Formulation of Novel Composite (Activated Nanoclay/Hydrocolloid of Nostoc sphaericum) and Its Application in the Removal of Heavy Metals from Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Treatment and Activation of Nanoclay
2.3. Determination of Cation Exchange Capacity
2.4. Clay and Hydrocolloid Characterization
2.5. Composite Preparation
2.6. Evaluation of Metal Adsorption
2.7. Determination of Adsorption Isotherms
2.8. Evaluation of Adsorption Kinetics
3. Results and Discussion
3.1. Clay Cation Exchange Capacity
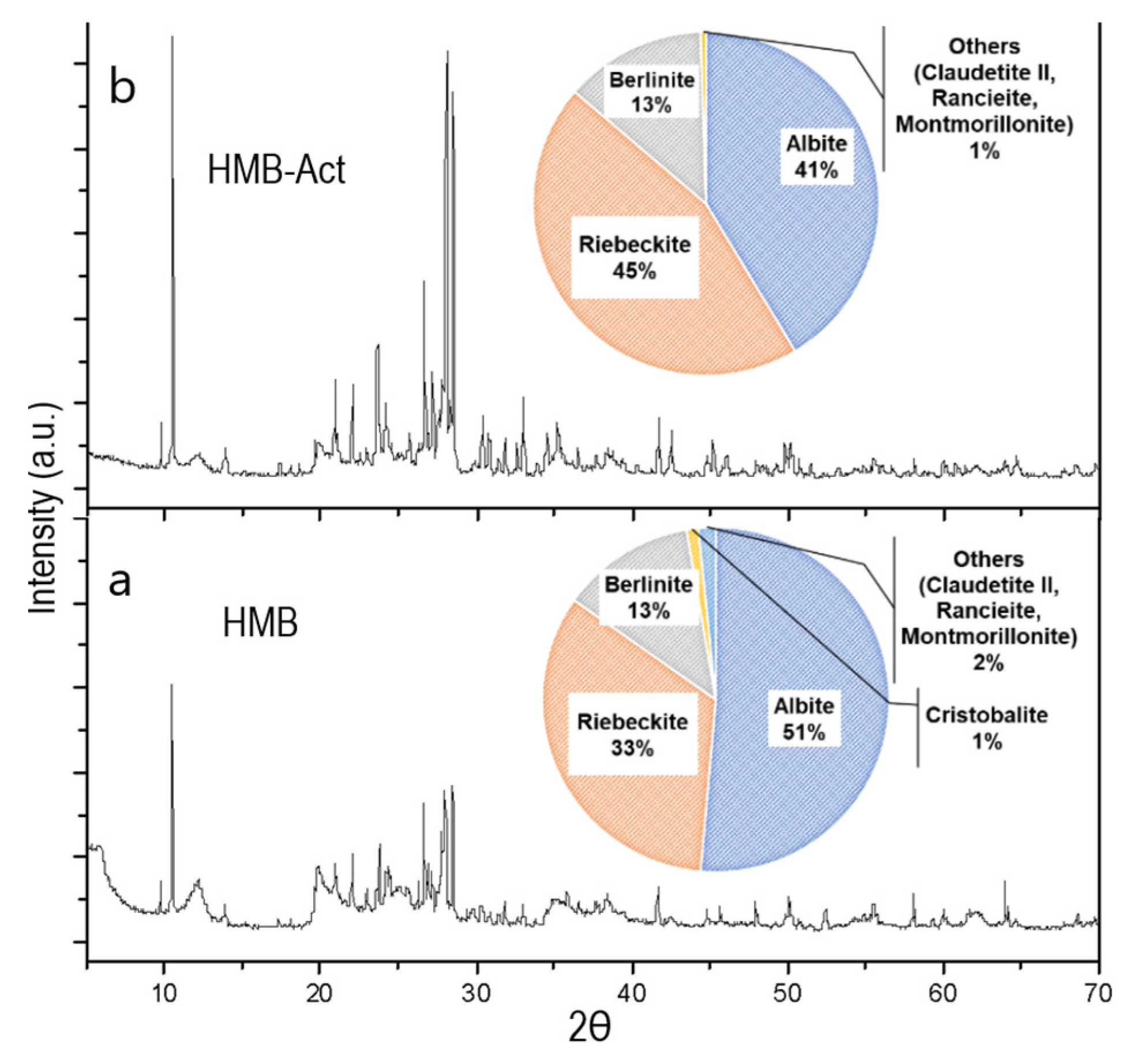
3.2. X-ray Analysis of Clay
3.3. Particle Size, ζ Potential, and SEM Images of the Clay and Hydrocolloid
3.4. Metal Adsorption
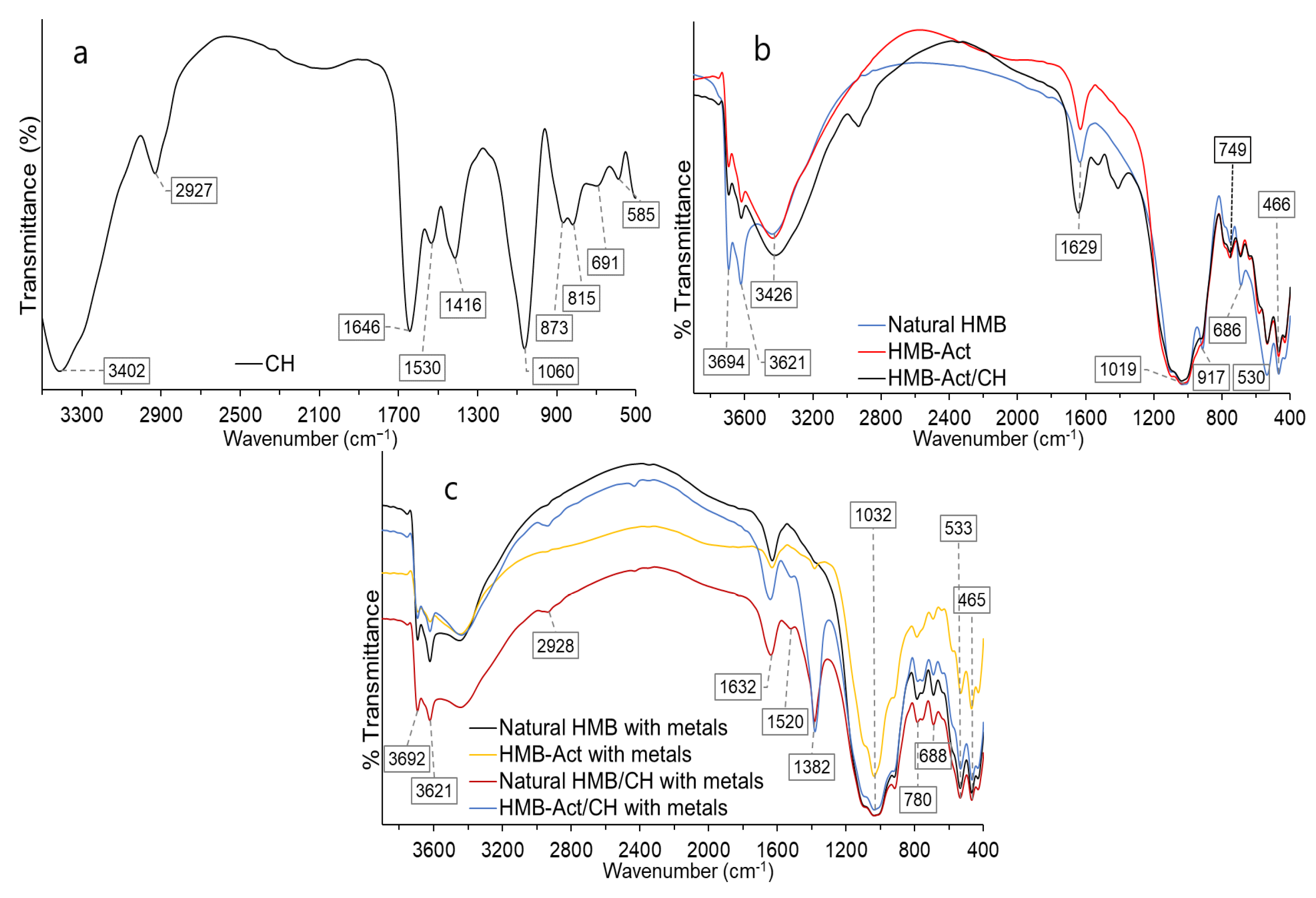
3.5. IR Analysis of Composites Subjected to Adsorption
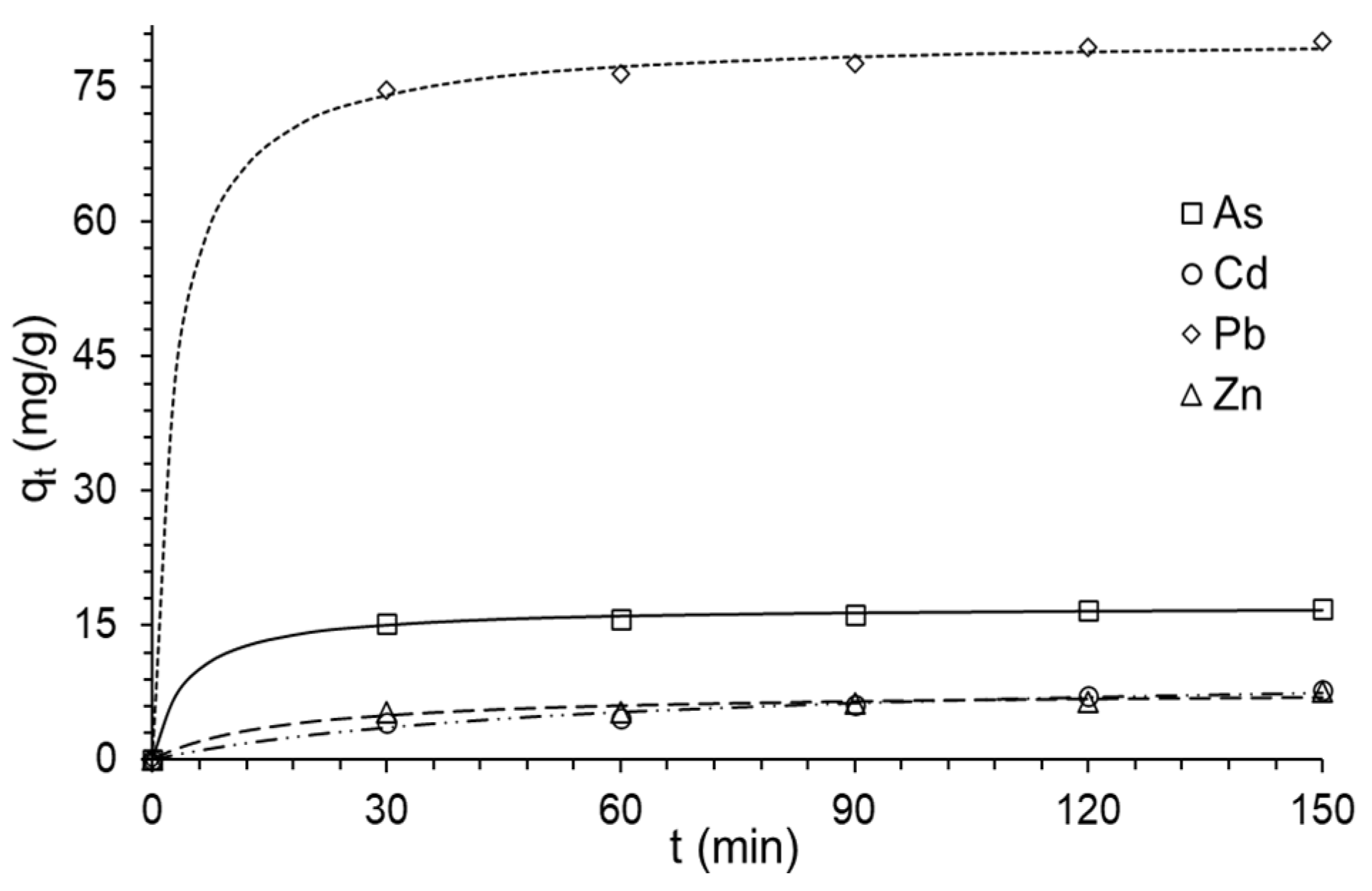
3.6. Metal Adsorption Kinetics in the Composite
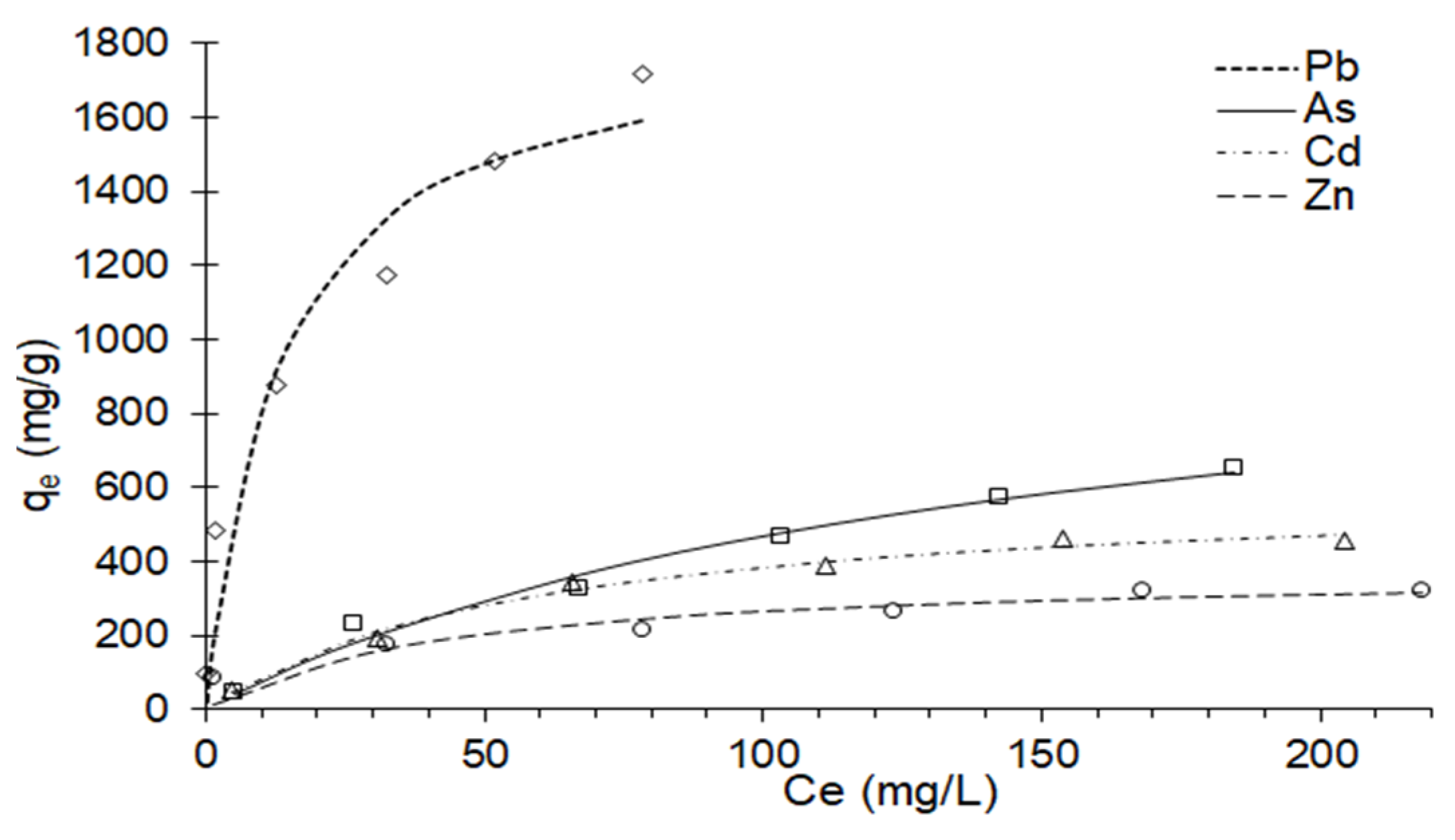
3.7. Metal Adsorption Isotherms in the Composite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choque-Quispe, D.; Masco-Arriola, M.L.; Ramos-Pacheco, B.S.; Ligarda-Samanez, C.A.; Solano-Reynoso, A.M.; Choque-Quispe, Y.; Alonzo-Lanado, J.F. Study of the pollution by surfactants in a river of a high Andean micro basin. DYNA 2021, 88, 9–12. [Google Scholar] [CrossRef]
- Choque-Quispe, D.; Ramos-Pacheco, B.S.; Ligarda-Samanez, C.A.; Barboza-Palomino, G.I.; Kari-Ferro, A.; Taipe-Pardo, F.; Choque-Quispe, Y. Heavy metal removal by biopolymers-based formulations with native potato starch/nopal mucilage. Rev. Fac. Ing. Univ. Antioq. 2022, 103, 44–50. [Google Scholar] [CrossRef]
- Pal, A.; He, Y.; Jekel, M.; Reinhard, M.; Gin, K.Y.-H. Emerging contaminants of public health significance as water quality indicator compounds in the urban water cycle. Environ. Int. 2014, 71, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Ramalingam, S.; Sathyaselvabala, V.; Kirupha, S.D.; Murugesan, A.; Sivanesan, S. Removal of Cd (II) from aqueous solution by agricultural waste cashew nut Shell. Korean J. Chem. Eng. 2012, 29, 756–768. [Google Scholar] [CrossRef]
- Barakat, M. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Hoang, A.T.; Nižetić, S.; Cheng, C.K.; Luque, R.; Thomas, S.; Banh, T.L.; Pham, V.V.; Nguyen, X.P. Heavy metal removal by biomass-derived carbon nanotubes as a greener environmental remediation: A comprehensive review. Chemosphere 2022, 287, 131959. [Google Scholar] [CrossRef]
- Ligarda-Samanez, C.A.; Choque-Quispe, D.; Palomino-Rincón, H.; Ramos-Pacheco, B.S.; Moscoso-Moscoso, E.; Huamán-Carrión, M.L.; Peralta-Guevara, D.E.; Obregón-Yupanqui, M.E.; Aroni-Huamán, J.; Bravo-Franco, E.Y.; et al. Modified Polymeric Biosorbents from Rumex acetosella for the Removal of Heavy Metals in Wastewater. Polymers 2022, 14, 2191. [Google Scholar] [CrossRef]
- Alawady, A.R.; Alshahrani, A.A.; Aouak, T.A.; Alandis, N.M. Polysulfone membranes with CNTs/Chitosan biopolymer nanocomposite as selective layer for remarkable heavy metal ions rejection capacity. Chem. Eng. J. 2020, 388, 124267. [Google Scholar] [CrossRef]
- Al Mahrouqi, D.; Vinogradov, J.; Jackson, M.D. Zeta potential of artificial and natural calcite in aqueous solution. Adv. Colloid Interface Sci. 2017, 240, 60–76. [Google Scholar] [CrossRef] [Green Version]
- Saravanan, D.; Sudha, P.N. Batch Adsorption Studies for the Removal of Copper from Wastewater using Natural Biopolymer. Int. J. ChemTech Res. 2014, 6, 3496–3508. [Google Scholar]
