Pembrolizumab-Induced Simultaneous and Refractory Systemic Capillary Leak and Cytokine Release Syndromes: A Case Report
Simple Summary
Abstract
1. Introduction
2. Case Report
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lorusso, D.; Xiang, Y.; Hasegawa, K.; Scambia, G.; Leiva, M.; Ramos-Elias, P.; Acevedo, A.; Sukhin, V.; Cloven, N.; Pereira de Santana Gomes, A.J.; et al. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): A randomised, double-blind, phase 3 clinical trial. Lancet 2024, 403, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keam, S.; Turner, N.; Kugeratski, F.G.; Rico, R.; Colunga-Minutti, J.; Poojary, R.; Alekseev, S.; Patel, A.B.; Li, Y.J.; Sheshadri, A.; et al. Toxicity in the era of immune checkpoint inhibitor therapy. Front. Immunol. 2024, 15, 1447021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qin, H.; Vlaminck, B.; Owoyemi, I.; Herrmann, S.M.; Leung, N.; Markovic, S.N. Successful Treatment of Pembrolizumab-Induced Severe Capillary Leak Syndrome and Lymphatic Capillary Dysfunction. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 670–674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sackstein, P.; Zaemes, J.; Kim, C. Pembrolizumab-induced cytokine release syndrome in a patient with metastatic lung adenocarcinoma: A case report. J. Immunother. Cancer 2021, 9, e002855. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clarkson, B.; Thompson, D.; Horwith, M.; Luckey, E.H. Cyclical edema and shock due to increased capillary permeability. Am. J. Med. 1960, 29, 193–216. [Google Scholar] [CrossRef] [PubMed]
- Ceschi, A.; Noseda, R.; Palin, K.; Verhamme, K. Immune Checkpoint Inhibitor-Related Cytokine Release Syndrome: Analysis of WHO Global Pharmacovigilance Database. Front. Pharmacol. 2020, 11, 557. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Druey, K.M.; Greipp, P.R. Narrative review: The systemic capillary leak syndrome. Ann. Intern. Med. 2010, 153, 90–98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong So, J.; Bouibede, F.; Jonville-Béra, A.P.; Maillot, F.; Barbier, F.; Largeau, B. Immune checkpoint inhibitor-associated capillary leak syndrome: A systematic review and a worldwide pharmacovigilance study. J. Intern. Med. 2023, 294, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.; Grupp, S.A.; Mackall, C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014, 