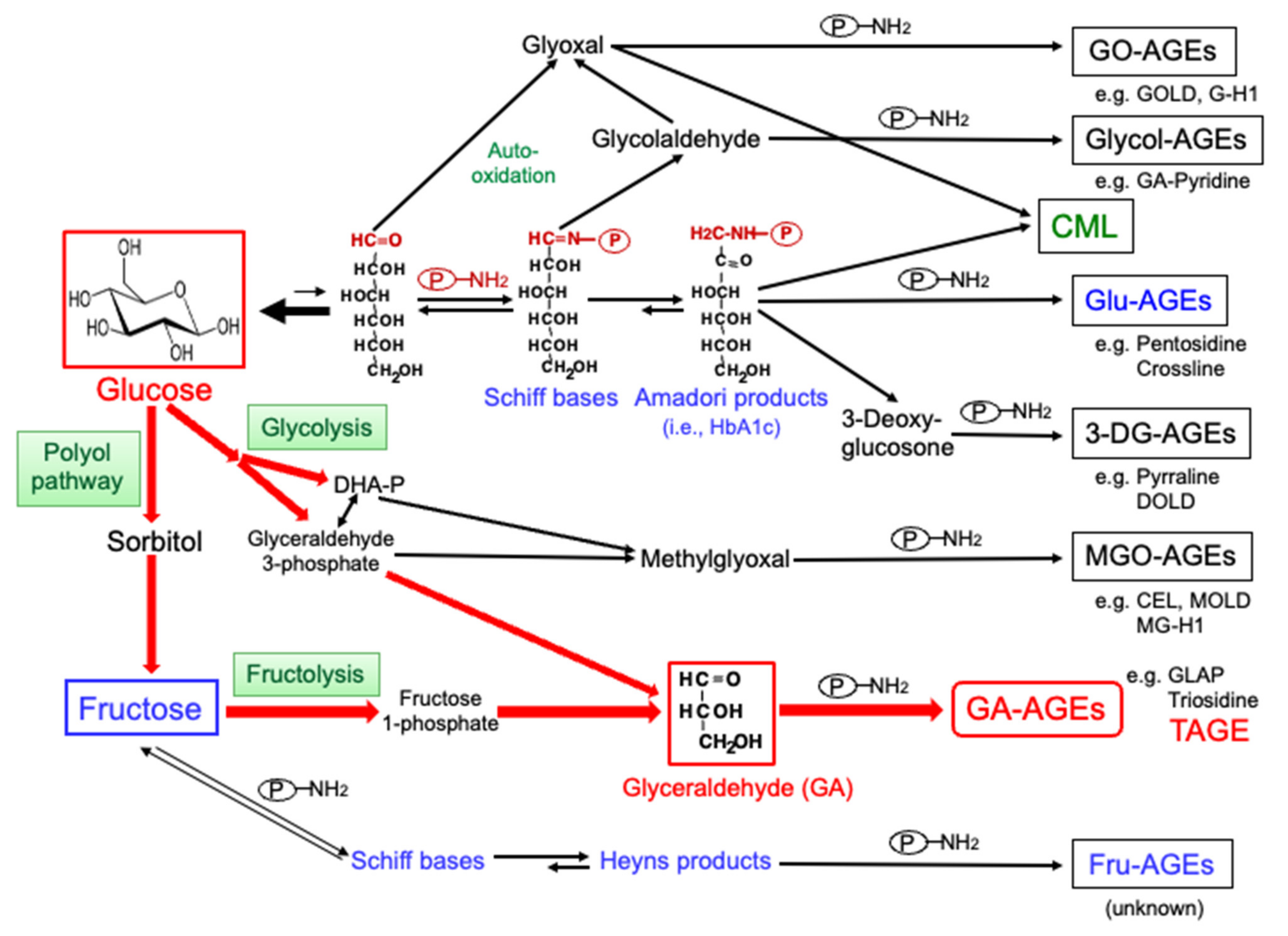
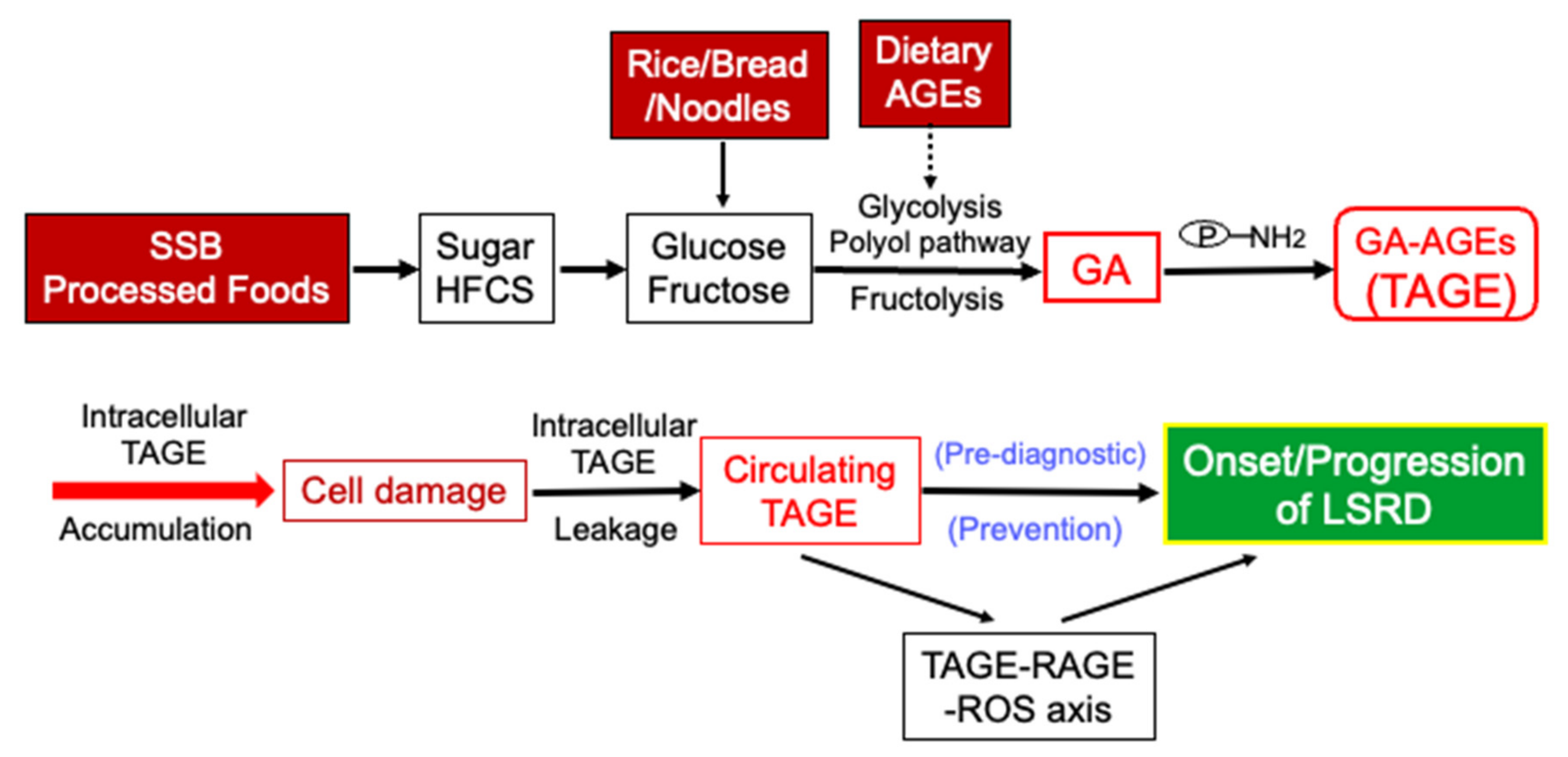
2. Production of AGEs in the Human Body
3. Detection of Intracellular and Circulating TAGE
3.1. Slot Blot (SB) Analysis of Intracellular TAGE Levels
3.2. Enzyme-Linked Immunosorbent Assay (ELISA) for TAGE Levels in Serum/Vitreous Fluid
4. Cytotoxicity of TAGE
4.1. TAGE Cytotoxicity in the Liver
4.1.1. Intracellular TAGE and Hepatocyte Cell Death
- i.