- Tejada, C.; Villabona, A.; Garcés, L. Adsorption of heavy metals in waste water using biological materials. TecnoLogicas 2015, 18, 109–123. [Google Scholar]
- Al Juhaiman, L.A.; Al-Enezi, D.A.; Mekhamer, W.K. Preparation and characterization of polystyrene/organoclay nanocomposites from raw clay. Dig. J. Nanomater. Biostructures 2016, 11, 105–114. [Google Scholar]
- Sebeia, N.; Jabli, M.; Ghith, A.; Elghoul, Y.; Alminderej, F.M. Production of cellulose from Aegagropila Linnaei macro-algae: Chemical modification, characterization and application for the bio-sorptionof cationic and anionic dyes from water. Int. J. Biol. Macromol. 2019, 135, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Gaur, J.P. Use of Algae for removing heavy metal ions from wastewater: Progress and Prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152. [Google Scholar] [CrossRef] [PubMed]
- Salama, E.-S.; Roh, H.-S.; Dev, S.; Khan, M.A.; Abou-Shanab, R.A.I.; Chang, S.W.; Jeon, B.-H. Algae as a green technology for heavy metals removal from various wastewater. World J. Microbiol. Biotechnol. 2019, 35, 75. [Google Scholar] [CrossRef]
- Efimova, N.V.; Krasnopyorova, A.P.; Yuhno, G.D.; Scheglovskaya, A.A. Sorption of heavy metals by natural biopolymers. Adsorpt. Sci. Technol. 2017, 35, 595–601. [Google Scholar] [CrossRef]
- Rani, K.; Gomathi, T.; Vijayalakshmi, K.; Saranya, M.; Sudha, P.N. Banana fiber Cellulose Nano Crystals grafted with butyl acrylate for heavy metal lead (II) removal. Int. J. Biol. Macromol. 2019, 131, 461–472. [Google Scholar] [CrossRef]
- Ahmed, Z.K.; Özlem, C.; Pontikes, Y.; Heath, A.; Patureau, P.; Bernal, S.A.; Marsh, L. Advances in alkali-activation of clay minerals. Cem. Concr. Res. 2020, 132, 106050. [Google Scholar] [CrossRef]
- Carbonel, D. Cadmium, Copper and Lead Adsorption on Natural and Modified Bentonite, Kaolin and Zeolite: A Review of Process Parameters, Isotherms and Kinetics. Ingeniería 2018, 23, 252–273. [Google Scholar] [CrossRef]
- Ismadji, S.; Soetaredjo, F.E.; Ayucitra, A. Clay Materials for Environmental Remediation, 2015a ed; Springer International Publishing: Berlin, Germany, 2015; pp. 39–56. [Google Scholar] [CrossRef]
- Abbou, B.; Lebkiri, I.; Ouaddari, H.; Kadiri, L.; Ouass, A.; Habsaoui, A.; Lebkiri, A.; Rifi, E.H. Removal of Cd(II), Cu(II), and Pb(II) by adsorption onto natural clay: A kinetic and thermodynamic study. Turk. J. Chem. 2021, 45, 362–376. [Google Scholar] [CrossRef]
- Obayomi, K.S.; Auta, M. Development of microporous activated Aloji clay for adsorption of lead (II) ions from aqueous solution. Heliyon 2019, 5, e02799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šuránek, M.; Melichová, Z.; Kureková, V.; Kljajević, L.; Nenadović, S. Removal of Nickel from Aqueous Solutions by Natural Bentonites from Slovakia. Materials 2021, 14, 282. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the Removal of Heavy Metals from Wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malayoglu, U. Removal of heavy metals by biopolymer (chitosan)/nanoclay composites. Sep. Sci. Technol. 2018, 53, 2741–2749. [Google Scholar] [CrossRef]
- Louati, S.; Baklouti, S.; Samet, B. Geopolymers Based on Phosphoric Acid and Illito-Kaolinitic Clay. Adv. Mater. Sci. Eng. 2016, 2016, 2359759. [Google Scholar] [CrossRef] [Green Version]
- Choque-Quispe, D.; Mojo-Quisani, A.; Ligarda-Samanez, C.A.; Calla-Florez, M.; Ramos-Pacheco, B.S.; Zamalloa-Puma, L.M.; Peralta-Guevara, D.E.; Solano-Reynoso, A.M.; Choque-Quispe, Y.; Zamalloa-Puma, A.; et al. Preliminary Characterization of a Spray-Dried Hydrocolloid from a High Andean Algae (Nostoc sphaericum). Foods 2022, 11, 1640. [Google Scholar] [CrossRef] [PubMed]