124, 188–195, Erratum in: Blood 2015, 126, 1048; Dosage Error in Article Text, Erratum in: Blood 2016, 128, 1533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shimabukuro-Vornhagen, A.; Gödel, P.; Subklewe, M.; Stemmler, H.J.; Schlößer, H.A.; Schlaak, M.; Kochanek, M.; Böll, B.; von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eo, T.S.; Chun, K.J.; Hong, S.J.; Kim, J.Y.; Lee, I.R.; Lee, K.H.; Eisenhut, M.; Kronbichler, A.; Shin, J.I. Clinical Presentation, Management, and Prognostic Factors of Idiopathic Systemic Capillary Leak Syndrome: A Systematic Review. J. Allergy Clin. Immunol. Pract. 2018, 6, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Huarte, E.; Peel, M.T.; Verbist, K.; Fay, B.L.; Bassett, R.; Albeituni, S.; Nichols, K.E.; Smith, P.A. Ruxolitinib, a JAK1/2 Inhibitor, Ameliorates Cytokine Storm in Experimental Models of Hyperinflammation Syndrome. Front. Pharmacol. 2021, 12, 650295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zi, F.M.; Ye, L.L.; Zheng, J.F.; Cheng, J.; Wang, Q.M. Using JAK inhibitor to treat cytokine release syndrome developed after chimeric antigen receptor T cell therapy for patients with refractory acute lymphoblastic leukemia: A case report. Medicine 2021, 100, e25786. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, C.; Kim, M.-J.; Kumar, A.; Lee, H.-W.; Yang, Y.; Kim, Y. Vascular endothelial growth factor signaling in health and disease: From molecular mechanisms to therapeutic perspectives. Signal Transduct. Target. Ther. 2025, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gödel, P.; Shimabukuro-Vornhagen, A.; von Bergwelt-Baildon, M. Understanding cytokine release syndrome. Intensive Care Med. 2018, 44, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef] [PubMed]
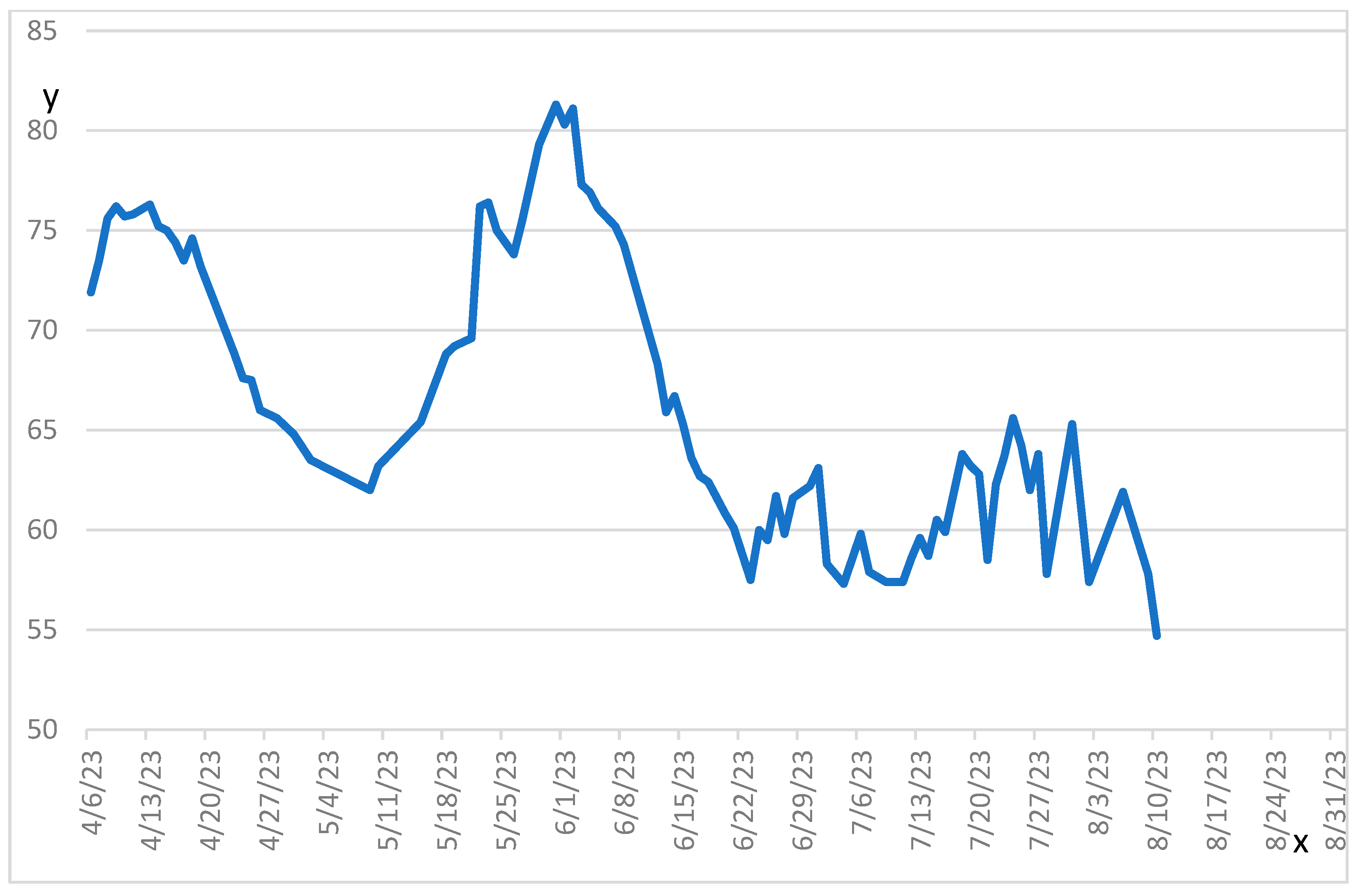
| Date | Hb (g/L) | Albumin (g/L) | Creatinine | ALT (U/L) | Troponin | Weight (kg) |
|---|---|---|---|---|---|---|
| Day 1 | 179 | 21 | 154 | 299 | 12 | N/A |
| Day 3 | 159 | N/A | 105 | 269 | N/A | 71.9 |
| Day 4 | 158 | 19 | 109 | 244 | N/A | 73.5 |
| Day 11 | 170 | 20 | 100 | 98 | 12,001 | 75.2 |
| Day 25 | 117 | 25 | 48 | 231 | 512 | 65.6 |
| Day 45 | 93 | 26 | 63 | 272 | 328 | 68.8 |
| Day 57 | 85 | 25 | 39 | 172 | N/A | 80.3 |
| Day 70 | 89 | 33 | 48 | 120 | N/A | 66.7 |
| Day 86 | 77 | 34 | 34 | 46 | N/A | 62.2 |
| Day 110 | 77 | 26 | 49 | 20 | N/A | 58.7 |