- The accumulation of TAGE has been shown to induce damage in HCC cell lines (HepG2 and Hep3B) and primary cultured hepatocytes [30,31,32]. The intracellular accumulation of TAGE induced by GA increased the mRNA expression levels of C-reactive protein and decreased the chaperone activity of Hep3B cells [30]. The intracellular TAGE accumulation also induced necrotic-type cell death associated with TAGE-modified caspase-3 [31], and promoted the production of ROS in HepG2 cells and primary cultured hepatocytes [32].
- ii.
- Heterogeneous nuclear ribonucleoprotein M (hnRNPM), an RNA-binding protein, was identified as a target protein for TAGE in Hep3B cells incubated in GA- or high fructose-containing media [59,60]. Furthermore, following the knockdown of hnRNPM, the up- or down-regulated expression of genes associated with extracellular exosome-containing extracellular spaces was observed [60].
- iii.
- HCC cell lines and primary cultured cells have been used as in vitro models of NASH. However, they have several disadvantages, such as specialized characteristics by immortalization or limited growth potential. A decrease was noted in the viability of hepatocyte-like cells (HLCs), which differentiated from human induced pluripotent stem cells (hiPSCs) (hiPSC-HLCs), as TAGE accumulated in cells, which was consistent with previous findings on HCC cells and primary cultured hepatocytes [33]. In addition, the accumulation of TAGE up-regulated the expression of inflammation-related genes (i.e., interleukin (IL)-6, IL-8, and monocyte chemoattractant protein (MCP)-1) in hiPSC-HLCs. Experimental data from hiPSC-HLCs are considered to more accurately reflect the pathology of humans.
4.1.2. Effects of Extracellular TAGE on HSC
4.2. Cytotoxicity of TAGE in Skeletal Muscle
4.3. Cytotoxicity of TAGE in Pancreatic Islet β-Cells (β-Cells)
4.4. Cytotoxicity of TAGE in Bone
4.5. Cytotoxicity of TAGE in the Heart
4.5.1. Intracellular TAGE and Cardiomyocyte Death
4.5.2. Intracellular TAGE and Human Cardiac Fibroblast (HCF) Cell Death
4.5.3. Extracellular TAGE and Cardiomyocytes and HCF
4.6. Cytotoxicity of TAGE in the Brain
4.6.1. Intracellular TAGE and Neuronal Cells
4.6.2. Extracellular TAGE on Neuronal Cells
4.6.3. Extracellular TAGE on Brain Vascular Endothelial Cells (EC)
4.7. Cytotoxicity of TAGE in Cancer
4.7.1. Melanoma
4.7.2. Lung Cancer
4.7.3. Pancreatic Cancer
4.7.4. Colorectal Cancer (CRC)
4.8. Limitation
4.9. Brief Summary and Perspectives
5. Clinical Relevance of Circulating TAGE Levels and LSRD
5.1. Healthy Population (Apparently Healthy/General Population)
5.2. Non-Diabetic General Population (Outpatients)
5.3. Diabetic Outpatients
5.4. CVD/Heart Failure
- i.
- Serum levels of TAGE, but not HbA1c or CML, independently correlated with vascular inflammation in outpatients using [18F] fluorodeoxyglucose-positron emission tomography [55];
- ii.
- Among pre-DM patients, circulating levels of TAGE were significantly higher in the high mean amplitude of the glycemic excursions (MAGE) group than in the low MAGE group [21]. TAGE and medication for hypertension were independently associated with area of visceral adipose tissues, whereas medication for TAGE, DM, intima-media thickness, and PEDF were independent correlates of subcutaneous adipose tissue areas [100].
5.5. Infertility
5.6. NASH
5.7. Cancer (Non-B or Non-C (NBNC)-HCC and CRC)
5.7.1. NBNC-HCC
5.7.2. CRC
5.8. Schizophrenia and AD
5.9. Other Diseases
5.10. Brief Summary and Perspectives
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Yu, M.; Fang, L.; Hu, R.Y. Association between sugar-sweetened beverages and type 2 diabetes: A meta-analysis. J. Diabetes Investig. 2015, 6, 360–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imamura, F.; O’Connor, L.; Ye, Z.; Mursu, J.; Hayashino, Y.; Bhupathiraju, S.N.; Forouhi, N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. Br. J. Sports Med. 2016, 50, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S. Sugar sweetened beverages and cardiometabolic health. Curr. Opin. Cardiol. 2017, 32, 572–579. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, M.; Makita, Z. Alternative routes for the formation of immunochemically distinct advanced glycation end-products in vivo. Curr. Mol. Med. 2001, 1, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 1037. [Google Scholar] [CrossRef] [PubMed]
- Brings, S.; Fleming, T.; Freichel, M.; Muckenthaler, M.U.; Herzig, S.; Nawroth, P.P. Dicarbonyls and advanced glycation end-products in the development of diabetic complications and targets for intervention. Int. J. Mol. Sci. 2017, 18, 984. [Google Scholar] [CrossRef] [Green Version]
- Jud, P.; Sourij, H. Therapeutic options to reduce advanced glycation end products in patients with diabetes mellitus: A review. Diabetes Res. Clin. Pract. 2019, 148, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M. Serum levels of toxic AGEs (TAGE) may be a promising novel biomarker for the onset/progression of lifestyle-related diseases. Diagnostics 2016, 6, 23. [Google Scholar] [CrossRef]
- Takeuchi, M. Toxic AGEs (TAGE) theory: A new concept for preventing the development of diseases related to lifestyle. Diabetol. Metab. Syndr. 2020, 12, 105. [Google Scholar] [CrossRef]
- Takeuchi, M.; Sakasai-Sakai, A.; Takata, T.; Takino, J.; Koriyama, Y.; Kikuchi, C.; Furukawa, A.; Nagamine, K.; Hori, T.; Matsunaga, T. Intracellular toxic AGEs (TAGE) triggers numerous types of cell damage. Biomolecules 2021, 11, 387. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Yamagishi, S. TAGE (toxic AGEs) hypothesis in various chronic diseases. Med. Hypotheses 2004, 63, 449–452. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamagishi, S.; Adachi, H.; Matsui, T.; Kurita-Nakamura, Y.; Takeuchi, M.; Inoue, H.; Imaizumi, T. Circulating advanced glycation end products (AGEs) and soluble form of receptor for AGEs (sRAGE) are independent determinants of serum monocyte chemoattractant protein-1 (MCP-1) levels in patients with type 2 diabetes. Diabetes Metab. Res. Rev. 2008, 24, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Tsunosue, M.; Mashiko, N.; Ohta, Y.; Matsuo, Y.; Ueda, K.; Ninomiya, M.; Tanaka, S.; Hoshiko, M.; Yoshiyama, Y.; Takeuchi, M.; et al. An α-glucosidase inhibitor, acarbose treatment decreases serum levels of glyceraldehyde-derived advanced glycation end products (AGEs) in patients with type 2 diabetes. Clin. Exp. Med. 2010, 10, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Takino, J.; Yamagishi, S. Involvement of the toxic AGEs (TAGE)-RAGE system in the pathogenesis of diabetic vascular complications: A novel therapeutic strategy. Curr. Drug Targets 2010, 11, 1468–1482. [Google Scholar] [CrossRef] [Green Version]