- Chorom, M.; Rengasamy, P. Dispersion and zeta potential of pure clays as related to net particle charge under varying pH, electrolyte concentration and cation type. Eur. J. Soil Sci. 2005, 46, 657–665. [Google Scholar] [CrossRef]
- Igwe, J.C. A Review of Potentially Low Cost Sorbents for Heavy Metal Removal and Recovery. Terr. Aquat. Environ. Toxicol. 2007, 1, 60–69. [Google Scholar]
- Singha, A.S.; Guleria, A. Chemical modification of cellulosic biopolymer and its use in removal of heavy metal ions from wastewater. Int. J. Biol. Macromol. 2014, 67, 409–417. [Google Scholar] [CrossRef]
- Saravanan, D.; Gomathi, T.; Sudha, P.N. Sorption studies on heavy metal removal using chitin/bentonite biocomposite. Int. J. Biol. Macromol. 2013, 53, 67–71. [Google Scholar] [CrossRef]
- Murillo, Y.S.; Giraldo, L.; Moreno, J.C. Determination of the 2,4-dinitrofenol adsorption kinetic on bovine bone char by UV-Vis spectrophotometry. Rev. Colomb. Química 2011, 40, 91–104. [Google Scholar]
- Rajeshwari, K.; Latha, S.; Gomathi, T.; Sangeetha, K.; Sudha, P.N. Adsorption of Heavy Metal Cr (VI) By a Ternary Biopolymer Blend. Mater. Today Proc. 2018, 5, 14628–14638. [Google Scholar] [CrossRef]
- Zgorelec, Z.; Grahovac, B.; Perčin, A.; Jurković, V.; Gandjaeva, L.; Maurović, N. Comparison of two different CEC determination methods regarding the soil properties. Agric. Conspec. Sci. 2019, 84, 151–158. [Google Scholar]
- Aran, D.; Maul, A.; Masfaraud, J.-F. A spectrophotometric measurement of soil cation exchange capacity based on cobaltihexamine chloride absorbance. Comptes Rendus Geosci. 2008, 340, 865–871. [Google Scholar] [CrossRef]
- Do Rosário, J.A.; Miguel, R.F.; Do Rosário, D.A.; Kunhen, N.C.; Riella, H.G. Factorial design applied to sodium activation of a Brazilian bentonite. Cerâmica 2019, 65, 185–189. [Google Scholar] [CrossRef]
- Bendou, S.; Amrani, M. Effect of hydrochloric acid on the structural of sodic-bentonite clay. J. Miner. Mater. Charact. Eng. 2014, 2, 404–413. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, T.; Chen, L.; Li, G. Study on Sodium Modification of Inferior Ca-Based Bentonite by Suspension Method. Int. Sch. Res. Not. 2011, 2011, 953132. [Google Scholar] [CrossRef] [Green Version]
- Makhoukhi, B.; Didi, M.A.; Villemin, D.; Azzouz, A. Acid activation of Bentonite for use as a vegetable oil bleaching agent. Grasas Aceites 2009, 60, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Drweesh, S.A.; Fathy, N.A.; Wahba, M.A.; Hanna, A.A.; Akarish, A.I.M.; Elzahany, E.A.M.; El-Sherif, I.Y.; Abou-El-Sherbini, K.S. Equilibrium, kinetic and thermodynamic studies of Pb(II) adsorption from aqueous solutions on HCl-treated Egyptian kaolin. J. Environ. Chem. Eng. 2016, 4, 1674–1684. [Google Scholar] [CrossRef]
- Pawar, R.R.; Lalhmunsiama; Bajaj, H.C.; Lee, S.-M. Activated bentonite as a low-cost adsorbent for the removal of Cu (II) and Pb (II) from aqueous solutions: Batch and column studies. J. Ind. Eng. Chem. 2016, 34, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Macías-Quiroga, I.F.; Giraldo-Gómez, G.I.; Sanabria-González, N.R. Characterization of Colombian clay and its potential use as adsorbent. Sci. World J. 2018, 2018, 5969178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novikova, L.; Ayrault, P.; Fontaine, C.; Chatel, G.; Jérôme, F.; Belchinskaya, L. Effect of low frequency ultrasound on the surface properties of natural aluminosilicates. Ultrason. Sonochem. 2016, 31, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Tuesta, E.G.; Vivas, M.; Sun, R.; Gutarra, A. Modificación química de arcillas y su aplicación en la retención de colorantes. Rev. Soc. Quím. Perú 2005, 71, 26–36. [Google Scholar]
