MG-Pe: A Novel Galectin-3 Ligand with Antimelanoma Properties and Adjuvant Effects to Dacarbazine
Abstract
:1. Introduction
2. Results and Discussion
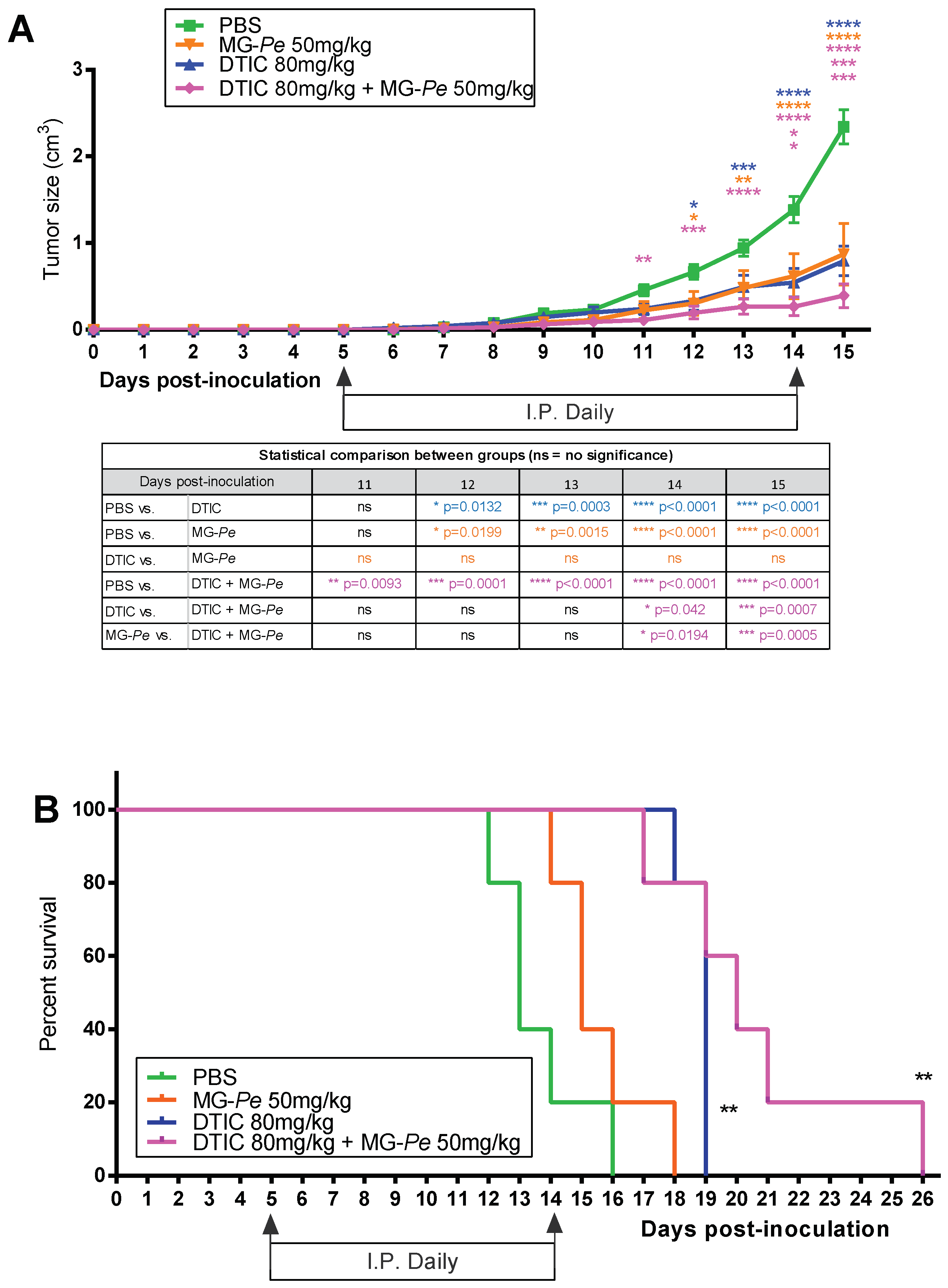
2.1. Adjuvant Effect and Increased Survival of Tumor-Bearing Mice Treated with MG-Pe
2.2. MG-Pe Reduces Experimental and Spontaneous Metastasis Development
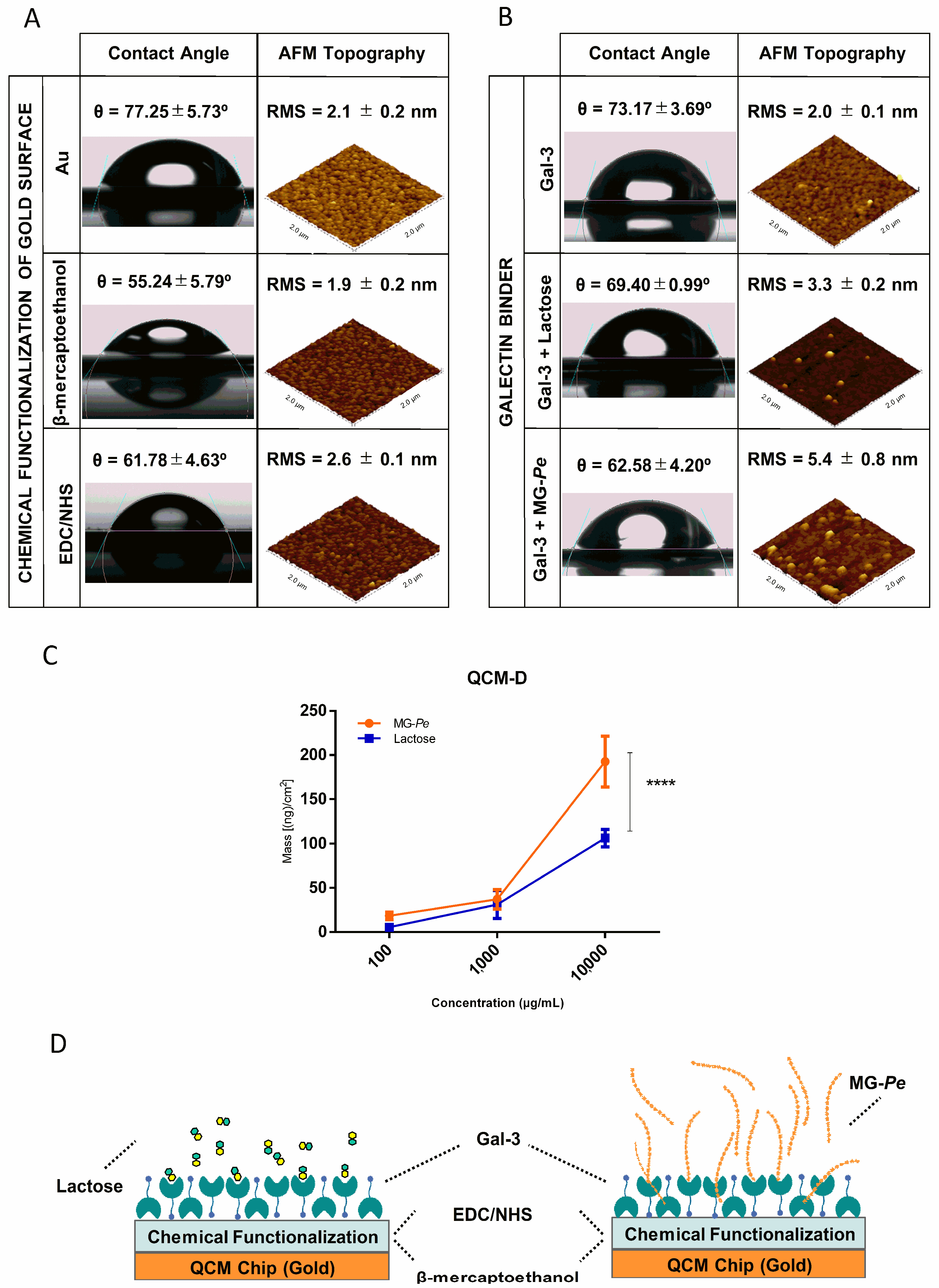
2.3. MG-Pe Binds Gal-3
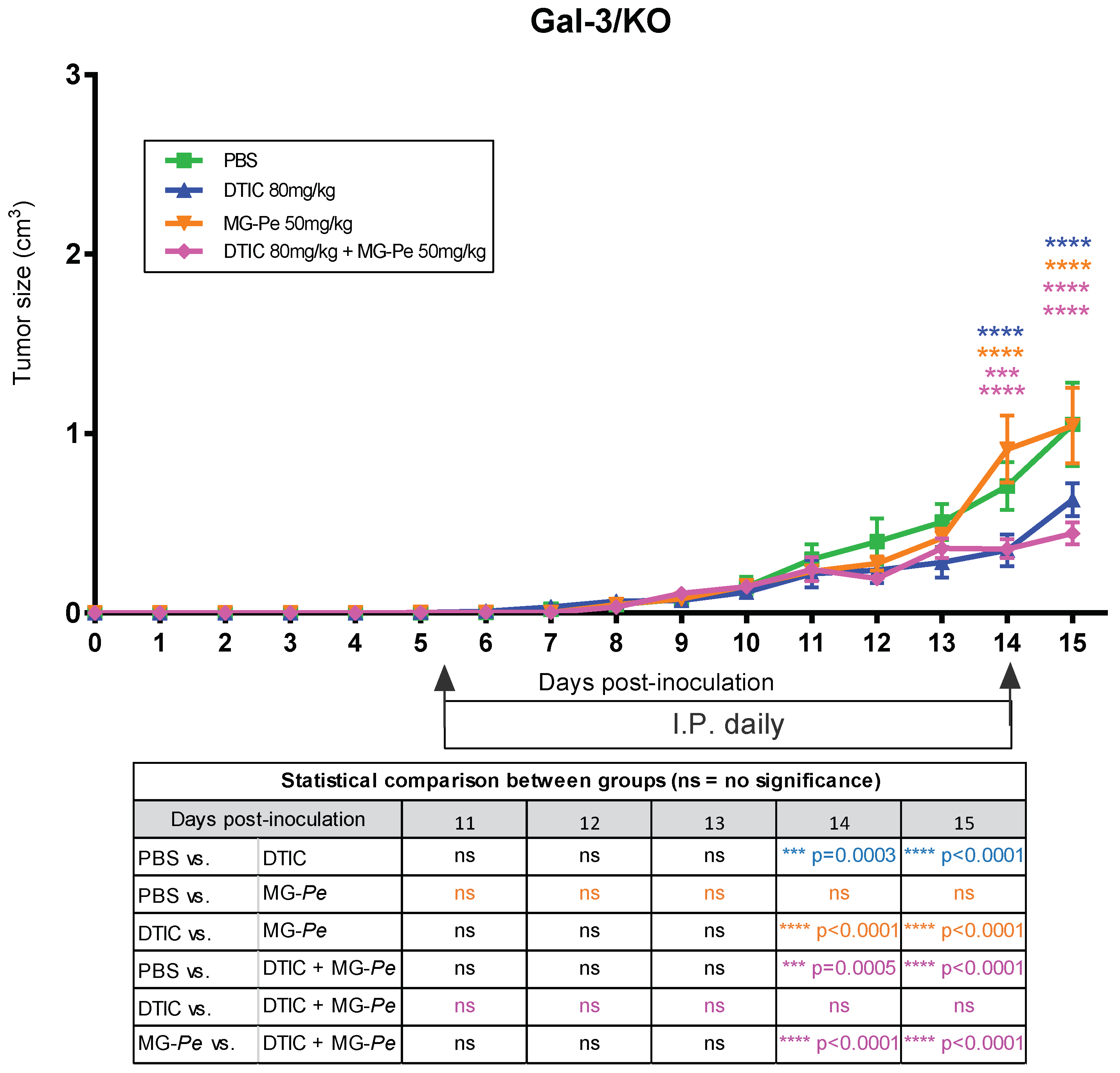