- Hyogo, H.; Yamagishi, S.; Iwamoto, K.; Arihiro, K.; Takeuchi, M.; Sato, T.; Ochi, H.; Nonaka, M.; Nabeshima, Y.; Inoue, M.; et al. Elevated levels of serum advanced glycation end-products in patients with nonalcoholic steatohepatitis. J. Gastroenterol. Hepatol. 2007, 22, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Hyogo, H.; Yamagishi, S.; Takeuchi, M.; Ishitobi, T.; Nabeshima, Y.; Arihiro, K.; Chayama, K. Atorvastatin decreases serum levels of advanced glycation endproducts (AGEs) in nonalcoholic steatohepatitis (NASH) patients with dyslipidemia: Clinical usefulness of AGEs as a biomarker for the attenuation of NASH. J. Gastroenterol. 2010, 45, 750–757. [Google Scholar] [CrossRef]
- Kan, H.; Yamagishi, S.; Ojima, A.; Fukami, K.; Ueda, S.; Takeuchi, M.; Hyogo, H.; Aikata, H.; Chayama, K. Elevation of serum levels of advanced glycation end products in patients with non-B or non-C hepatocellular carcinoma. J. Clin. Lab. Anal. 2015, 29, 480–484. [Google Scholar] [CrossRef]
- Takeuchi, M.; Takino, J.; Sakasai-Sakai, A.; Takata, T.; Tsutsumi, M. Toxic AGE (TAGE) theory for the pathophysiology of the onset/progression of NAFLD and ALD. Nutrients 2017, 9, 634. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, Y.; Daida, H.; Morimoto, T.; Kasai, T.; Miyauchi, K.; Yamagishi, S.; Takeuchi, M.; Hiro, T.; Kimura, T.; Nakagawa, Y.; et al. Relationship between advanced glycation end products and plaque progression in patients with acute coronary syndrome: The JAPAN-ACS sub-study. Cardiovasc. Diabetol. 2013, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Kawai, Y.; Kitayama, M.; Akao, H.; Motoyama, A.; Wakasa, M.; Saito, R.; Aoki, H.; Fujibayashi, K.; Tsuchiya, T.; et al. Diurnal glycemic fluctuation is associated with severity of coronary artery disease in prediabetic patients: Possible role of nitrotyrosine and glyceraldehyde-derived advanced glycation end products. J. Cardiol. 2017, 69, 625–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomosugi, N.; Yamamoto, S.; Takeuchi, M.; Yonekura, H.; Ishigaki, Y.; Numata, N.; Katsuda, S.; Sakai, Y. Effect of collagen tripeptide on atherosclerosis in healthy humans. J. Atheroscler. Thromb. 2017, 24, 530–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, M.; Bucala, R.; Suzuki, T.; Ohkubo, T.; Yamazaki, M.; Koike, T.; Kameda, Y.; Makita, Z. Neurotoxicity of advanced glycation end-products for cultured cortical neurons. J. Neuropathol. Exp. Neurol. 2000, 59, 1094–1105. [Google Scholar] [CrossRef] [Green Version]
- Choei, H.; Sasaki, N.; Takeuchi, M.; Yoshida, T.; Ukai, W.; Yamagishi, S.; Kikuchi, S.; Saito, T. Glyceraldehyde-derived advanced glycation end products in Alzheimer’s disease. Acta Neuropathol. 2004, 108, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Yamagishi, S. Involvement of toxic AGEs (TAGE) in the pathogenesis of diabetic vascular complications and Alzheimer’s disease. J. Alzheimers Dis. 2009, 16, 845–858. [Google Scholar] [CrossRef]
- Abe, R.; Shimizu, T.; Sugawara, H.; Watanabe, H.; Nakamura, H.; Choei, H.; Sasaki, N.; Yamagishi, S.; Takeuchi, M.; Shimizu, H. Regulation of human melanoma growth and metastasis by AGE-AGE receptor interactions. J. Investig. Dermatol. 2004, 122, 461–467. [Google Scholar] [CrossRef] [Green Version]
- Takino, J.; Yamagishi, S.; Takeuchi, M. Cancer malignancy is enhanced by glyceraldehyde-derived advanced glycation end-products. J. Oncol. 2010, 2010, 739852. [Google Scholar] [CrossRef]
- Takino, J.; Nagamine, K.; Hori, T.; Sakasai-Sakai, A.; Takeuchi, M. Contribution of the toxic advanced glycation end-products-receptor axis in nonalcoholic steatohepatitis-related hepatocellular carcinoma. World J. Hepatol. 2015, 7, 2459–2469. [Google Scholar] [CrossRef]
- Kong, S.Y.; Takeuchi, M.; Hyogo, H.; McKeown-Eyssen, G.; Yamagishi, S.; Chayama, K.; O’Brien, P.J.; Ferrari, P.; Overvad, K.; Olsen, A.; et al. The association between glyceraldehyde-derived advanced glycation end-products and colorectal cancer risk. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1855–1863. [Google Scholar] [CrossRef] [Green Version]
- Takino, J.; Kobayashi, Y.; Takeuchi, M. The formation of intracellular glyceraldehyde-derived advanced glycation end-products and cytotoxicity. J. Gastroenterol. 2010, 45, 646–655. [Google Scholar] [CrossRef]
- Sakasai-Sakai, A.; Takata, T.; Takino, J.; Takeuchi, M. Impact of intracellular glyceraldehyde-derived advanced glycation end-products on human hepatocyte cell death. Sci. Rep. 2017, 7, 14282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakasai-Sakai, A.; Takata, T.; Takeuchi, M. Intracellular toxic advanced glycation end-products promote the production of reactive oxygen species in HepG2 cells. Int. J. Mol. Sci. 2020, 21, 4861. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, C.; Sakasai-Sakai, A.; Okimura, R.; Tanaka, H.; Takata, T.; Takeuchi, M.; Matsunaga, T. Accumulation of toxic advanced glycation end-products induces cytotoxicity and inflammation in hepatocyte-like cells differentiated from human induced pluripotent stem cells. Biol. Pharm. Bull. 2021, 44, 1399–1402. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Sakasai-Sakai, A.; Takeuchi, M. Impact of intracellular toxic advanced glycation end-products (TAGE) on murine myoblast cell death. Diabetol. Metab. Syndr. 2020, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Sakasai-Sakai, A.; Takeuchi, M. Intracellular toxic advanced glycation end-products 1.4E7 cell line induce death with reduction of microtubule-associated protein 1 light chain 3 and p62. Nutrients 2022, 14, 332. [Google Scholar] [CrossRef]
- Sakasai-Sakai, A.; Takata, T.; Takeuchi, M. The association between accumulation of toxic advanced glycation end-products and cytotoxic effect in MC3T3-E1 cells. Nutrients 2022, 14, 990. [Google Scholar] [CrossRef]
- Takata, T.; Sakasai-Sakai, A.; Ueda, T.; Takeuchi, M. Intracellular toxic advanced glycation end-products in cardiomyocytes may cause cardiovascular disease. Sci. Rep. 2019, 9, 2121. [Google Scholar] [CrossRef] [Green Version]
- Takata, T.; Sakasai-Sakai, A.; Takeuchi, M. Intracellular toxic advanced glycation end-products may induce cell death and suppress cardiac fibroblasts. Metabolites 2022, 12, 615. [Google Scholar] [CrossRef]
- Koriyama, Y.; Furukawa, A.; Muramatsu, M.; Takino, J.; Takeuchi, M. Glyceraldehyde caused Alzheimer’s disease-like alterations in diagnostic marker levels in SH-SY5Y human neuroblastoma cells. Sci. Rep. 2015, 5, 13313. [Google Scholar] [CrossRef] [Green Version]
