Treatment Disparities, Heterogeneities, and Barriers to Access for Patients with Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer: A National Survey from Brazil
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
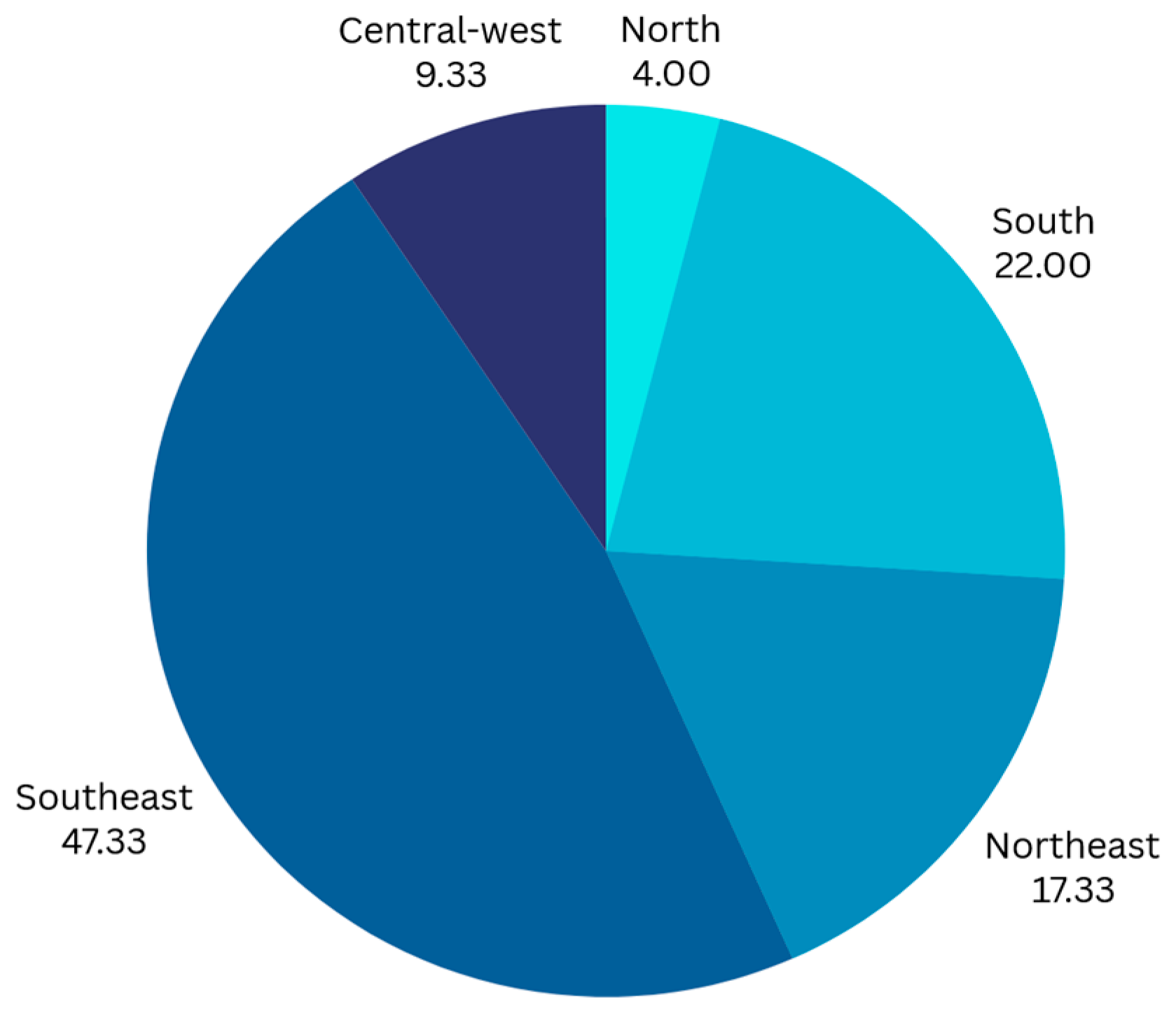
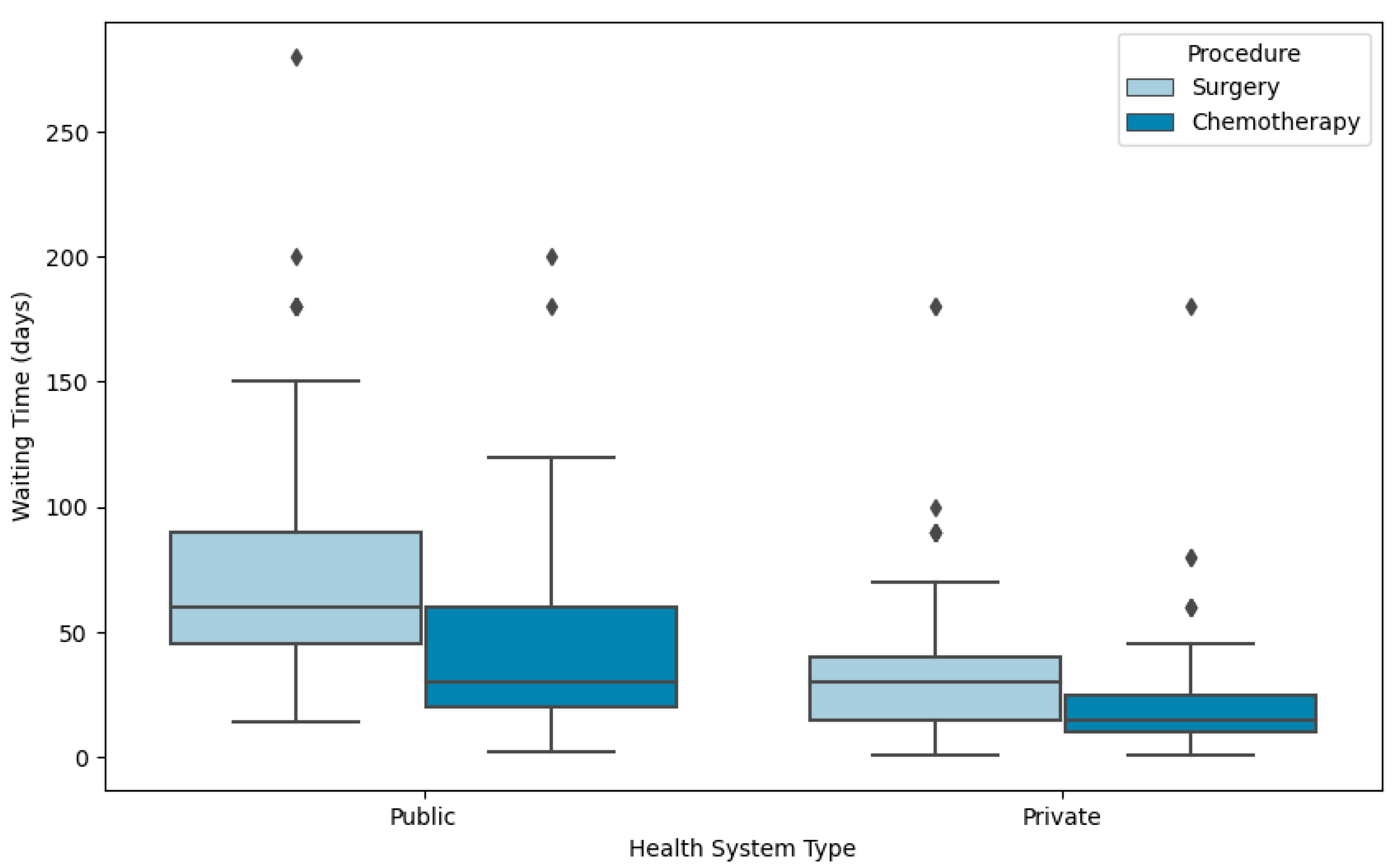
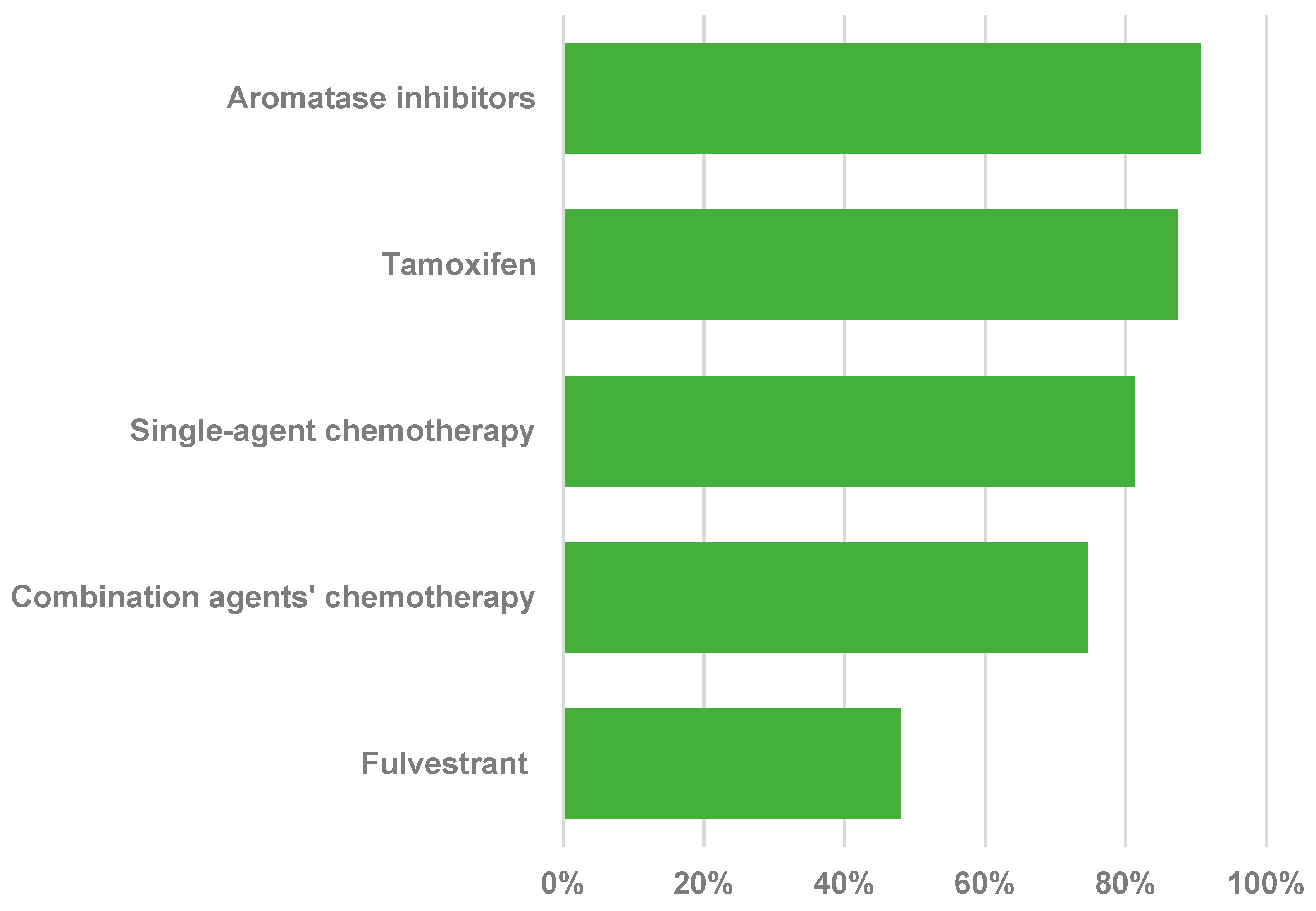
3. Results
3.1. Waiting Time to Initial Treatment
3.2. Use and Availability of Treatments
3.3. Operational Aspects of Oncology Care Delivery in the Public System
3.4. Availability of Multidisciplinary and Palliative Care
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Breast Cancer |
| mBC | Metastatic Breast Cancer |
| SUS | Sistema Único de Saúde (Unified Health System, Brazil’s public health system) |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HR | Hormone Receptor |
| WT | Waiting Time |
| INCA | Instituto Nacional do Câncer (National Cancer Institute, Brazil) |
| LMICs | Low- and Middle-Income Countries |
| CACONs | Centros de Assistência de Alta Complexidade em Oncologia (High Complexity Oncology Care Centers) |