- Medina-López, S.V.; Zuluaga-Domínguez, C.M.; Fernández-Trujillo, J.P.; Hernández-Gómez, M.S. Nonconventional Hydrocolloids’ Technological and Functional Potential for Food Applications. Foods 2022, 11, 401. [Google Scholar] [CrossRef]
- Putro, J.N.; Lunardi, V.B.; Soetaredjo, F.E.; Yuliana, M.; Santoso, S.P.; Wenten, I.G.; Ismadji, S.A. Review of Gum Hydrocolloid Polyelectrolyte Complexes (PEC) for Biomedical Applications: Their Properties and Drug Delivery Studies. Processes 2021, 9, 1796. [Google Scholar] [CrossRef]
- Shahzad, S.A.; Hussain, S.; Alamri, M.S.; Mohamed, A.A.; Ahmed, A.S.; Ibraheem, M.A.; Qasem, A.A.A. Use of hydrocolloid gums to modify the pasting, thermal, rheological, and textural properties of sweet potato starch. Int. J. Polym. Sci. 2019, 2019, 6308591. [Google Scholar] [CrossRef]
- Lin, J.; Cai, X.; Tang, M.; Wang, S. Preparation and evaluation of the chelating nanocomposite fabricated with marine algae Schizochytrium sp. protein hydrolysate and calcium. J. Agric. Food Chem. 2015, 63, 9704–9714. [Google Scholar] [CrossRef]
- Shashikant, C.D.; Sudhir, G.W.; Avinash, K.D. Behavior of suspending and wetting agents in aqueous environment. Asian J. Pharm. 2009, 3, 9–12. [Google Scholar] [CrossRef]
- Schramm, L.L. Emulsions, Foams, and Suspensions: Fundamentals and Applications; Wiley-VCH Verlag GmbH y Co. KGaA: Weinheim, Germany, 2005; ISBN 9783527307432. [Google Scholar]
- Malhotra, A.; Coupland, J.N. The effect of surfactants on the solubility, zeta potential, and viscosity of soy protein isolates. Food Hydrocoll. 2004, 18, 101–108. [Google Scholar] [CrossRef]
- Fijałkowska, G.; Wiśniewska, M.; Szewczuk-Karpisz, K.; Jędruchniewicz, K.; Oleszczuk, P. Comparison of Lead (II) ions accumulation and bioavailability on the montmorillonite and kaolinite surfaces in the presence of polyacrylamide soil flocculant. Chemosphere 2021, 276, 130088. [Google Scholar] [CrossRef]
- Contreras-Lozano, K.P.; Ciro-Velásquez, H.J.; Arango-Tobón, J.C. Hydrocolloids as stabilizers in beverages from sweet corn (Zea mays var. Saccharata) and aloe vera gel (Aloe barbadensis Miller). Rev. UDCA Actual. Divulg. Cient. 2019, 22, 1–9. [Google Scholar] [CrossRef]
- Furusawa, K.; Uchiyama, K. Collaborative studies of zeta-potential measurements and electrophoretic measurements using reference sample. Colloids Surf. A Physicochem. Eng. Asp. 1998, 140, 217–226. [Google Scholar] [CrossRef]
- Souza, T.G.F.; Ciminelli, V.S.T.; Mohallem, N.D.S. A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles. J. Phys. Conf. Ser. 2016, 733, 012039. [Google Scholar] [CrossRef] [Green Version]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef] [Green Version]
- Kaya, A.; Yukselen, Y. Zeta potential of clay minerals and quartz contaminated by heavy metals. Can. Geotech. J. 2005, 42, 1280–1289. [Google Scholar] [CrossRef]
- Yukselen, Y.; Kaya, A. Zeta potential of kaolinite in the presence of alkali, alkaline earth and hydrolyzable metal ions. Water Air Soil Pollut. 2003, 145, 155–168. [Google Scholar] [CrossRef]
- Zendelska, A.; Golomeova, M. Effect of competing cations (Cu, Zn, Mn, Pb) adsorbed by natural zeolite. Int. J. Eng. Technol. 2014, 2, 483–492. [Google Scholar]