- Nasu, R.; Furukawa, A.; Suzuki, K.; Takeuchi, M.; Koriyama, Y. The effect of glyceraldehyde-derived advanced glycation end-products on β-tubulin-inhibited neurite outgrowth in SH-SY5Y human neuroblastoma cells. Nutrients 2020, 12, 2958. [Google Scholar] [CrossRef]
- Ooi, H.; Nasu, R.; Furukawa, A.; Takeuchi, M.; Koriyama, Y. Pyridoxamine and aminoguanidine attenuate the abnormal aggregation of β-tubulin and suppression of neurite outgrowth by glyceraldehyde-derived toxic advanced glycation end-products. Front. Pharmacol. 2022, 13, 921611. [Google Scholar] [CrossRef]
- Takata, T.; Ueda, T.; Sakasai-Sakai, A.; Takeuchi, M. Generation of glyceraldehyde-derived advanced glycation end-products in pancreatic cancer cells and the potential of tumor promotion. World J. Gastroenterol. 2017, 23, 4910–4919. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative stress and advanced lipoxidation and glycation end products (ALEs and AGEs) in aging and age-related diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brownlee, M. Advanced protein glycosylation in diabetes and aging. Ann. Rev. Med. 1995, 46, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Yamagishi, S.; Imaizumi, T. Diabetic vascular complications: Pathophysiology, biochemical basis and potential therapeutic strategy. Curr. Pharm. Des. 2005, 11, 2279–2299. [Google Scholar] [CrossRef]
- Takeuchi, M.; Iwaki, M.; Takino, J.; Shirai, H.; Kawakami, M.; Bucala, R.; Yamagishi, S. Immunological detection of fructose-derived advanced glycation end-products. Lab. Invest. 2010, 90, 1117–1127. [Google Scholar] [CrossRef] [Green Version]
- Yonekura, H.; Yamamoto, Y.; Sakurai, S.; Petrove, R.G.; Abedin, M.J.; Li, H.; Yasui, K.; Takeuchi, M.; Makita, Z.; Takasawa, S.; et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem. J. 2003, 370, 1097–1109. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yonekura, H.; Watanabe, T.; Sakurai, S.; Li, H.; Harashima, A.; Myint, K.M.; Osawa, M.; Takeuchi, A.; Takeuchi, M.; et al. Short-chain aldehyde-derived ligands for RAGE and their actions on endothelial cells. Diabetes Res. Clin. Pract. 2007, 77 (Suppl. S1), S30–S40. [Google Scholar] [CrossRef]
- Takino, J.; Yamagishi, S.; Takeuchi, M. Glycer-AGEs-RAGE signaling enhances the angiogenic potential of HCC by upregulating VEGF expression. World J. Gastroenterol. 2012, 18, 1781–1788. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Matsui, T.; Takeuchi, M.; Yamagishi, S. Metformin inhibits advanced glycation end products (AGEs)-induced growth and VEGF expression in MCF-7 breast cancer cells by suppressing AGEs receptor expression via AMP-activated protein kinase. Horm. Metab. Res. 2013, 45, 387–390. [Google Scholar] [CrossRef]
- Takeuchi, M.; Makita, Z.; Bucala, R.; Suzuki, T.; Koike, T.; Kameda, Y. Immunological evidence that non-carboxymethyllysine advanced glycation end-products are produced from short chain sugars and dicarbonyl compounds in vivo. Mol. Med. 2000, 6, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Usui, T.; Hayase, F. Isolation and identification of the 3-hydroxy-5-hydroxymethyl-pyridinium compound as a novel advanced glycation end product on glyceraldehyde-related Maillard reaction. Biosci. Biotechnol. Biochem. 2003, 67, 930–932. [Google Scholar] [CrossRef]
- Tessier, F.J.; Monnier, V.M.; Sayre, L.M.; Kornfield, J.A. Triosidines: Novel Maillard reaction products and cross-links from the reaction of triose sugars with lysine and arginine residues. Biochem. J. 2003, 369, 705–719. [Google Scholar] [CrossRef] [Green Version]
- Yamagishi, S.; Nakamura, K.; Matsui, T.; Inagaki, Y.; Takenaka, K.; Jinnouchi, Y.; Yoshida, Y.; Matsuura, T.; Narama, I.; Motomiya, Y.; et al. Pigment epithelium-derived factor inhibits advanced glycation end product-induced retinal vascular hyperpermeability by blocking reactive oxygen species-mediated vascular endothelial growth factor expression. J. Biol. Chem. 2006, 281, 20213–20220. [Google Scholar] [CrossRef] [Green Version]
- Tahara, N.; Yamagishi, S.; Takeuchi, M.; Honda, A.; Tahara, A.; Nitta, Y.; Kodama, N.; Mizoguchi, M.; Kaida, H.; Ishibashi, M.; et al. Positive association between serum level of glyceraldehyde-derived advanced glycation end products (AGEs) and vascular inflammation evaluated by 18F-fluorodeoxyglucose positron emission tomography (FDG-PET). Diabetes Care 2012, 35, 2618–2625. [Google Scholar] [CrossRef] [Green Version]
- Jinno, M.; Takeuchi, M.; Watanabe, A.; Teruya, K.; Hirohama, J.; Eguchi, N.; Miyazaki, A. Advanced glycation end-products accumulation compromises embryonic development and achievement of pregnancy by assisted reproductive technology. Hum. Reprod. 2011, 26, 604–610. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, M.; Takino, J.; Shirai, H.; Kawakami, M.; Furuno, S.; Kobayashi, Y. Assessment of total sugar and glucose concentrations in commonly consumed beverages in Japan. Nutr. Food Technol. 2015, 1, 2. [Google Scholar]
- Takeuchi, M.; Takino, J.; Furuno, S.; Shirai, H.; Kawakami, M.; Muramatsu, M.; Kobayashi, Y.; Yamagishi, S. Assessment of the concentrations of various advanced glycation end-products in beverages and foods that are commonly consumed in Japan. PLoS ONE 2015, 10, e0118652. [Google Scholar] [CrossRef] [Green Version]
- Takino, J.; Nagamine, K.; Takeuchi, M.; Hori, T. In vitro identification of nonalcoholic fatty liver disease-related protein hnRNPM. World J. Gastroenterol. 2015, 21, 1784–1793. [Google Scholar] [CrossRef]
- Takino, J.; Nagamine, K.; Suzuki, M.; Sakasai-Sakai, A.; Takeuchi, M.; Hori, T. Gene expression changes associated with the loss of heterogeneous nuclear ribonucleoprotein M function. Am. J. Mol. Biol. 2017, 7, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, K.; Kanno, K.; Hyogo, H.; Yamagishi, S.; Takeuchi, M.; Tazuma, S.; Chayama, K. Advanced glycation end products enhance the proliferation and activation of hepatic stellate cells. J. Gastroenterol. 2008, 43, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Takino, J.; Sato, T.; Nagamine, K.; Sakasai-Sakai, A.; Takeuchi, M.; Hori, T. Suppression of hepatic stellate cell death by toxic advanced glycation end-products. Biol. Pharm. Bull. 2021, 44, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Adachi, N.; Kanazawa, I.; Tanaka, K.; Takeno, A.; Notsu, M.; Tanaka, S.; Sugimoto, T. Insulinlike growth factor-I protects against the detrimental effects of advanced glycation end products and high glucose in myoblastic C2C12 cell. Calcif. Tissue Int. 2019, 105, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Bhanji, R.A.; Narayanan, P.; Moynagh, M.R.; Takahashi, N.; Angirekula, M.; Kennedy, C.C.; Mara, K.C.; Dierkhising, R.A.; Watt, K.D. Differing impact of sarcopenia and frailty in nonalcoholic steatohepatitis and alcoholic liver disease. Liver Transpl. 2019, 25, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Ciminnisi, S.; Di Marco, V.; Cabibi, D.; Cammà, C.; Licata, A.; Marchesini, G.; Craxi, A. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2017, 45, 510–518. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Funaki, A.; Kimura, Y.; Sumitomo, M.; Yoshida, H.; Fukata, H.; Ueno, K. Ethanol extract of Cyclolepis genistoides D. Don (palo azul) induces formation of myotubes, which involves differentiation of C2C12 myoblast cells. Nutr. Res. 2016, 36, 731–741. [Google Scholar] [CrossRef]