| Day 127 | 95 | 29 | 57 | 44 | N/A | 65.3 |
| Day 147 | 76 | 26 | 48 | 90 | N/A | 54.7 |
| Day 151 | 78 | 24 | 52 | 115 | N/A | N/A |
| Day 165 | 83 | 26 | 64 | 132 | N/A | N/A |
| Day 195 | 97 | 28 | 79 | 62 | N/A | N/A |
| Cytokine | Value (pg/mL) | Reference Value (pg/mL) |
|---|---|---|
| Group A: Innate autoimmune inflammation | ||
| IL-1 alpha | 59.7 | 0–58.3 |
| IFN-alpha2 | <2.5 | 5–134 |
| IL-17E/IL-25 | 1925 | 37–1194 |
| Group B2: T helper cell mediated inflammation | ||
| IL-12p70 | 57.7 | 0–14.6 |
| Group B3: Innate inflammation/Cytokine storm | ||
| IL-6 | 12.9 | 0.2–10.2 |
| IL-18 | 4.6 | 7–109 |
| IP-10 | 472 | 15–220 |
| M-CSF | 196 | 2–192 |
| Group D: Type 2/Type 3 immune response | ||
| IL-28A | 483 | 0–272 |
| TGF alpha | 32.5 | 1–31.2 |
| Group F: Hematopoietic growth factors | ||
| IL-7 | 29.3 | 0–20.5 |
| Group G: Homeostatic chemokines | ||
| CTAK | 1859 | 292–1685 |
| Group H: Platelet activation/wound healing | ||
| PDGF-AA | 5015 | 173–3619 |
| PDGF-AB/BB | >37,500 | 6653–34,584 |
| All other cytokine levels were within normal range | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberge-Maltais, E.; Lévesque, E.; Castonguay, V.; Marcoux, N.; Grenier, L.-P.; Veilleux, M. Pembrolizumab-Induced Simultaneous and Refractory Systemic Capillary Leak and Cytokine Release Syndromes: A Case Report. Curr. Oncol. 2025, 32, 469. https://doi.org/10.3390/curroncol32080469
Roberge-Maltais E, Lévesque E, Castonguay V, Marcoux N, Grenier L-P, Veilleux M. Pembrolizumab-Induced Simultaneous and Refractory Systemic Capillary Leak and Cytokine Release Syndromes: A Case Report. Current Oncology. 2025; 32(8):469. https://doi.org/10.3390/curroncol32080469
Chicago/Turabian StyleRoberge-Maltais, Eugénie, Eric Lévesque, Vincent Castonguay, Nicolas Marcoux, Louis-Philippe Grenier, and Martin Veilleux. 2025. "Pembrolizumab-Induced Simultaneous and Refractory Systemic Capillary Leak and Cytokine Release Syndromes: A Case Report" Current Oncology 32, no. 8: 469. https://doi.org/10.3390/curroncol32080469
APA StyleRoberge-Maltais, E., Lévesque, E., Castonguay, V., Marcoux, N., Grenier, L.-P., & Veilleux, M. (2025). Pembrolizumab-Induced Simultaneous and Refractory Systemic Capillary Leak and Cytokine Release Syndromes: A Case Report. Current Oncology, 32(8), 469. https://doi.org/10.3390/curroncol32080469