2.4. The Antitumoral Effects of MG-Pe Were Mediated by Inhibition of Gal-3
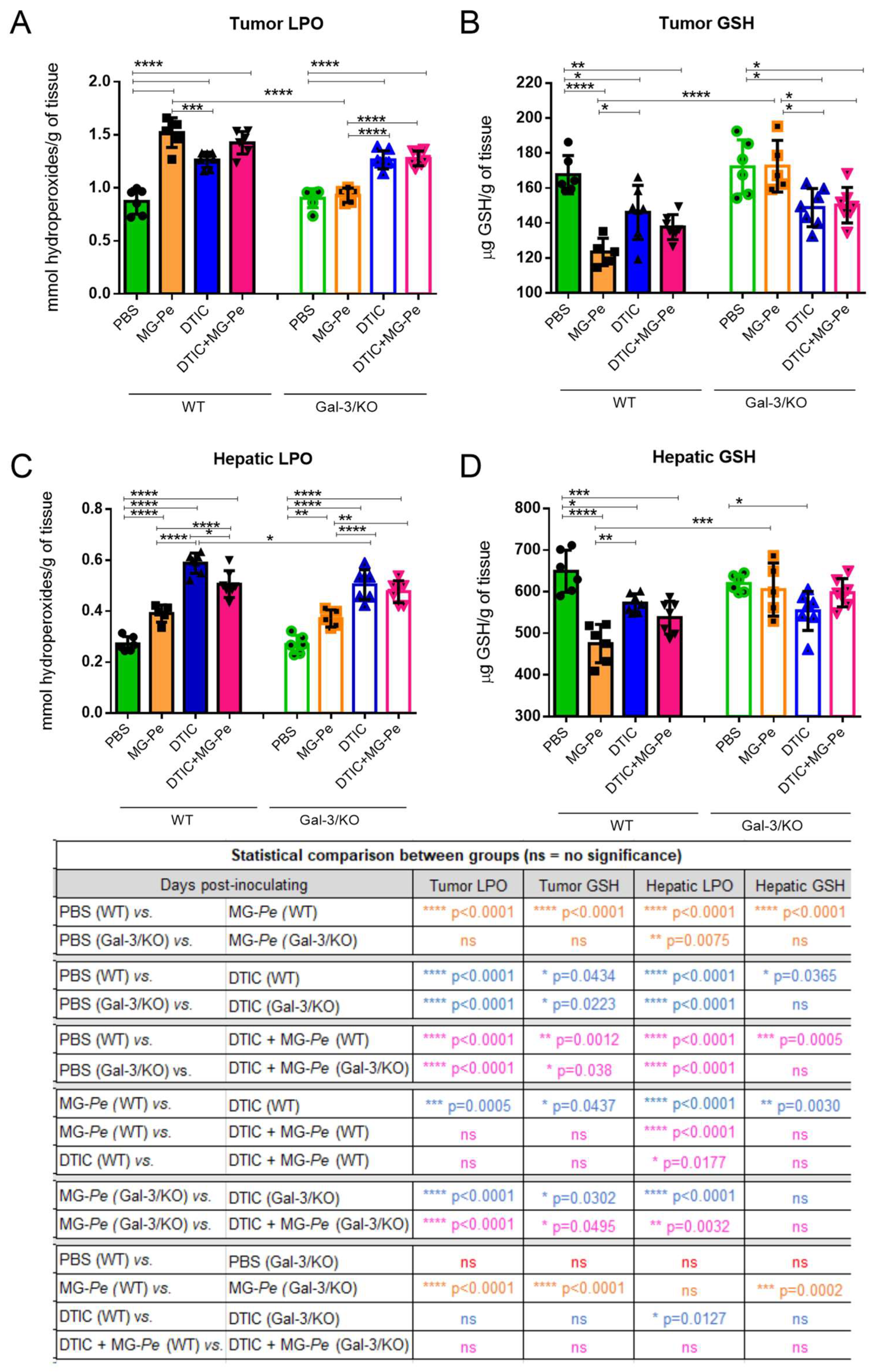
2.5. MG-Pe Modulated Oxidative Stress in Tumor and Liver Tissue, Mediated by Gal-3
3. Materials and Methods
3.1. Partially Methylated Mannogalactan (MG-Pe)
3.2. Cell Culture
3.3. In Vivo Experiments and Treatment Groups
3.3.1. Primary Tumor Model
3.3.2. Survival Assay
3.3.3. Metastases Assays—Experimental and Spontaneous
3.4. Galectin Binding Assay
3.4.1. Expression and Purification of Recombinant Human Gal-3
3.4.2. QCM (Quartz Crystal Microbalance)
3.4.3. AFM (Atomic Force Microscopy)
3.4.4. Contact Angle (CA) Tensiometer
3.5. Effect of MG-Pe via Gal-3
3.6. Oxidative Stress Assay
3.7. Statistical Analyses
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO—World Health Organization. Available online: https://www.who.int/en/news-room/fact-sheets/detail/cancer (accessed on 28 March 2022).