- Byun, S.K.; An, T.H.; Son, M.J.; Lee, D.S.; Kang, H.S.; Lee, E.W.; Han, B.S.; Kim, W.K.; Bae, K.H.; Oh, K.J.; et al. HDAC11 inhibits myoblast differentiation through repression of MyoD-dependent transcription. Mol. Cells 2017, 40, 667–676. [Google Scholar]
- Bohuslavova, R.; Smolik, O.; Malfatti, J.; Berkova, Z.; Novakova, Z.; Saudek, F.; Pavlinkova, G. NUEROD1 is required for the early and endocrine differentiation in the pancreas. Int. J. Mol. Sci. 2021, 22, 6713. [Google Scholar] [CrossRef]
- Ji, S.; Zhu, C.; Gao, S.; Shao, X.; Zhang, H.; Tang, D. Morus alba leaves ethanol extract protects pancreatic islet cells against dysfunction and death by inducing autophagy in type 2 diabetes. Phytomedicine 2021, 83, 153478. [Google Scholar] [CrossRef]
- Costes, S.; Bertrand, G.; Raier, M.A. Mechanisms of beta-cell apoptosis in type 2 diabetes-prone situations and potential protection by GLP-1-based therapies. Int. J. Mol. Sci. 2021, 22, 5303. [Google Scholar] [CrossRef] [PubMed]
- Romero-Díaz, C.; Duarte-Montero, D.; Gutiérrez-Romero, S.A.; Mendivil, C.O. Diabetes and bone fragility. Diabetes Ther. 2021, 12, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Johnell, O.; Kanis, J.A.; Odén, A.; Sernbo, I.; Redlund-Johnell, I.; Petterson, C.; De Laet, C.; Jönsson, B. Mortality after osteoporotic fractures. Osteoporos Int. 2004, 15, 38–42. [Google Scholar] [CrossRef]
- Asadipooya, K.; Uy, E.M. Advanced glycation end products (AGEs), receptor for AGEs, diabetes, and bone: Review of the literature. J. Endocr. Soc. 2019, 3, 1799–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enomoto, M.; Adachi, H.; Yamagishi, S.; Takeuchi, M.; Furuki, K.; Hino, A.; Hiratsuka, A.; Takajo, Y.; Imaizumi, T. Positive association of serum levels of advanced glycation end products with thrombogenic markers in humans. Metabolism 2006, 55, 912–917. [Google Scholar] [CrossRef]
- Mittal, K.; Katre, D.P. Shared links between type 2 diabetes mellitus and Alzheimer’s disease: A review. Diabetes Metab. Syndr. 2016, 10 (Suppl. S1), S144–S149. [Google Scholar] [CrossRef]
- Chatterjee, S.; Peters, S.A.; Woodward, M.; Mejia Arango, S.; Batty, G.D.; Beckett, N.; Beiser, A.; Borenstein, A.R.; Crane, P.K.; Haan, M.; et al. Type 2 diabetes as a risk factor for dementia in women compared with men: A pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 2016, 39, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Steele, M.; Stuchbury, G.; Münch, G. The molecular basis of the prevention of Alzheimer’s disease through healthy nutrition. Exp. Geront. 2007, 42, 28–36. [Google Scholar] [CrossRef]
- Takeuchi, M.; Kikuchi, S.; Sasaki, N.; Suzuki, T.; Watai, T.; Iwaki, M.; Bucala, R.; Yamagishi, S. Involvement of advanced glycation end-products (AGEs) in Alzheimer’s disease. Curr. Alzheimer Res. 2004, 1, 39–46. [Google Scholar] [CrossRef]
- Mazzola, J.L.; Sirover, M.A. Reduction of glyceraldehyde-3-phosphate dehydrogenase activity in Alzheimer’s disease and in Huntington’s disease fibroblasts. J. Neurochem. 2001, 76, 442–449. [Google Scholar] [CrossRef] [Green Version]
- Mazzola, J.L.; Sirover, M.A. Subcellular alteration of glyceraldehyde-3-phosphate dehydrogenase in Alzheimer’s disease fibroblasts. J. Neurosci. Res. 2003, 71, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Niiya, Y.; Abumiya, T.; Shichinohe, H.; Kuroda, S.; Kikuchi, S.; Ieko, M.; Yamagishi, S.; Takeuchi, M.; Sato, T.; Iwasaki, Y. Susceptibility of brain microvascular endothelial cells to advanced glycation end products-induced tissue factor upregulation is associated with intracellular reactive oxygen species. Brain Res. 2006, 1108, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Niiya, Y.; Abumiya, T.; Yamagishi, S.; Takino, J.; Takeuchi, M. Advanced glycation end products increase permeability of brain microvascular endothelial cells through reactive oxygen species-induced vascular endothelial growth factor expression. J. Stroke Cerebrovasc. Dis. 2012, 21, 293–298. [Google Scholar] [CrossRef]
- Takino, J.; Sato, T.; Kanetaka, T.; Okihara, K.; Nagamine, K.; Takeuchi, M.; Hori, T. RasGRP2 inhibits glyceraldehyde-derived toxic advanced glycation end-products from inducing permeability in vascular endothelial cells. Sci. Rep. 2021, 11, 2959. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Spinelli, S.; Trichilo, V.; Loddo, S.; Sarikas, A.; Pusch, M.; Dossena, S.; Marino, A.; Morabito, R.J. d-Galactose induced early aging in human erythrocytes: Role of band 3 protein. J. Cell. Physiol. 2022, 237, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Yamagishi, S.; Matsui, T.; Noda, Y.; Ueda, S.; Jinnouchi, Y.; Sasaki, K.; Takeuchi, M.; Imaizumi, T. Serum levels of advanced glycation end products (AGEs) are inversely associated with the number and migratory activity of circulating endothelial progenitor cells in apparently healthy subjects. Cardiovasc. Ther. 2012, 30, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Adachi, H.; Takeuchi, M.; Enomoto, M.; Furuki, K.; Matsui, T.; Nakamura, K.; Imaizumi, T. Serum level of advanced glycation end-products (AGEs) is an independent determinant of plasminogen activator inhibitor-1 (PAI-1) in nondiabetic general population. Horm. Metab. Res. 2007, 39, 845–848. [Google Scholar] [CrossRef]
- Kajikawa, M.; Nakashima, A.; Fujimura, N.; Maruhashi, T.; Iwamoto, Y.; Iwamoto, A.; Matsumoto, T.; Oda, N.; Hidaka, T.; Kihara, Y.; et al. Ratio of serum levels of AGEs to soluble form of RAGE is a predictor of endothelial function. Diabetes Care 2015, 38, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Yamagishi, S.; Adachi, H.; Nakamura, K.; Matsui, T.; Jinnouchi, Y.; Takenaka, K.; Takeuchi, M.; Enomoto, M.; Furuki, K.; Hino, A.; et al. Positive association between serum levels of advanced glycation end products and the soluble form of receptor for advanced glycation end products in nondiabetic subjects. Metabolism 2006, 55, 1227–1231. [Google Scholar] [CrossRef]