| UNACON | Unidade de Assistência de Alta Complexidade em Oncologia (High Complexity Oncology Care Unit) |
| SIA-SUS | Sistema de Informação Ambulatorial do SUS (Outpatient Information System of SUS) |
| APAC | Autorização Procedimento de Alto Custo (Authorization for High-Cost Procedures) |
| CONITEC | Comissão Nacional de Incorporação de Tecnologias (National Commission for the Incorporation of Technologies) |
| ANS | Agência Nacional de Saúde Suplementar (National Supplementary Health Agency) |
| ANVISA | Agência Nacional de Vigilância Sanitária (National Health Surveillance Agency) |
| CDK 4/6 | Cyclin-Dependent Kinase 4/6 |
| AIs | Aromatase Inhibitors |
| PLD | Pegylated Liposomal Doxorubicin |
| GDP | Gross Domestic Product |
| OECD | Organisation for Economic Co-operation and Development |
| SBOC | Sociedade Brasileira de Oncologia Clínica (Brazilian Society of Clinical Oncology) |
| WHO | World Health Organization |
| ICD | International Classification of Diseases |
| HICs | High-Income Countries |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- de Câncer, I.N. Estimativa 2023: Incidência de Câncer no Brasil; Instituto Nacional De Câncer: Rio de Janeiro, Brazil, 2023; ISBN 978-65-88517-10-9. [Google Scholar]
- Rosa, D.D.; Bines, J.; Werutsky, G.; Barrios, C.H.; Cronemberger, E.; Queiroz, G.S.; De Lima, V.C.C.; Freitas-Júnior, R.; Couto, J.D.; Emerenciano, K.; et al. The Impact of Sociodemographic Factors and Health Insurance Coverage in the Diagnosis and Clinicopathological Characteristics of Breast Cancer in Brazil: AMAZONA III Study (GBECAM 0115). Breast Cancer Res. Treat. 2020, 183, 749–757. [Google Scholar] [CrossRef]
- Werutsky, G.; Rosa, D.D.; Reinert, T.; Barroso-Sousa, R.; Resende, H.; Signorini, P.; Cronemberger, E.; Fagundes, J.G.M.; Leal, J.H.S.; Guimarães, M.B.; et al. Real-World Data on First-Line Treatment of Hormone Receptor-Positive, HER2-Negative, Metastatic Breast Cancer in Brazil (BRAVE Study). Clin. Breast Cancer 2025, S1526820925001624. [Google Scholar] [CrossRef] [PubMed]