- WHO. WHO|Skin Cancers. Available online: http://www.who.int/uv/faq/skincancer/en/index1.html (accessed on 19 April 2022).
- Sandru, A.; Voinea, S.; Panaitescu, E.; Blidaru, A. Survival rates of patients with metastatic malignant melanoma. J. Med. Life 2014, 7, 572–576. [Google Scholar] [PubMed]
- Mishra, H.; Mishra, P.K.; Ekielski, A.; Jaggi, M.; Iqbal, Z.; Talegaonkar, S. Melanoma treatment: From conventional to nanotechnology. J. Cancer Res. Clin. Oncol. 2018, 144, 2283–2302. [Google Scholar] [CrossRef] [PubMed]
- Domingues, B.; Lopes, J.; Soares, P.; Populo, H. Melanoma treatment in review. ImmunoTargets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Ministério da Saúde do Brasil. Portaria n° 357 de 8 de abril de 2013-Diretrizes Diagnósticas e Terapêuticas do Melanoma Maligno Cutâneo; Ministério da Saúde do Brasil: Brazil, 2013; p. 16. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/sas/2013/prt0357_08_04_2013.html (accessed on 4 July 2022).
- de Miranda Corrêa, F.; Guerra, R.L.; Fernandes, R.R.A.; Souza, M.C.D.; Zimmermann, I.R. Terapia-alvo versus dacarbazina no tratamento de primeira linha do melanoma avançado não cirúrgico e metastático: Análise de impacto orçamentário na perspectiva do Sistema Único de Saúde, 2018–2020*. Epidemiol. Serviços Saúde 2019, 28, e2018325. [Google Scholar] [CrossRef] [Green Version]
- Guerra, R.L.; de Miranda Corrêa, F.; Fernandes, R.R.A.; Zimmerman, I.R. Cost Utility of Target Therapies Compared to Dacarbazine for First-Line Treatment of Advanced Non-Surgical and Metastatic Melanoma in the Brazilian National Health System. Value Health Reg. Issues 2019, 20, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Al-Badr, A.A.; Alodhaib, M.M. Dacarbazine. Profiles Drug Subst. Excip. Relat. Methodol. 2016, 41, 323–377. [Google Scholar] [PubMed]
- Sun, Y. Tumor microenvironment and cancer therapy resistance. Cancer Lett. 2015, 380, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef] [Green Version]
- Falcone, I.; Conciatori, F.; Bazzichetto, C.; Ferretti, G.; Cognetti, F.; Ciuffreda, L.; Milella, M. Tumor Microenvironment: Implications in Melanoma Resistance to Targeted Therapy and Immunotherapy. Cancers 2020, 12, 2870. [Google Scholar] [CrossRef]
- Marusyk, A.; Janiszewska, M.; Polyak, K. Intratumor Heterogeneity: The Rosetta Stone of Therapy Resistance. Cancer Cell 2020, 37, 471–484. [Google Scholar] [CrossRef]
- Jardim, D.L.; De Melo Gagliato, D.; Nikanjam, M.; Barkauskas, D.A.; Kurzrock, R. Efficacy and safety of anticancer drug combinations: A meta-analysis of randomized trials with a focus on immunotherapeutics and gene-targeted compounds. Oncoimmunology 2020, 9, 1710052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biscaia, S.M.P.; Carbonero, E.R.; Bellan, D.L.; Borges, B.S.; Costa, C.R.; Rossi, G.R.; Gonçalves, J.P.; Melo, C.M.; Lívero, F.A.R.; Ruthes, A.C.; et al. Safe therapeutics of murine melanoma model using a novel antineoplasic, the partially methylated mannogalactan from Pleurotus eryngii. Carbohydr. Polym. 2017, 178, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Nangia-Makker, P.; Hogan, V.; Raz, A. Galectin-3 and cancer stemness. Glycobiology 2018, 28, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.R.; Doig, T.; Anderson, N.; Brenn, T.; Doherty, V.; Xu, Y.; Bartlett, J.M.S.; Smyth, J.F.; Melton, D.W. Association of galectin-3 expression with melanoma progression and prognosis. Eur. J. Cancer 2012, 48, 865–874. [Google Scholar] [CrossRef]
- Braeuer, R.R.; Zigler, M.; Kamiya, T.; Dobroff, A.S.; Huang, L.; Choi, W.; McConkey, D.J.; Shoshan, E.; Mobley, A.K.; Song, R.; et al. Galectin-3 Contributes to Melanoma Growth and Metastasis via Regulation of NFAT1 and Autotaxin. Cancer Res. 2012, 72, 5757–5766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortuna-Costa, A.; Gomes, A.M.; Kozlowski, E.O.; Stelling, M.P.; Pavão, M.S.G. Extracellular Galectin-3 in Tumor Progression and Metastasis. Front. Oncol. 2014, 4, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comodo, A.N.; Soares, M.F.; de Paulo Castro Teixeira, V.; Franco, M.; Lacerda Bachi, A.L. Galectin-3 expression favors metastasis in murine melanoma. Adv. Biosci. Biotechnol. 2013, 4, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Harazono, Y.; Kho, D.H.; Balan, V.; Nakajima, K.; Hogan, V.; Raz, A. Extracellular galectin-3 programs multidrug resistance through Na+/K+-ATPase and P-glycoprotein signaling. Oncotarget 2015, 6, 19592–19604. [Google Scholar] [CrossRef] [Green Version]