- Yamagishi, S.; Adachi, H.; Matsui, T.; Nakamura, K.; Takeuchi, M.; Enomoto, M.; Fukami, A.; Otsuka, M.; Kumagae, S.; Nanjo, Y.; et al. Low-density lipoprotein levels are one of the independent determinants of circulating levels of advanced glycation end products in nondiabetic subjects. Clin. Cardiol. 2009, 32, E12–E15. [Google Scholar] [CrossRef]
- Yamagishi, S.; Matsui, T.; Adachi, H.; Takeuchi, M. Positive association of circulating level of advanced glycation end products (AGEs) with pigment epithelium-derived factor (PEDF) in a general population. Pharmacol. Res. 2010, 61, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Tahara, N.; Yamagishi, S.; Matsui, T.; Takeuchi, M.; Nitta, Y.; Kodama, N.; Mizoguchi, M.; Imaizumi, T. Serum levels of advanced glycation end products (AGEs) are independent correlates of insulin resistance in non-diabetic subjects. Cardiovasc. Ther. 2012, 30, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Tahara, N.; Yamagishi, S.; Tahara, A.; Ishibashi, M.; Hayabuchi, N.; Takeuchi, M.; Imaizumi, T. Adiponectin is inversely associated with ratio of serum levels of AGEs to sRAGE and vascular inflammation. Int. J. Cardiol. 2012, 158, 461–462. [Google Scholar] [CrossRef] [PubMed]
- Tahara, N.; Yamagishi, S.; Tahara, A.; Takeuchi, M.; Imaizumi, T. Serum levels of pigment epithelium-derived factor, a novel marker of insulin resistance, are independently associated with fasting apolipoprotein B48 levels in humans. Clin. Biochem. 2012, 45, 1404–1408. [Google Scholar] [CrossRef]
- Tahara, N.; Yamagishi, S.; Takeuchi, M.; Tahara, A.; Kaifu, K.; Ueda, S.; Okuda, S.; Imaizumi, T. Serum levels of advanced glycation end products (AGEs) are independently correlated with circulating levels of dipeptidyl peptidase-4 (DPP-4) in humans. Clin. Biochem. 2013, 46, 300–303. [Google Scholar] [CrossRef]
- Tahara, N.; Imaizumi, T.; Takeuchi, M.; Yamagishi, S. Insulin resistance is an independent correlate of high serum levels of advanced glycation end products (AGEs) and low testosterone in non-diabetic men. Oxid. Med. Cell. Longev. 2010, 3, 262–265. [Google Scholar] [CrossRef]
- Nakamura, T.; Sato, E.; Fujiwara, N.; Kawagoe, Y.; Ueda, Y.; Suzuki, T.; Yamada, S.; Takeuchi, M.; Fukami, K.; Ueda, S.; et al. Positive association of serum levels of advanced glycation end products and high mobility group box-1 with asymmetric dimethylarginine in nondiabetic chronic kidney disease patients. Metabolism 2009, 58, 1624–1628. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamagishi, S.; Matsui, T.; Adachi, H.; Takeuchi, M.; Imaizumi, T. Serum levels of soluble form of receptor for advanced glycation end products (sRAGE) are correlated with AGEs in both diabetic and non-diabetic subjects. Clin. Exp. Med. 2007, 7, 188–190. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamagishi, S.; Adachi, H.; Matsui, T.; Kurita-Nakamura, Y.; Takeuchi, M.; Inoue, H.; Imaizumi, T. Serum levels of soluble form of receptor for advanced glycation end products (sRAGE) are positively associated with circulating AGEs and soluble form of VCAM-1 in patients with type 2 diabetes. Microvasc. Res. 2008, 76, 52–56. [Google Scholar] [CrossRef]
- Tahara, N.; Yamagishi, S.; Kodama, N.; Tahara, A.; Honda, A.; Nitta, Y.; Igata, S.; Matsui, T.; Takeuchi, M.; Kaida, H.; et al. Clinical and biochemical factors associated with area and metabolic activity in the visceral and subcutaneous adipose tissues by FDG-PET/CT. J. Clin. Endocrinol. Metab. 2015, 100, E739–E747. [Google Scholar] [CrossRef] [Green Version]
- Tahara, A.; Tahara, N.; Yamagishi, S.; Honda, A.; Igata, S.; Nitta, Y.; Bekki, M.; Nakamura, T.; Sugiyama, Y.; Sun, J.; et al. Ratio of serum levels of AGEs to soluble RAGE is correlated with trimethylamine-N-oxide in non-diabetic subjects. Int. J. Food Sci. Nutr. 2017, 68, 1013–1020. [Google Scholar] [CrossRef]
- Yasuda, Y.; Aoki, H.; Fujita, W.; Fujibayashi, K.; Wakasa, M.; Kawai, Y.; Nakanishi, H.; Saito, K.; Yamada, S.; Takeuchi, M.; et al. Glyceraldehyde-derived advanced glycation end-products are associated with left ventricular ejection fraction and brain natriuretic peptide in patients with diabetic adverse cardiac remodeling. Scand. Cardiovasc. J. 2022, 56, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Ohnuma, T.; Takeuchi, M.; Katsuta, N.; Maeshima, H.; Takebayashi, Y.; Higa, M.; Nakamura, T.; Nishimon, S.; Sannohe, T.; et al. Altered serum glyceraldehyde-derived advanced glycation end product (AGE) and soluble AGE receptor levels indicate carbonyl stress in patients with schizophrenia. Neurosci. Lett. 2015, 593, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sato, E.; Fujiwara, N.; Kawagoe, Y.; Suzuki, T.; Ueda, Y.; Yamada, S.; Shoji, H.; Takeuchi, M.; Ueda, S.; et al. Circulating levels of advanced glycation end products (AGE) and interleukin-6 (IL-6) are independent determinants of serum asymmetric dimethylarginine (ADMA) levels in patients with septic shock. Pharmacol. Res. 2009, 60, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Iwata, D.; Kitaichi, N.; Takeuchi, M.; Sato, M.; Endo, N.; Iwabuchi, K.; Ando, R.; Fukuhara, J.; Kinoshita, S.; et al. Amelioration of experimental autoimmune uveoretinitis by inhibition of glyceraldehyde-derived advanced glycation end-product formation. J. Leukoc. Biol. 2014, 96, 1077–1085. [Google Scholar] [CrossRef]
- Miura, J.; Uchigata, Y.; Yamamoto, Y.; Takeuchi, M.; Sakurai, S.; Watanabe, T.; Yonekura, H.; Yamagishi, S.; Makita, Z.; Sato, A.; et al. AGE down-regulation of monocyte RAGE expression and its association with diabetic complications in type 1 diabetes. J. Diabetes Complicat. 2004, 18, 53–59. [Google Scholar] [CrossRef]
- Yokoi, M.; Yamagishi, S.; Takeuchi, M.; Ohgami, K.; Okamoto, T.; Saito, W.; Muramatsu, M.; Imaizumi, T.; Ohno, S. Elevations of AGE and vascular endothelial growth factor with decreased total antioxidant status in the vitreous fluid of diabetic patients with retinopathy. Br. J. Ophthalmol. 2005, 89, 673–675. [Google Scholar] [CrossRef]
- Nakamura, T.; Sato, E.; Fujiwara, N.; Kawagoe, Y.; Takeuchi, M.; Maeda, S.; Yamagishi, S. Atorvastain reduces proteinuria in non-diabetic chronic kidney disease patients partly via lowering serum levels of advanced glycation end products (AGEs). Oxid. Med. Cell. Longev. 2010, 3, 304–307. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Sato, E.; Fujiwara, N.; Kawagoe, Y.; Koide, H.; Ueda, Y.; Takeuchi, M.; Yamagishi, S. Calcium channel blocker inhibition of AGE and RAGE axis limits renal injury in nondiabetic patients with stage I or II chronic kidney disease. Clin. Cardiol. 2011, 34, 372–377. [Google Scholar] [CrossRef]