- Alexander, J.A.; Zaini, M.A.A.; Abdulsalam, S.; El-Nafaty, U.A.; Aroke, U.O. Isotherm studies of Lead (II), Manganese (II), and Cadmium (II) adsorption by Nigerian bentonite clay in single and multimetal solutions. J. Part. Sci. Technol. 2019, 37, 403–413. [Google Scholar] [CrossRef]
- Alexander, J.A.; Surajudeen, A.; Aliyu, E.; Omeiza, A.; Zaini, M. Multi metals column adsorption of Lead(II), Cadmium(II) and Manganese(II) onto natural bentonite clay. Water Sci. Technol. 2017, 76, 2232–2241. [Google Scholar] [CrossRef]
- Pacheco, M.E.; Pimentel, J.P.; Roque, W.F. Biosorption kinetic of cadmium (II) and lead (II) ions from aqueous solutions by biomass residual of coffee. Rev. Soc. Quím. 2010, 76, 279–292. [Google Scholar]
- Kozera-Sucharda, B.; Gworek, B.; Kondzielski, I.; Chojnicki, J. The Comparison of the Efficacy of Natural and Synthetic Aluminosilicates, Including Zeolites, in Concurrent Elimination of Lead and Copper from Multi-Component Aqueous Solutions. Processes 2021, 9, 812. [Google Scholar] [CrossRef]
- Eloussaief, M.; Hamza, W.; Kallel, N.; Benzin, M. Wastewaters decontamination: Mechanisms of Pb(II), Zn(II), and Cd(II) competitive adsorption on tunisian smectite in single and multi-solute systems. Environ. Prog. Sustain. Energy 2013, 32, 223–238. [Google Scholar] [CrossRef]
- De Luca, P.; Bernaudo, I.; Elliani, R.; Tagarelli, A.; Nagy, J.B.; Macario, A. Industrial Waste Treatment by ETS-10 Ion Exchanger Material. Materials 2018, 11, 2316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budsaereechai, S.; Kamwialisak, K.; Ngernyen, Y. Adsorption of lead, cadmium and copper on natural and acid activated bentonite clay. Asia-Pac. J. Sci. Technol. 2014, 17, 800–810. [Google Scholar]
- Jiang, M.-Q.; Jin, X.-Y.; Lu, X.-Q.; Chen, Z.-I. Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination 2010, 252, 33–39. [Google Scholar] [CrossRef]
- Rodriguez, S.; Gonzales, K.N.; Romero, E.G.; Troncoso, O.P.; Torres, F.G. Unusual reversible elastomeric gels from Nostoc commune. Int. J. Biol. Macromol. 2017, 97, 411–417. [Google Scholar] [CrossRef]
- Rodriguez, S.; Torres, F.G.; López, D. Preparation and characterization of polysaccharide films from the cyanobacteria Nostoc commune. Polym. Renew. Resour. 2017, 8, 133–150. [Google Scholar] [CrossRef]
- Aftab, K.; Hameed, S.; Umbreen, H.; Ali, S.; Rizwan, M.; Alkahtani, S.; Abdel-Daim, M.M. Physicochemical and Functional Potential of Hydrocolloids Extracted from Some Solanaceae Plants. J. Chem. 2020, 2020, 3563945. [Google Scholar] [CrossRef]
- Hamrun, N.; Talib, B.; Ruslin, M.; Pangeran, H.; Hatta, M.; Marlina, E.; Yusuf, A.S.H.; Saito, T.; Ou, K.-L. A Promising Potential of Brown Algae Sargassum polycystum as Irreversible Hydrocolloid Impression Material. Mar. Drugs 2022, 20, 55. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lee, H.V.; Juan, J.C.; Phang, S.-M. Production of new cellulose nanomaterial from red algae marine biomass Gelidiumelegans. Carbohydr. Polym. 2016, 151, 1210–1219. [Google Scholar] [CrossRef]
- Bureau, S.; Cozzolino, D.; Clark, C.J. Contributions of Fourier-transform mid infrared (FT-MIR) spectroscopy to the study of fruit and vegetables: A review. Postharvest Biol. Technol. 2019, 148, 1–14. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, C.; Mastalerz, M.; Hu, S.; Gasaway, C.; Tao, X. Applications of Micro-Fourier Transform Infrared Spectroscopy (FTIR) in the Geological Sciences—A Review. Int. J. Mol. Sci. 2015, 16, 30223–30250. [Google Scholar] [CrossRef] [PubMed]