- Bretas, G.; Renna, N.L.; Bines, J. Practical Considerations for Expediting Breast Cancer Treatment in Brazil. Lancet Reg. Health—Am. 2021, 2, 100028. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.; Freitas-Junior, R.; Martins, S.; Bines, J.; Estevez-Diz, M.D.P.; Caleffi, M. Challenge of Incorporating New Drugs for Breast Cancer in Brazil: A Proposed Framework for Improving Access to Innovative Therapies. JCO Glob. Oncol. 2021, 7, 474–485. [Google Scholar] [CrossRef]
- Agência Nacional de Saúde Suplementar (ANS). General Data Beneficiaries of Private Health Plans, by Assistance Coverage (Brasil—2015–2025); Agência Nacional de Saúde Suplementar (ANS): Rio de Janeiro, Brazil, 2025. [Google Scholar]
- Werutsky, G.; Gössling, G.; Pellegrini, R.A.; Ampuero, G.A.S.; Rebelatto, T. Socioeconomic Impact of Cancer in Latin America and The Caribbean. Arch. Med. Res. 2022, 53, 818–825. [Google Scholar] [CrossRef]
- Collor, F.; Guerra, A. Law No. 8080, of 19 September 1990. Available online: https://www.planalto.gov.br/ccivil_03/leis/l8080.htm (accessed on 1 August 2025).