- Sakata, K.; Hayakawa, M.; Yano, Y.; Tamaki, N.; Yokota, N.; Eto, T.; Watanabe, R.; Hirayama, N.; Matsuo, T.; Kuroki, K.; et al. Efficacy of alogliptin, a dipeptidyl peptidase-4 inhibitor, on glucose parameters, the activity of the advanced glycation end product (AGE)-receptor for AGE (RAGE) axis and albuminuria in Japanese type 2 diabetes. Diabetes Metab. Res. Rev. 2013, 29, 624–630. [Google Scholar] [CrossRef]
- Nakamura, I.; Oyama, J.; Komoda, H.; Shiraki, A.; Sakamoto, Y.; Taguchi, I.; Hiwatashi, A.; Komatsu, A.; Takeuchi, M.; Yamagishi, S.; et al. Possible effects of glimepiride beyond glycemic control in patients with type 2 diabetes: A preliminary report. Cardiovasc. Diabetol. 2014, 13, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagisawa, K.; Ashihara, J.; Obata, S.; Wada, N.; Takeuchi, M.; Nishino, Y.; Maeda, S.; Ishibashi, Y.; Yamagishi, S. Switching to multiple daily injection therapy with glulisine improves glycemic control, vascular damage and treatment satisfaction in basal insulin glargine-injected diabetic patients. Diabetes Metab. Res. Rev. 2014, 30, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Jinnouchi, Y.; Yamagishi, S.; Takeuchi, M.; Ishida, S.; Jinnouchi, Y.; Jinnouchi, J.; Imaizumi, T. Atorvastatin decreases serum levels of advanced glycation end products (AGEs) in patients with type 2 diabetes. Clin. Exp. Med. 2006, 6, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, M.; Oyama, J.; Takeuchi, M.; Shibata, Y.; Yamamoto, Y.; Kawasaki, T.; Komoda, H.; Kodama, K.; Sakuma, M.; Toyoda, S.; et al. Acute effects of statin on reduction of angiopoietin-like 2 and glyceraldehyde-derived advanced glycation end-products levels in patients with acute myocardial infarction: A message from SAMIT (Statin for Acute Myocardial Infarction Trial). Heart Vessel. 2016, 31, 1583–1589. [Google Scholar] [CrossRef]
- Sakasai-Sakai, A.; Jinno, M.; Takeuchi, M. Usefulness of toxic AGEs (TAGE) as a biomarker for fertility treatment. J. Jpn. Mibyou Assoc. 2015, 21, 96. [Google Scholar]
- Jinno, M.; Nagai, R.; Takeuchi, M.; Watanabe, A.; Teruya, K.; Sugawa, H.; Hatakeyama, N.; Jinno, Y. Trapa bispinosa Roxb. extract lowers advanced glycation end-products and increases live births in older patients with assisted reproductive technology: A randomized controlled trial. Reprod. Biol. Endocrinol. 2021, 19, 149. [Google Scholar] [CrossRef]
- Ueda, S.; Yamagishi, S.; Takeuchi, M.; Kohno, K.; Shibata, R.; Matsumoto, Y.; Kaneyuki, U.; Fujimura, T.; Hayashida, A.; Okuda, S. Oral adsorbent AST-120 decreases serum levels of AGEs in patients with chronic renal failure. Mol. Med. 2006, 12, 180–184. [Google Scholar] [CrossRef]
- Fukami, K.; Yamagishi, S.; Sakai, K.; Kaida, Y.; Yokoro, M.; Ueda, S.; Wada, Y.; Takeuchi, M.; Shimizu, M.; Yamazaki, H.; et al. Oral L-carnitine supplementation increases trimethylamine-N-oxide but reduces markers of vascular injury in hemodialysis patients. J. Cardiovasc. Pharmacol. 2015, 65, 289–295. [Google Scholar] [CrossRef]
- Yokoi, M.; Yamagishi, S.; Takeuchi, M.; Matsui, T.; Yoshida, Y.; Ohgami, K.; Amano-Okamoto, T.; Ohno, S. Positive correlation between vitreous levels of advanced glycation end products and vascular endothelial growth factor in patients with diabetic retinopathy sufficiently treated with photocoagulation. Br. J. Ophthalmol. 2007, 91, 397–398. [Google Scholar] [CrossRef]
- Blennow, K.; Hampel, H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003, 2, 605–613. [Google Scholar] [CrossRef]
- Kang, J.H.; Ryoo, N.Y.; Shin, D.W.; Trojanowski, J.Q.; Shaw, L.M. Role of cerebrospinal fluid biomarkers in clinical trials for Alzheimer’s disease modifying therapies. Korean J. Physiol. Pharmacol. 2014, 18, 447–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarkowski, E.; Issa, R.; Sjögren, M.; Wallin, A.; Blennow, K.; Tarkowski, A.; Kumar, P. Increased intrathecal levels of the angiogenic factors VEGF and TGF-beta in Alzheimer’s disease and vascular dementia. Neurobiol. Aging 2002, 23, 237–243. [Google Scholar] [CrossRef]
- Zetterberg, H.; Andreasen, N.; Blennow, K. Increased cerebrospinal fluid levels of transforming growth factor-β1 in Alzheimer’s disease. Neurosci. Lett. 2004, 367, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Sato, T.; Takino, J.; Kobayashi, Y.; Furuno, S.; Kikuchi, S.; Yamagishi, S. Diagnostic utility of serum or cerebrospinal fluid levels of toxic advanced glycation end-products (TAGE) in early detection of Alzheimer’s disease. Med. Hypotheses 2007, 69, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
| Subjects | Main Findings | Ref. |
|---|---|---|
| Apparently Healthy | Serum TAGE levels independently correlated with decreases in the number and migratory activity of circulating EPC in apparently healthy subjects. | [86] |
| Health Examination (General Population) | Serum TAGE levels were independent determinants of PAI-1 in the general population. | [87] |
| A positive relationship was observed between fibrinogen levels and serum TAGE levels. TAGE-associated thrombogenic abnormalities may be involved in atherogenesis. | [75] | |
| Flow-mediated vasodilation correlated with serum levels of TAGE and sRAGE, and the ratio of TAGE to sRAGE. | [88] | |
| Non-Diabetic General Population (Outpatients) | Serum sRAGE levels were positively associated with serum TAGE levels. | [89] |
| LDL-C levels were independent determinants of serum TAGE levels. | [90] | |
| Serum TAGE levels independently correlated with serum PEDF levels. PEDF levels may be elevated in response to TAGE levels as a counter system against TAGE-elicited tissue damage. | [91] | |
| Serum TAGE levels independently correlated with the HOMA-IR index, suggesting that TAGE play a pathological role in insulin resistance. | [92] | |
| Adiponectin was inversely associated with the ratio of serum levels of TAGE to sRAGE and vascular inflammation. | [93] | |
| Serum levels of apoB48 were correlated with TAGE, PEDF, and adiponectin (inversely). | [94] | |
| Serum DPP-4 levels were independently associated with various metabolic parameters. TAGE may up-regulate cellular DPP-4 expression and subsequently increase circulating levels of DPP-4. | [95] | |
| Non-Diabetic Men | HOMA-IR was independently associated with high serum levels of TAGE and low testosterone. | [96] |