- Djebbar, M.; Djafri, F.; Bouchekara, M.; Djafri, A. Adsorption of phenol on natural clay. Appl. Water Sci. 2012, 2, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Xi, Y.; Ding, Z.; He, H.; Frost, R.L. Infrared spectroscopy of organoclays synthesized with the surfactant octadecyltrimethylammonium bromide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Patel, H.A.; Somani, R.S.; Bajaj, H.C.; Jasra, R.V. Nanoclays for polymer nanocomposites, paints, inks, greases and cosmetics formulations, drug delivery vehicle and waste water treatment. Bull. Mater. Sci. 2006, 29, 133–145. [Google Scholar] [CrossRef]
- Criado, M.; Fernández-Jiménez, A.A.; Palomo, A. Alkaliactivation of fly ash: Effect of the SiO2/Na2O ratio: Part I: FTIR study. Microporous Mesoporous Mater. 2007, 106, 180–191. [Google Scholar] [CrossRef]
- Dekhil, A.V.; Hannachi, Y.; Ghorbel, A.; Boubaker, T. Removal of lead and cadmium ions from aqueous solutions using dried marine green macroalga [caulerpa racemosa]. Int. J. Environ. Res. 2011, 5, 725–732. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Immobilization of Pb(II), Cd(II) and Ni(II) ions on kaolinite and montmorillonite surfaces from aqueous medium. J. Environ. Manag. 2008, 87, 46–58. [Google Scholar] [CrossRef]
- Mamera, M.; Van Tol, J.J.; Aghoghovwia, M.P.; Kotze, E. Sensitivity and Calibration of the FT-IR Spectroscopy on Concentration of Heavy Metal Ions in River and Borehole. Water Sources. Appl. Sci. 2020, 10, 7785. [Google Scholar] [CrossRef]
- Tajuddin, N.A.; Rosli, N.H.; Abdullah, N.; Yusoff, M.Y.M.; Salimon, J. Estolide ester from ricinus communis L. seed oil for bio lubricant purpose. Malays. J. Anal. Sci. 2014, 18, 85–93. [Google Scholar]
- Choi, H.-J.; Yu, S.-W.; Kim, K.H. Efficient use of Mg-modified zeolite in the treatment of aqueous solution contaminated with heavy metal toxic ions. J. Taiwan Inst. Chem. Eng. 2016, 63, 482–489. [Google Scholar] [CrossRef]
- Chuquilín, R.C.; Rosales, D.D. Study of the biosorption of Cd (II) AND Pb (II) using as adsorbent Nostoc sphaericum Vaucher. Rev. Soc. Quím. Perú 2016, 82, 49–60. [Google Scholar]
- Ismael, I.S.; Melegy, A.; Kratochvíl, T. Lead Removal from Aqueous Solution by Natural and Pretreated Zeolites. Geotech. Geol. Eng. 2012, 30, 253–262. [Google Scholar] [CrossRef]
- Alandis, N.M.; Aldayel, O.A.; Mekhemer, W.K.; Hefne, J.A.; Jokhab, H.A. Thermodynamic and Kinetic Studies for the Adsorption of Fe(III) and Ni(II) Ions From Aqueous Solution Using Natural Bentonite. J. Dispers. Sci. Technol. 2010, 31, 1526–1534. [Google Scholar] [CrossRef]
- Matouq, M.; Jildeh, N.; Qtaishat, M.; Hindiyeh, M.; Syouf, M.Q.A. The adsorption kinetics and modeling for heavy metals removal from wastewater by Moringa pods. J. Environ. Chem. Eng. 2015, 3, 775–784. [Google Scholar] [CrossRef]
- Çavuş, S.; Gürdag, G. Noncompetitive Removal of Heavy Metal Ions from Aqueous Solutions by Poly[2-(acrylamido)-2-methyl-1-propanesulfonic acid-co-itaconic acid] Hydrogel. Ind. Eng. Chem. Res. 2009, 48, 2652–2658. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W. Evaluating the adsorption of Shanghai silty clay to Cd(II), Pb(II), As(V), and Cr(VI): Kinetic, equilibrium, and thermodynamic studies. Environ. Monit. Assess. 2021, 193, 131. [Google Scholar] [CrossRef]
- Ghogomu, J.N.; Noufame, T.D.; Ketcha, M.J.; Ndi, N.J. Removal of Pb(II) Ions from Aqueous Solutions byKaolinite and Metakaolinite Materials. Curr. J. Appl. Sci. Technol. 2013, 3, 942–961. [Google Scholar] [CrossRef]