- Castro, M.C.; Massuda, A.; Almeida, G.; Menezes-Filho, N.A.; Andrade, M.V.; De Souza Noronha, K.V.M.; Rocha, R.; Macinko, J.; Hone, T.; Tasca, R.; et al. Brazil’s Unified Health System: The First 30 Years and Prospects for the Future. Lancet 2019, 394, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Andrade, D.A.P.; Veneziani, A.C.; Paiva, C.E.; Reis, R.D.; Filho, C.A.F.; Sanches, A.O.N.; Barroso, A.W.A.; Paz, A.C.M.C.; Kons, G.C.D.M.; Preto, D.D.; et al. Discrepancies in Breast Cancer’s Oncological Outcomes between Public and Private Institutions in the Southeast Region of Brazil: A Retrospective Cohort Study. Front. Oncol. 2023, 13, 1169982. [Google Scholar] [CrossRef]
- Gadelha, M.I.P. A Assistência Oncológica e Os 30 Anos Do Sistema Único de Saúde. Rev. Bras. Cancerol. 2019, 64, 237–245. [Google Scholar] [CrossRef]
- Serra, J. Ordinance No. 3535 of 2 September 1998. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/gm/1998/prt3535_02_09_1998_revog.html (accessed on 1 August 2025).
- Serra, J. Ordinance No. 3536, of 2 September 1998. Available online: https://pesquisa.in.gov.br/imprensa/jsp/visualiza/index.jsp?data=03/09/1998&jornal=1&pagina=167&totalArquivos=202 (accessed on 1 August 2025).
- Temporao, J.G. Ordinance No. 741 of 19 December 2005. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/sas/2005/prt0741_19_12_2005.html (accessed on 1 August 2025).
- Rousseff, D.; Mantega, G.; Padilha, A.R.S. Law No. 12401, of 28 April 2011. Available online: https://www.planalto.gov.br/ccivil_03/_ato2011-2014/2011/lei/l12401.htm (accessed on 1 August 2025).
- Barreto, M.L.; Rasella, D.; Machado, D.B.; Aquino, R.; Lima, D.; Garcia, L.P.; Boing, A.C.; Santos, J.; Escalante, J.; Aquino, E.M.L.; et al. Monitoring and Evaluating Progress towards Universal Health Coverage in Brazil. PLoS Med. 2014, 11, e1001692. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.; De Lima Lopes, G.; Yusof, M.M.; Rubagumya, F.; Rutkowski, P.; Sengar, M. Barriers in Access to Oncology Drugs—A Global Crisis. Nat. Rev. Clin. Oncol. 2023, 20, 7–15. [Google Scholar] [CrossRef] [PubMed]
- CFM-DF. Demografia Médica no Brasil 2018; CFM-DF: São Paulo, Brazil, 2018; ISBN 978-85-87077-55-4. [Google Scholar]
- Rousseff, D.; Cardozo, J.E.; Padilha, A.R.S. Law No. 12732 of 22 November 2012. Available online: http://www.planalto.gov.br/ccivil_03/_ato2011-2014/2012/lei/l12732.htm (accessed on 1 August 2025).