- Takenaka, Y.; Fukumori, T.; Raz, A. Galectin-3 and metastasis. Glycoconj. J. 2002, 19, 543–549. [Google Scholar] [CrossRef]
- Ahmed, H.; Guha, P.; Kaptan, E.; Bandyopadhyaya, G. Galectin-3: A potential target for cancer prevention. Trends Carbohydr. Res. 2011, 3, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Jeethy Ram, T.; Lekshmi, A.; Somanathan, T.; Sujathan, K. Galectin-3: A factotum in carcinogenesis bestowing an archery for prevention. Tumour Biol. 2021, 43, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Ikemori, R.Y.; Machado, C.M.L.; Furuzawa, K.M.; Nonogaki, S.; Osinaga, E.; Umezawa, K.; De Carvalho, M.A.; Verinaud, L.; Chammas, R. Galectin-3 up-regulation in hypoxic and nutrient deprived microenvironments promotes cell survival. PLoS ONE 2014, 9, e111592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimian, R.; Béland, L.C.; Sato, S.; Kriz, J. Microglia-derived galectin-3 in neuroinflammation; a bittersweet ligand? Med. Res. Rev. 2021, 41, 2582–2589. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, R.; Béland, L.C.; Kriz, J. Galectin-3: Mediator of microglia responses in injured brain. Drug Discov. Today 2018, 23, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, R.; Lively, S.; Abdelhamid, E.; Lalancette-Hebert, M.; Schlichter, L.; Sato, S.; Kriz, J. Delayed Galectin-3-Mediated Reprogramming of Microglia After Stroke is Protective. Mol. Neurobiol. 2019, 56, 6371–6385. [Google Scholar] [CrossRef]
- Wdowiak, K.; Francuz, T.; Gallego-Colon, E.; Ruiz-Agamez, N.; Kubeczko, M.; Grochoła, I.; Wojnar, J. Galectin Targeted Therapy in Oncology: Current Knowledge and Perspectives. Int. J. Mol. Sci. 2018, 19, 210. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, H.; Yu, X.; Collins, P.M.; Bum-Erdene, K. Galectin-3 inhibitors: A patent review (2008–present). Expert Opin. Ther. Pat. 2014, 24, 1053–1065. [Google Scholar] [CrossRef]
- NIH. Galectin Inhibitor (GR-MD-02) and Ipilimumab in Patients with Metastatic Melanoma-NCT02117362. Available online: https://clinicaltrials.gov/ct2/show/study/NCT02117362?term=gr-md-02&cond=cancer&draw=2&rank=3 (accessed on 23 June 2022).
- Lee, Y. Reconstitution of galectin-3 alters glutathione content and potentiates TRAIL-induced cytotoxicity by dephosphorylation of Akt. Exp. Cell Res. 2003, 288, 21–34. [Google Scholar] [CrossRef]
- Wu, W.-S. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006, 25, 695–705. [Google Scholar] [CrossRef]
- Ziech, D.; Franco, R.; Pappa, A.; Panayiotidis, M.I. Reactive Oxygen Species (ROS)––Induced genetic and epigenetic alterations in human carcinogenesis. Mutat. Res. Mol. Mech. Mutagen. 2011, 711, 167–173. [Google Scholar] [CrossRef]
- Liu-Smith, F.; Dellinger, R.; Meyskens, F.L. Updates of reactive oxygen species in melanoma etiology and progression. Arch. Biochem. Biophys. 2014, 563, 51–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sander, C.S.; Hamm, F.; Elsner, P.; Thiele, J.J. Oxidative stress in malignant melanoma and non-melanoma skin cancer. Br. J. Dermatol. 2003, 148, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Milkovic, L.; Cipak Gasparovic, A.; Cindric, M.; Mouthuy, P.-A.; Zarkovic, N. Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells 2019, 8, 793. [Google Scholar] [CrossRef] [Green Version]
- Wittgen, H.G.M.; van Kempen, L.C.L.T. Reactive oxygen species in melanoma and its therapeutic implications. Melanoma Res. 2007, 17, 400–409. [Google Scholar] [CrossRef] [PubMed]
- NIH. Safety of GM-CT-01 with and without 5-Fluorouracil in Patients with Solid Tumors-NCT00054977. Available online: https://clinicaltrials.gov/ct2/show/NCT00054977?term=gm-ct-01&cond=cancer&draw=2&rank=4 (accessed on 23 June 2022).
- Platt, D.; Klyosov, A. Patent-Selectively Depolymerized Galactomannan Polysaccharide. U.S. Patent No. 7,893,252 B2, 22 February 2011. Available online: https://patentimages.storage.googleapis.com/79/ad/80/04ce3e1adad964/US7893252.pdf (accessed on 4 July 2022).
- NIH. A Phase III Study to Investigate Toripalimab versus Dacarbazine as the First Line Therapy for Unresectable or Metastatic Melanoma-NCT03430297. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03430297?cond=dacarbazine&draw=2&rank=6 (accessed on 23 June 2022).