| Non-Diabetic Chronic Kidney Disease (CKD) | Serum TAGE and sRAGE levels correlated with each other, and TAGE and HMGB-1 were independently associated with ADMA. | [97] |
| Type 2 DM | Serum sRAGE levels were positively associated with serum TAGE levels. | [98] |
| Serum sRAGE levels were positively associated with serum TAGE and sVCAM-1 levels. | [99] | |
| Serum levels of TAGE and sRAGE were independent determinants of serum MCP-1 levels. | [13] | |
| Cardiovascular Disease (CVD)/Heart Failure | Serum TAGE levels were independently associated with vascular inflammation evaluated by FDG-PET, suggesting that serum TAGE levels are a biomarker that reflects vascular inflammation within an area of atherosclerosis. | [55] |
| Diurnal glycemic fluctuations (GF) were associated with the severity of CAD, even in prediabetic patients. GF and TAGE levels may play a pathological role in the progression of CAD. | [21] | |
| TAGE and medication for hypertension were independently associated with area of visceral adipose tissues, whereas medication for TAGE, DM, IMT, and PEDF were independent correlates of subcutaneous adipose tissue areas. | [100] | |
| Serum TAGE levels were independently associated with log TMA. The TAGE to sRAGE ratio correlated with log TMAO, a marker of cardiometabolic disorders. | [101] | |
| Serum TAGE and TNF-α levels were associated with LVEF and BNP values in patients with diabetic adverse cardiac remodeling. | [102] | |
| Infertile Women | Serum TAGE levels correlated with poor follicular and embryonic development and a lower likelihood of ongoing pregnancy. | [56] |
| Non-Alcoholic Steatohepatitis (NASH) | Serum TAGE levels were significantly higher in NASH patients than in NAFL or healthy controls. Moreover, TAGE inversely correlated with adiponectin. | [16] |
| Non-B or Non-C-Hepatocellular Carcinoma | Serum TAGE levels were significantly higher in NBNC-HCC patients than in NASH and control subjects. | [18] |
| Colorectal Cancer | Serum TAGE levels were not associated with the risk of colon cancer, but showed a positive association with the risk of rectal cancer. | [29] |
| Schizophrenia | Serum TAGE levels were significantly higher, and sRAGE levels were significantly lower in patients with acute schizophrenia than in healthy controls. | [103] |
| Septic Shock Patients | Serum ADMA levels were significantly elevated in patients with septic shock, and serum TAGE levels were independent determinants of ADMA. | [104] |
| Autoimmune Uveoretinitis | Serum TAGE levels were significantly higher for each etiology of uveitis (HLA-B27, VKH disease, Bechet’s disease, and sarcoidosis) than in healthy controls. | [105] |
| Diabetic Retinopathy/Nephropathy | In diabetic patients, serum TAGE levels increased as the stages of retinopathy and nephropathy developed. | [106] |
| Diabetic Retinopathy | A positive correlation was observed between vitreous levels of TAGE and VEGF in patients with diabetic retinopathy. | [107] |
| Subjects | Therapeutic Agents | Correlation Factor | Ref. |
|---|---|---|---|
| Healthy Humans | Collagen tripeptide (CTP) | A significant reduction in serum TAGE levels was observed in all subjects and in the high-risk group after the CTP treatment. | [22] |
| Non-Diabetic CKD | Statin (Atorvastatin) | Atorvastatin may attenuate proteinuria in non-diabetic CKD with dyslipidemia partly by reducing serum TAGE levels. | [108] |
| Non-Diabetic Hypertensive CKD | Calcium channel blocker (Azelnidipine) | A treatment with azelnidipine decreased serum levels of TAGE, sRAGE, and proteinuria. | [109] |
| Type 2 DM | α-Glucosidase inhibitor (Acarbose) | A treatment with acarbose significantly decreased serum TAGE and free fatty acid levels. | [14] |
| DPP-4 inhibitor (Alogliptin) | Serum TAGE levels were only reduced in patients with baseline TAGE >7 U/mL after a treatment with alogliptin. | [110] | |
| Sulfonyl urea (Glimepiride) | Glimepiride may repair tissue damage by decreasing serum TAGE levels. | [111] | |
| Insulin (Glulisine) | Switching to multiple daily injection therapy with glulisine decreased serum levels of TAGE and sRAGE. | [112] | |
| Statin (Atorvastatin) | Atorvastatin decreased serum TAGE levels in hypercholesterolaemic T2DM patients. | [113] | |
| JAPAN-ACS Sub-Study | Statin (Pitavastatin/Atorvastatin) | Serum TAGE levels significantly decreased with statin therapy, whereas sRAGE levels did not change. | [20] |
| SAMIT (Statin for Acute Myocardial Infarction Trial) | Statin (Atorvastatin) | Statin therapy initiated early after the onset reduced serum TAGE levels, and may exert cardioprotective effects in patients with AMI. | [114] |
| Infertile Women | DPP-4 inhibitor (Sitagliptin) | Ovarian dysfunction was attenuated, and ongoing pregnancy rates were significantly increased in the group treated with sitagliptin, which decreased serum TAGE levels. | [115] |
| Hishi (Trapa bispinosa Roxb.) extract | Hishi lowered serum TAGE levels and increased live births in older patients with ART. | [116] | |
| NASH with Dyslipidemia | Statin (Atorvastatin) | Atorvastatin decreased serum TAGE levels in NASH patients with dyslipidemia. | [17] |
| Non-Diabetic CRF Patients | Oral adsorbent (AST-120/Kremedin) | The administration of AST-120 significantly decreased serum TAGE levels in non-diabetic CRF patients. | [117] |
| Hemodialysis (HD) Patients | L-Carnitine | The vasculoprotective properties of L-carnitine in HD patients may be partly attributed to its inhibitory effects on TAGE. | [118] |
| Diabetic Retinopathy | Photocoagulation | A positive correlation was observed between vitreous levels of TAGE and VEGF in patients with diabetic retinopathy sufficiently treated with photocoagulation. | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeuchi, M.; Sakasai-Sakai, A.; Takata, T.; Takino, J.-i.; Koriyama, Y. Effects of Toxic AGEs (TAGE) on Human Health. Cells 2022, 11, 2178. https://doi.org/10.3390/cells11142178
Takeuchi M, Sakasai-Sakai A, Takata T, Takino J-i, Koriyama Y. Effects of Toxic AGEs (TAGE) on Human Health. Cells. 2022; 11(14):2178. https://doi.org/10.3390/cells11142178
Chicago/Turabian StyleTakeuchi, Masayoshi, Akiko Sakasai-Sakai, Takanobu Takata, Jun-ichi Takino, and Yoshiki Koriyama. 2022. "Effects of Toxic AGEs (TAGE) on Human Health" Cells 11, no. 14: 2178. https://doi.org/10.3390/cells11142178
APA StyleTakeuchi, M., Sakasai-Sakai, A., Takata, T., Takino, J.-i., & Koriyama, Y. (2022). Effects of Toxic AGEs (TAGE) on Human Health. Cells, 11(14), 2178. https://doi.org/10.3390/cells11142178