- Borja, N.A.; Villegas, V.R.; Ojeda, A.G.; Lezama, E.G.; García, H.J. Kinetic study of lead (II) biosorption on Ascophyllum nodosum seaweed. Rev. Soc. Quím. Perú 2015, 81, 212–223. [Google Scholar]
- El-Shafey, O.I.; Fathy, N.A.; El-Nabarawy, T.A. Sorption of Ammonium Ions onto Natural and Modified Egyptian Kaolinites: Kinetic and Equilibrium Studies. Adv. Phys. Chem. 2014, 2014, 935854. [Google Scholar] [CrossRef] [Green Version]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Kozera-Sucharda, B.; Gworek, B.; Kondzielski, I. The simultaneous removal of Zinc and Cadmium from multicomponent aqueous solutions by their sorption onto selected natural and synthetic zeolites. Minerals 2020, 10, 343. [Google Scholar] [CrossRef] [Green Version]
- Hajeeth, T.; Sudha, P.N.; Vijayalakshmi, K.; Gomathi, T. Sorption studies on Cr (VI) removal from aqueous solution using cellulose grafted with acrylonitrile monomer. Int. J. Biol. Macromol. 2014, 66, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Khoshnevisan, N. Optimization of process parameters for removal of heavy metals by biomass of Cu and Co-doped alginate-coated chitosan nanoparticles. Bioresour. Technol. 2016, 218, 650–658. [Google Scholar] [CrossRef]
- Allen, S.J.; Mckay, G.; Porter, J.F. Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. J. Colloid Interface Sci. 2004, 280, 322–333. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die adsorption in lösungen (Adsorption in Solution). J. Z. Für Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Nnaji, C.C.; Agim, A.E.; Mama, C.N.; Emenike, P.G.C.; Ogarekpe, N.M. Equilibrium and thermodynamic investigation of biosorption of nickel from water by activated carbon made from palm kernel chaff. Sci. Rep. 2021, 11, 7808. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]

| Community | District | Region | Coordinates | Altitude (m) | Collected Period | |
|---|---|---|---|---|---|---|
| S | W | |||||
| Huancabamba | José María Arguedas | Apurímac | 13°43′58″ | 73°20′38″ | 3682 | April/2021 |
| Material | NICOMP Distribution | Gaussian Distribution | ζ Potential (mV) | ||||
|---|---|---|---|---|---|---|---|
| Peak | Size (nm) | % | SD | CV (%) | |||
| CH | 1 | 43.4 | 4.2 | 454.0 | 269.2 | 59.3 | −27.14 |
| 2 | 421.7 | 95.8 | |||||
| HMB | 1 | 37.6 | 0.6 | 372.7 | 200.2 | 53.7 | −19.31 |
| 2 | 239.8 | 46.4 | |||||
| 3 | 839.5 | 53 | |||||
| HMB-Act | 1 | 65 | 2.6 | 686.4 | 505.2 | 73.6 | −39.91 |
| 2 | 487.5 | 97.4 | |||||
| Clay or Composite | As (WL 197.262 nm) | Cd (WL 326.106 nm) | Pb (WL 405.783 nm) | Zn (WL 213.856 nm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qe (mg/g) | CV (%) | qe (mg/g) | CV (%) | qe (mg/g) | CV (%) | qe (mg/g) | CV (%) | |||||
| Natural HMB | 18.97 | 22.25 ± 0.24a * | 1.10 | 2.40 | 3.88 ± 0.09a | 2.41 | 85.14 | 78.35 ± 0.10a | 0.13 | 3.67 | 5.23 ± 0.30a | 5.68 |
| HMB-Act | 21.23 | 24.91 ± 0.51b | 2.05 | 4.17 | 6.73 ± 0.16b | 2.40 | 88.49 | 81.43 ± 0.06b | 0.07 | 5.23 | 7.46 ± 0.51b | 6.89 |
| Natural HMB/CH | 17.87 | 30.61 ± 0.45c | 1.48 | 6.07 | 12.21 ± 0.60c | 4.95 | 107.13 | 98.59 ± 0.44c | 0.47 | 5.40 | 8.61 ± 0.33c | 3.85 |
| HMB-Act/CH | 18.87 | 32.32 ± 0.51d | 1.59 | 7.03 | 14.16 ± 0.42d | 2.96 | 108.14 | 99.51 ± 0.53d | 0.54 | 6.47 | 10.31 ± 0.40d | 3.89 |
| Metal Ion | Pseudo First-Order | Pseudo Second-Order | Intraparticle Diffusion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qe | K1 | R2 | Chi-sq | qe | k2 | R2 | Chi-sq | kid | C | R2 | Chi-sq | |
| As | 16.57 | 0.10 | 0.99 | 0.04 | 16.99 | 0.03 | 1.00 | 0.01 | 1.32 | 3.58 | 0.76 | 2.81 |