- Jiang, G.; Li, R.-H.; Sun, C.; Liu, Y.-Q.; Zheng, J.-N. Dacarbazine Combined Targeted Therapy versus Dacarbazine Alone in Patients with Malignant Melanoma: A Meta-Analysis. PLoS ONE 2014, 9, e111920. [Google Scholar] [CrossRef] [Green Version]
- Adami, E.R.; Corso, C.R.; Turin-Oliveira, N.M.; Galindo, C.M.; Milani, L.; Stipp, M.C.; da Silva, L.C.M.; do Nascimento, G.E.; Chaves, P.F.P.; Chequin, A.; et al. Polysaccharides from green sweet pepper increase the antineoplastic effect of methotrexate on mammary tumor cells. Int. J. Biol. Macromol. 2020, 158, 1071–1081. [Google Scholar] [CrossRef]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [Green Version]
- Bellan, D.L.; Biscaia, S.M.P.; Rossi, G.R.; Cristal, A.M.; Gonçalves, J.P.; Oliveira, C.C.; Simas, F.F.; Sabry, D.A.; Rocha, H.A.O.; Franco, C.R.C.; et al. Green does not always mean go: A sulfated galactan from Codium isthmocladum green seaweed reduces melanoma metastasis through direct regulation of malignancy features. Carbohydr. Polym. 2020, 250, 116869. [Google Scholar] [CrossRef]
- Bellan, D.L.; Mazepa, E.; Biscaia, S.M.P.; Gonçalves, J.P.; Oliveira, C.C.; Rossi, G.R.; Ferreira, L.G.; Noseda, M.D.; Trindade, E.S.; Duarte, M.E.R.; et al. Non-Cytotoxic Sulfated Heterorhamnan from Gayralia brasiliensis Green Seaweed Reduces Driver Features of Melanoma Metastatic Progression. Mar. Biotechnol. 2020, 22, 194–206. [Google Scholar] [CrossRef]
- Yan, J.; Meng, Y.; Zhang, M.; Zhou, X.; Cheng, H.; Sun, L.; Zhou, Y. A 3-O-methylated heterogalactan from Pleurotus eryngii activates macrophages. Carbohydr. Polym. 2019, 206, 706–715. [Google Scholar] [CrossRef]
- Nesmelova, I.V.; Dings, R.P.M.; Mayo, K.H. Understanding Galectin Structure–Function Relationships to Design Effective Antagonists. In Galectins; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 33–69. ISBN 9780470373187. [Google Scholar]
- Miller, M.C.; Klyosov, A.; Mayo, K.H. The α-galactomannan Davanat binds galectin-1 at a site different from the conventional galectin carbohydrate binding domain. Glycobiology 2009, 19, 1034–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethi, A.; Sanam, S.; Alvala, R.; Alvala, M. An updated patent review of galectin-1 and galectin-3 inhibitors and their potential therapeutic applications (2016–present). Expert Opin. Ther. Pat. 2021, 31, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, Y.; Zhang, F.; Linhardt, R.J.; Zeng, G.; Zhang, A. Extraction, structure and bioactivities of the polysaccharides from Pleurotus eryngii: A review. Int. J. Biol. Macromol. 2020, 150, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.C.; Ippel, H.; Suylen, D.; Klyosov, A.A.; Traber, P.G.; Hackeng, T.; Mayo, K.H. Binding of polysaccharides to human galectin-3 at a noncanonical site in its carbohydrate recognition domain. Glycobiology 2015, 26, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Hara, A.; Niwa, M.; Noguchi, K.; Kanayama, T.; Niwa, A.; Matsuo, M.; Hatano, Y.; Tomita, H. Galectin-3 as a next-generation biomarker for detecting early stage of various diseases. Biomolecules 2020, 10, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capone, E.; Iacobelli, S.; Sala, G. Role of galectin 3 binding protein in cancer progression: A potential novel therapeutic target. J. Transl. Med. 2021, 19, 1–18. [Google Scholar] [CrossRef]
- Newlaczyl, A.U.; Yu, L.-G. Galectin-3—A jack-of-all-trades in cancer. Cancer Lett. 2011, 313, 123–128. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, L.; Liao, W.; Chen, H.; Du, Z.; Shao, C.; Wang, P.; Ding, K. HH1-1, a novel Galectin-3 inhibitor, exerts anti-pancreatic cancer activity by blocking Galectin-3/EGFR/AKT/FOXO3 signaling pathway. Carbohydr. Polym. 2019, 204, 111–123. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Qin, Y.; Cong, Q.; Shao, C.; Du, Z.; Ni, X.; Li, P.; Ding, K. RN1, a novel galectin-3 inhibitor, inhibits pancreatic cancer cell growth in vitro and in vivo via blocking galectin-3 associated signaling pathways. Oncogene 2017, 36, 1297–1308. [Google Scholar] [CrossRef]
- Cardoso, A.C.F.; de Sousa Andrade, L.N.; Bustos, S.O.; Chammas, R. Galectin-3 Determines Tumor Cell Adaptive Strategies in Stressed Tumor Microenvironments. Front. Oncol. 2016, 6, 127. [Google Scholar] [CrossRef] [Green Version]
- Machado, C.M.L.; Andrade, L.N.S.; Teixeira, V.R.; Costa, F.F.; Melo, C.M.; dos Santos, S.N.; Nonogaki, S.; Liu, F.T.; Bernardes, E.S.; Camargo, A.A.; et al. Galectin-3 disruption impaired tumoral angiogenesis by reducing VEGF secretion from TGFb1-induced macrophages. Cancer Med. 2014, 3, 201–214. [Google Scholar] [CrossRef] [PubMed]
- NIH. Dacarbazine; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548913/ (accessed on 4 July 2022).