- Resende, H.; Aguiar, V.; Jacob, L.; Renó, A.; Cunha, A.; Assis, B.; Pereira, V.; Tureta, L.; Eler, L.; Oliveira, M.; et al. The Journey of Breast Cancer Patient from Self-Perception of Breast Abnormalities to First Cancer Treatment—A Sectional Study in Sul Fluminense Region-RJ-Brazil. Med. Res. Arch. 2023, 11. [Google Scholar] [CrossRef]
- Ferreira, N.A.S.; Schoueri, J.H.M.; Sorpreso, I.C.E.; Adami, F.; Dos Santos Figueiredo, F.W. Waiting Time between Breast Cancer Diagnosis and Treatment in Brazilian Women: An Analysis of Cases from 1998 to 2012. Int. J. Environ. Res. Public Health 2020, 17, 4030. [Google Scholar] [CrossRef] [PubMed]
- Maia De Castro, F.C.; Jobim, F.C.; Flores Jacobi, L. Analysis of the Waiting Time of the Diagnosis for the First Breast Cancer Treatment in Southern Brazil. Women Health 2021, 61, 542–549. [Google Scholar] [CrossRef]
- Ceschin, M. Normative Resolution No. 259, of 17 June 2011. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/ans/2011/res0259_17_06_2011.htm (accessed on 1 August 2025).
- Mosconi, C.E.V. Ordinance No. 171 of 17 December 1993. Available online: http://www.oncoguia.org.br/pub/15_oncoguia_noticias/Portaria_171.pdf (accessed on 1 August 2025).
- Mosconi, C.E.V. Ordinance No. 170 of 17 December 1993. Available online: http://www.oncoguia.org.br/pub/15_oncoguia_noticias/Portaria_170.pdf (accessed on 1 August 2025).
- Padilha, A.R.S. Ordinance No. 2947 of 21 December 2012. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/gm/2012/prt2947_21_12_2012.html (accessed on 1 August 2025).
- SIASUS. Sistema de Informações Ambulatoriais Do SUS. Available online: http://sia.datasus.gov.br/documentos/listar_ftp_apac.php (accessed on 1 August 2025).
- Ministério da Saúde Manual de Bases Técnicas Da Oncologia—Sistema de Informações Ambulatoriais (SIA/SUS), 30a Edição 2022. Available online: https://www.inca.gov.br/sites/ufu.sti.inca.local/files//media/document//manual_oncologia_30a_edicao_agosto_2022_25_08_2022_-_26-08-2022.pdf (accessed on 1 August 2025).
- Editora Atheneu. Manual de Condutas em Oncologia; Editora Atheneu: São Paulo, Brazil, 2021; ISBN 978-85-388-0892-3. [Google Scholar]
- Rugo, H.S.; Rumble, R.B.; Macrae, E.; Barton, D.L.; Connolly, H.K.; Dickler, M.N.; Fallowfield, L.; Fowble, B.; Ingle, J.N.; Jahanzeb, M.; et al. Endocrine Therapy for Hormone Receptor–Positive Metastatic Breast Cancer: American Society of Clinical Oncology Guideline. J. Clin. Oncol. 2016, 34, 3069–3103. [Google Scholar] [CrossRef]
- Comissão Nacional de Incorporação de Tecnologias no Sistema Único de Saúde—CONITEC. Abemaciclibe, Palbociclibe e Succinato de Ribociclibe Para o Tratamento de Pacientes Adultas Com Câncer de Mama Avançado Ou Metastático Com HR+ e HER2; CONITEC: Brasilia, Brazil, 2021. [Google Scholar]
- Sociedade Brasileira de Oncologia Clínica (SBOC) Proposta SBOC Para Garantir o Efetivo Acesso a Antineoplásicos Incorporados Ao SUS 2023. Available online: https://sboc.org.br/images/pdf/075-Proposta-SBOC-Iniquidade-Pos-Conferencia.pdf (accessed on 1 August 2025).
- Gadelha, C.A.G. Ordinance No. 18 of 25 July 2012. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/sctie/2012/prt0018_25_07_2012.html (accessed on 1 August 2025).
- Rai, A.K. Access to Biomedical Innovation—Inside the Legal Labyrinth. N. Engl. J. Med. 2023, 388, 1537–1540. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.L.B.; Pepe, V.L.E.; Osorio-de-Castro, C.G.S. Public Procurement of Antineoplastic Agents Used for Treating Breast Cancer in Brazil between 2013 and 2019. BMC Cancer 2022, 22, 769. [Google Scholar] [CrossRef]
- Martins, R.R.; Zara, A.L.D.S.A.; Melo, D.O.D.; Simone, A.L.M. Financial Impact of Centralized Purchasing of Rituximab by the Brazilian Ministry of Health for Lymphoma Treatment, 2015–2022: An Exploratory Study. Epidemiol. Serv. Saúde 2025, 34, e20240203. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária (ANVISA). Listas de Preços de Medicamentos—CMED. Available online: https://www.gov.br/anvisa/pt-br/assuntos/medicamentos/cmed/precos/capa-listas-de-precos (accessed on 1 August 2025).