| Cd | 7.63 | 0.02 | 0.97 | 0.27 | 9.85 | 0.00 | 0.98 | 0.17 | 0.60 | 0.21 | 0.85 | 3.82 |
| Pb | 78.545 | 0.0989 | 0.99 | 0.09 | 80.76 | 0.00 | 0.99 | 0.03 | 6.260 | 16.841 | 0.77 | 15.15 |
| Zn | 5.98 | 0.0345 | 0.94 | 0.28 | 7.00 | 0.01 | 0.99 | 0.17 | 0.508 | 0.571 | 0.93 | 0.57 |
| Metal Ion | Langmuir Isotherm | Freundlich Isotherm | Redlich–Peterson Isotherm | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qmax | KL | R2 | Chi-sq | KF | 1/n | n | R2 | Chi-sq | KR | aR | g | R2 | Chi-sq | |
| As | 1117.78 | 0.01 | 0.98 | 21.43 | 27.55 | 0.61 | 1.64 | 0.99 | 16.00 | 0.58 | −1.02 | −0.04 | 0.96 | 11.95 |
| Pb | 1855.51 | 0.08 | 0.94 | 51.99 | 332.39 | 0.38 | 2.66 | 0.99 | 41.71 | 14.65 | −2.28 | −0.41 | 0.83 | 18.24 |
| Cd | 606.08 | 0.02 | 0.99 | 7.82 | 44.95 | 0.45 | 2.22 | 0.95 | 26.81 | 8.13 | 0.00 | 1.24 | 0.99 | 12.81 |
| Zn | 374.13 | 0.02 | 0.92 | 57.42 | 64.11 | 0.30 | 3.35 | 0.96 | 9.73 | 415.13 | 6.18 | 0.71 | 0.96 | 14.43 |
| Initial Concentration, C0 (mg/L) | As | Pb | Cd | Zn | ||||
|---|---|---|---|---|---|---|---|---|
| Final Concentration, Cf (mg/L) | RL | Final Concentration, Cf (mg/L) | RL | Final Concentration, Cf (mg/L) | RL | Final Concentration, Cf (mg/L) | RL | |
| 10.0 | 5.15 | 0.93 | 0.17 | 0.56 | 4.52 | 0.85 | 1.23 | 0.81 |
| 50.0 | 26.78 | 0.73 | 1.87 | 0.20 | 31.04 | 0.54 | 32.63 | 0.46 |
| 100.0 | 67.21 | 0.57 | 12.53 | 0.11 | 65.89 | 0.37 | 78.56 | 0.30 |
| 150.0 | 103.35 | 0.47 | 32.50 | 0.08 | 111.11 | 0.28 | 123.56 | 0.22 |
| 200.0 | 142.52 | 0.40 | 51.87 | 0.06 | 153.97 | 0.23 | 168.12 | 0.18 |
| 250.0 | 184.55 | 0.35 | 78.40 | 0.05 | 204.47 | 0.20 | 218.08 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choque-Quispe, D.; Ligarda-Samanez, C.A.; Ramos-Pacheco, B.S.; Solano-Reynoso, A.M.; Quispe-Marcatoma, J.; Choque-Quispe, Y.; Peralta-Guevara, D.E.; Martínez-Huamán, E.L.; Correa-Cuba, O.; Masco-Arriola, M.L.; et al. Formulation of Novel Composite (Activated Nanoclay/Hydrocolloid of Nostoc sphaericum) and Its Application in the Removal of Heavy Metals from Wastewater. Polymers 2022, 14, 2803. https://doi.org/10.3390/polym14142803
Choque-Quispe D, Ligarda-Samanez CA, Ramos-Pacheco BS, Solano-Reynoso AM, Quispe-Marcatoma J, Choque-Quispe Y, Peralta-Guevara DE, Martínez-Huamán EL, Correa-Cuba O, Masco-Arriola ML, et al. Formulation of Novel Composite (Activated Nanoclay/Hydrocolloid of Nostoc sphaericum) and Its Application in the Removal of Heavy Metals from Wastewater. Polymers. 2022; 14(14):2803. https://doi.org/10.3390/polym14142803
Chicago/Turabian StyleChoque-Quispe, David, Carlos A. Ligarda-Samanez, Betsy S. Ramos-Pacheco, Aydeé M. Solano-Reynoso, Justiniano Quispe-Marcatoma, Yudith Choque-Quispe, Diego E. Peralta-Guevara, Edgar L. Martínez-Huamán, Odilon Correa-Cuba, Mery Luz Masco-Arriola, and et al. 2022. "Formulation of Novel Composite (Activated Nanoclay/Hydrocolloid of Nostoc sphaericum) and Its Application in the Removal of Heavy Metals from Wastewater" Polymers 14, no. 14: 2803. https://doi.org/10.3390/polym14142803
APA StyleChoque-Quispe, D., Ligarda-Samanez, C. A., Ramos-Pacheco, B. S., Solano-Reynoso, A. M., Quispe-Marcatoma, J., Choque-Quispe, Y., Peralta-Guevara, D. E., Martínez-Huamán, E. L., Correa-Cuba, O., Masco-Arriola, M. L., Lechuga-Canal, W. J., & Montalvo Amanca, F. (2022). Formulation of Novel Composite (Activated Nanoclay/Hydrocolloid of Nostoc sphaericum) and Its Application in the Removal of Heavy Metals from Wastewater. Polymers, 14(14), 2803. https://doi.org/10.3390/polym14142803