- Woiniak, A.; Drewa, G.; Woźniak, B.; Schachtschabel, D.O.; Mila-Kierzenkowska, C.; Drewa, T.; Olszewska-Słonina, D.; Sopońska, M. The effect of antitumor drugs on oxidative stress in B16 and S91 melanoma cells in vitro. Med. Sci. Monit. 2005, 11, BR22–BR29. [Google Scholar] [PubMed]
- Deneke, S.M.; Fanburg, B.L. Regulation of cellular glutathione. Am. J. Physiol. Cell. Mol. Physiol. 1989, 257, L163–L173. [Google Scholar] [CrossRef] [PubMed]
- Gaté, L.; Paul, J.; Ba, G.N.; Tew, K.D.; Tapiero, H. Oxidative stress induced in pathologies: The role of antioxidants. Biomed. Pharmacother. 1999, 53, 169–180. [Google Scholar] [CrossRef]
- Barrera, G. Oxidative Stress and Lipid Peroxidation Products in Cancer Progression and Therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landriscina, M.; Maddalena, F.; Laudiero, G.; Esposito, F. Adaptation to Oxidative Stress, Chemoresistance, and Cell Survival. Antioxid. Redox Signal. 2009, 11, 2701–2716. [Google Scholar] [CrossRef]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of Glutathione in Cancer Progression and Chemoresistance. Oxid. Med. Cell. Longev. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Calvert, P.; Yao, K.-S.; Hamilton, T.C.; O’Dwyer, P.J. Clinical studies of reversal of drug resistance based on glutathione. Chem. Biol. Interact. 1998, 111–112, 213–224. [Google Scholar] [CrossRef]
- Estrela, J.M.; Ortega, A.; Obrador, E. Glutathione in Cancer Biology and Therapy. Crit. Rev. Clin. Lab. Sci. 2006, 43, 143–181. [Google Scholar] [CrossRef]
- De Visser, K.E.; Jonkers, J. Towards Understanding the Role of Cancer-Associated Inflammation in Chemoresistance. Curr. Pharm. Des. 2009, 15, 1844–1853. [Google Scholar] [CrossRef]
- Glasauer, A.; Chandel, N.S. Targeting antioxidants for cancer therapy. Biochem. Pharmacol. 2014, 92, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Matarrese, P.; Tinari, N.; Semeraro, M.L.; Natoli, C.; Iacobelli, S.; Malorni, W. Galectin-3 overexpression protects from cell damage and death by influencing mitochondrial homeostasis. FEBS Lett. 2000, 473, 311–315. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-S.; Li, X.-T.; Yu, L.-G.; Wang, L.; Shi, Z.-Y.; Guo, X.-L. Roles of galectin-3 in metabolic disorders and tumor cell metabolism. Int. J. Biol. Macromol. 2020, 142, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.-K.; Lee, Y.J.; Battle, P.; Jessup, J.M.; Raz, A.; Kim, H.-R.C. Galectin-3 Protects Human Breast Carcinoma Cells against Nitric Oxide-Induced Apoptosis. Am. J. Pathol. 2001, 159, 1055–1060. [Google Scholar] [CrossRef]
- Song, Y.K.; Billiar, T.R.; Lee, Y.J. Role of Galectin-3 in Breast Cancer Metastasis. Am. J. Pathol. 2002, 160, 1069–1075. [Google Scholar] [CrossRef]
- Sun, W.; Li, L.; Li, L.J.; Yang, Q.Q.; Zhang, Z.R.; Huang, Y. Two birds, one stone: Dual targeting of the cancer cell surface and subcellular mitochondria by the galectin-3-binding peptide G3-C12. Acta Pharmacol. Sin. 2017, 38, 806–822. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Tamta, A.K.; Sarikhani, M.; Desingu, P.A.; Kizkekra, S.M.; Pandit, A.S.; Kumar, S.; Khan, D.; Raghavan, S.C.; Sundaresan, N.R. Subcutaneous Ehrlich Ascites Carcinoma mice model for studying cancer-induced cardiomyopathy. Sci. Rep. 2018, 8, 5599. [Google Scholar] [CrossRef]
- Hsu, D.K.; Yang, R.-Y.; Pan, Z.; Yu, L.; Salomon, D.R.; Fung-Leung, W.-P.; Liu, F.-T. Targeted Disruption of the Galectin-3 Gene Results in Attenuated Peritoneal Inflammatory Responses. Am. J. Pathol. 2000, 156, 1073–1083. [Google Scholar] [CrossRef] [Green Version]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Hunt, J.V.; Wolff, S.P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal. Biochem. 1992, 202, 384–389. [Google Scholar] [CrossRef]
- Biscaia, S.M.P.; Carbonero, E.R.; Trindade, E.S.; Franco, C.R.C.; de Oliveira, C.C.; Chammas, R.; Iacomini, M. Patent application: Manogalactana parcialmente metilada de cogumelo Pleurotus eryngii possui atividade antitumoral-BR 102018005595-0 A2. 2018. Available online: https://spin.ufpr.br/wp-content/uploads/2022/03/Publicacao_PI0458_BR1020180055950.pdf (accessed on 4 July 2022).