- Okane, S.Y. Ordinance No. 2, of 3 January 2022. Available online: https://www.in.gov.br/en/web/dou/-/portaria-n-2-de-3-de-janeiro-de-2022-371727353 (accessed on 1 August 2025).
- Gomez, H.L.; Castañeda, C.; Valencia, F.; Muñoz-Bermeo, R.; Torrico, M.D.C.; Neciosup, S. ABC4 Consensus: First Latin American Meeting—Assessment, Comments, and Application of Its Recommendations. JCO Glob. Oncol. 2020, 6, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Murillo, R. Cancer Plans Should Consider Local Needs. Lancet Glob. Health 2025, 13, e181–e182. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD Reviews of Health Systems: Brazil 2021; OECD Reviews of Health Systems; OECD: Paris, France, 2021; ISBN 978-92-64-66073-1. [Google Scholar]
- OECD. Institutionalising Health Accounts in Brazil: A Review of Methods, Data and Policy Relevance; OECD Reviews of Health Systems; OECD Publishing: Paris, France, 2025; ISBN 978-92-64-58400-6. [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD) OECD. Stat Database. Available online: https://stats.oecd.org/ (accessed on 1 August 2025).
- OECD; The World Bank. Health at a Glance: Latin America and the Caribbean 2023; OECD: Paris, France, 2023; ISBN 978-92-64-93641-6. [Google Scholar]
- Goss, P.E.; Lee, B.L.; Badovinac-Crnjevic, T.; Strasser-Weippl, K.; Chavarri-Guerra, Y.; Louis, J.S.; Villarreal-Garza, C.; Unger-Saldaña, K.; Ferreyra, M.; Debiasi, M.; et al. Planning Cancer Control in Latin America and the Caribbean. Lancet Oncol. 2013, 14, 391–436. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Measure (N = 150) |
|---|---|
| Years working as oncologist—median (range) | 8 (1.–30.0) |
| Percentage of time dedicated to SUS *—median (range) | 50 (10.00–95.00) |
| Percentage of time dedicated to clinical practice—median (range) | 90 (30.00–100.00) |
| Responsible for treatment decision—n (%) | 147 (98.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resende, H.; Aguiar, V.d.Q.; Santos, N.F.d.A.; Jardim, J.V.S.; Ornelas, A. Treatment Disparities, Heterogeneities, and Barriers to Access for Patients with Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer: A National Survey from Brazil. Curr. Oncol. 2025, 32, 471. https://doi.org/10.3390/curroncol32080471
Resende H, Aguiar VdQ, Santos NFdA, Jardim JVS, Ornelas A. Treatment Disparities, Heterogeneities, and Barriers to Access for Patients with Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer: A National Survey from Brazil. Current Oncology. 2025; 32(8):471. https://doi.org/10.3390/curroncol32080471
Chicago/Turabian StyleResende, Heloisa, Vinícius de Q. Aguiar, Nataline F. de A. Santos, João Vitor Siqueira Jardim, and André Ornelas. 2025. "Treatment Disparities, Heterogeneities, and Barriers to Access for Patients with Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer: A National Survey from Brazil" Current Oncology 32, no. 8: 471. https://doi.org/10.3390/curroncol32080471
APA StyleResende, H., Aguiar, V. d. Q., Santos, N. F. d. A., Jardim, J. V. S., & Ornelas, A. (2025). Treatment Disparities, Heterogeneities, and Barriers to Access for Patients with Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer: A National Survey from Brazil. Current Oncology, 32(8), 471. https://doi.org/10.3390/curroncol32080471