- Salomonsson, E.; Thijssen, V.L.; Griffioen, A.W.; Nilsson, U.J.; Leffler, H. The Anti-angiogenic Peptide Anginex Greatly Enhances Galectin-1 Binding Affinity for Glycoproteins. J. Biol. Chem. 2011, 286, 13801–13804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dings, R.P.M.; Kumar, N.; Miller, M.C.; Loren, M.; Rangwala, H.; Hoye, T.R.; Mayo, K.H. Structure-Based Optimization of Angiostatic Agent 6DBF7, an Allosteric Antagonist of Galectin-1. J. Pharmacol. Exp. Ther. 2013, 344, 589–599. [Google Scholar] [CrossRef] [Green Version]
- Astorgues-Xerri, L.; Riveiro, M.E.; Tijeras-Raballand, A.; Serova, M.; Rabinovich, G.A.; Bieche, I.; Vidaud, M.; de Gramont, A.; Martinet, M.; Cvitkovic, E.; et al. OTX008, a selective small-molecule inhibitor of galectin-1, downregulates cancer cell proliferation, invasion and tumour angiogenesis. Eur. J. Cancer 2014, 50, 2463–2477. [Google Scholar] [CrossRef] [PubMed]
- Dings, R.P.M.; Miller, M.C.; Nesmelova, I.; Astorgues-Xerri, L.; Kumar, N.; Serova, M.; Chen, X.; Raymond, E.; Hoye, T.R.; Mayo, K.H. Antitumor Agent Calixarene 0118 Targets Human Galectin-1 as an Allosteric Inhibitor of Carbohydrate Binding. J. Med. Chem. 2012, 55, 5121–5129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NIH. Study Evaluating the Efficacy and Safety of Belapectin for the Prevention of Esophageal Varices in NASH Cirrhosis-NCT04365868. Available online: https://clinicaltrials.gov/ct2/show/NCT04365868 (accessed on 23 June 2022).
- Fang, T.; Liu, D.; Ning, H.; Liu, D.; Sun, J.; Huang, X.; Dong, Y.; Geng, M.; Yun, S.; Yan, J.; et al. Modified citrus pectin inhibited bladder tumor growth through downregulation of galectin-3. Acta Pharmacol. Sin. 2018, 39, 1885–1893. [Google Scholar] [CrossRef] [Green Version]
- NIH. A Study to Test the Efficacy and Safety of Inhaled GB0139 in Subjects with Idiopathic Pulmonary Fibrosis (IPF)-NCT03832946. Available online: https://clinicaltrials.gov/ct2/show/NCT03832946 (accessed on 23 June 2022).
- NIH. DEFINE-Evaluating Therapies for COVID-19-NCT04473053. Available online: https://clinicaltrials.gov/ct2/show/NCT04473053 (accessed on 23 June 2022).
- NIH. A First in Human Study to Evaluate the Safety, Tolerability and PK of GB1211 in Healthy Subjects-NCT03809052. Available online: https://clinicaltrials.gov/ct2/show/NCT03809052 (accessed on 23 June 2022).
- NIH. Galecto’s Oral Galectin-3 Inhibitor GB1211 Well Tolerated in Phase I Clinical Data Presented Recently at Several Scientific Conferences—Galecto, Inc. Available online: https://galecto.com/gb1211-data/ (accessed on 23 June 2022).
- NIH. Study of Efficacy and Safety of MBG453 in Combination with Azacitidine in Subjects with Intermediate, High or Very High Risk Myelodysplastic Syndrome (MDS) as Per IPSS-R, or Chronic Myelomonocytic Leukemia-2 (CMML-2)-NCT04266301. Available online: https://clinicaltrials.gov/ct2/show/NCT04266301 (accessed on 23 June 2022).
- NIH. A Study of MBG453 in Combination with Azacitidine and Venetoclax in AML Patients Unfit for Chemotherapy-NCT04150029. Available online: https://clinicaltrials.gov/ct2/show/NCT04150029 (accessed on 23 June 2022).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biscaia, S.M.P.; Pires, C.; Lívero, F.A.R.; Bellan, D.L.; Bini, I.; Bustos, S.O.; Vasconcelos, R.O.; Acco, A.; Iacomini, M.; Carbonero, E.R.; et al. MG-Pe: A Novel Galectin-3 Ligand with Antimelanoma Properties and Adjuvant Effects to Dacarbazine. Int. J. Mol. Sci. 2022, 23, 7635. https://doi.org/10.3390/ijms23147635
Biscaia SMP, Pires C, Lívero FAR, Bellan DL, Bini I, Bustos SO, Vasconcelos RO, Acco A, Iacomini M, Carbonero ER, et al. MG-Pe: A Novel Galectin-3 Ligand with Antimelanoma Properties and Adjuvant Effects to Dacarbazine. International Journal of Molecular Sciences. 2022; 23(14):7635. https://doi.org/10.3390/ijms23147635
Chicago/Turabian StyleBiscaia, Stellee M. P., Cassiano Pires, Francislaine A. R. Lívero, Daniel L. Bellan, Israel Bini, Silvina O. Bustos, Renata O. Vasconcelos, Alexandra Acco, Marcello Iacomini, Elaine R. Carbonero, and et al. 2022. "MG-Pe: A Novel Galectin-3 Ligand with Antimelanoma Properties and Adjuvant Effects to Dacarbazine" International Journal of Molecular Sciences 23, no. 14: 7635. https://doi.org/10.3390/ijms23147635






