Targeting Senescence in Oncology: An Emerging Therapeutic Avenue for Cancer
Simple Summary
Abstract
1. Introduction
2. Hallmarks of Cellular Senescence
2.1. General Features of Senescent Cells
2.2. Senescence-Associated Secretory Phenotype
3. Senescence Suppresses Cancer
3.1. Tumor Cell Growth Arrest
3.2. Recruitment of Immune Cells
3.3. Alteration of the Surface Proteome
4. Senescence Promotes Cancer
4.1. SASP-Driven Cancer Promotion
4.2. Ligands on Senescent Cells to Evade Immune Attack
4.3. Senescence in Stroma: Senescent Cancer-Associated Fibroblasts and Senescent Endothelial Cells
4.4. Immunosenescence
5. Therapy-Induced Senescence
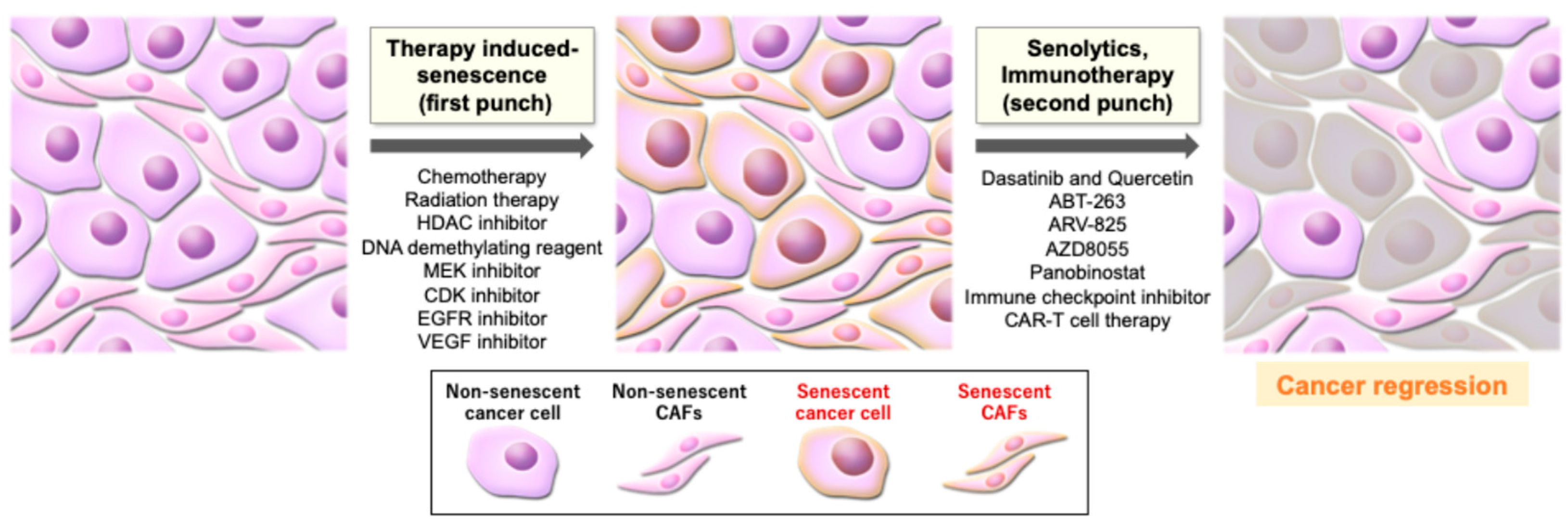
6. Senescence-Targeting Therapy in Cancer
6.1. Senotherapeutics: Senolytics and Senomorphics
6.2. Immune-Dependent Clearance of Senescent Cells
6.3. Perspectives on Clinical Applications
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McHugh, D.; Durán, I.; Gil, J. Senescence as a therapeutic target in cancer and age-related diseases. Nat. Rev. Drug Discov. 2025, 24, 57–71. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; di Fagagna, F.D. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Meguro, S.; Nakanishi, M. Cellular senescence in the cancer microenvironment. J. Biochem. 2025, 177, 171–176. [Google Scholar] [CrossRef]
- Schmitt, C.A.; Wang, B.; Demaria, M. Senescence and cancer—Role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 619–636. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Evangelou, K.; Lougiakis, N.; Rizou, S.V.; Kotsinas, A.; Kletsas, D.; Muñoz-Espín, D.; Kastrinakis, N.G.; Pouli, N.; Marakos, P.; Townsend, P.; et al. Robust, universal biomarker assay to detect senescent cells in biological specimens. Aging Cell 2017, 16, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Sharpless, N.E.; Sherr, C.J. Forging a signature of in vivo senescence. Nat. Rev. Cancer 2015, 15, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, Y.; Shao, L.; Laberge, R.-M.; DeMaria, M.; Campisi, J.; Janakiraman, K.; Sharpless, N.E.; Ding, S.; Feng, W.; et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016, 22, 78–83. [Google Scholar] [CrossRef]
- Yosef, R.; Pilpel, N.; Tokarsky-Amiel, R.; Biran, A.; Ovadya, Y.; Cohen, S.; Vadai, E.; Dassa, L.; Shahar, E.; Condiotti, R.; et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 2016, 7, 11190. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Shah, P.P.; Donahue, G.; Otte, G.L.; Capell, B.C.; Nelson, D.M.; Cao, K.; Aggarwala, V.; Cruickshanks, H.A.; Rai, T.S.; McBryan, T.; et al. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 2013, 27, 1787–1799. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Freund, A.; Laberge, R.-M.; Demaria, M.; Campisi, J.; Magin, T.M. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell 2012, 23, 2066–2075. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Han, J.; Elisseeff, J.H.; Demaria, M. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat. Rev. Mol. Cell Biol. 2024, 25, 958–978. [Google Scholar] [CrossRef] [PubMed]
- Laberge, R.-M.; Sun, Y.; Orjalo, A.V.; Patil, C.K.; Freund, A.; Zhou, L.; Curran, S.C.; Davalos, A.R.; Wilson-Edell, K.A.; Liu, S.; et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 2015, 17, 1049–1061, Erratum in Nat. Cell Biol. 2021, 23, 564–565. [Google Scholar] [CrossRef]
- Orjalo, A.V.; Bhaumik, D.; Gengler, B.K.; Scott, G.K.; Campisi, J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc. Natl. Acad. Sci. USA 2009, 106, 17031–17036. [Google Scholar] [CrossRef] [PubMed]
- Kuilman, T.; Michaloglou, C.; Vredeveld, L.C.; Douma, S.; van Doorn, R.; Desmet, C.J.; Aarden, L.A.; Mooi, W.J.; Peeper, D.S. Oncogene-Induced Senescence Relayed by an Interleukin-Dependent Inflammatory Network. Cell 2008, 133, 1019–1031. [Google Scholar] [CrossRef]
- Meguro, S.; Johmura, Y.; Wang, T.-W.; Kawakami, S.; Tanimoto, S.; Omori, S.; Okamura, Y.T.; Hoshi, S.; Kayama, E.; Yamaguchi, K.; et al. Preexisting senescent fibroblasts in the aged bladder create a tumor-permissive niche through CXCL12 secretion. Nat. Aging 2024, 4, 1582–1597. [Google Scholar] [CrossRef]
- Takikawa, T.; Hamada, S.; Matsumoto, R.; Tanaka, Y.; Kataoka, F.; Sasaki, A.; Masamune, A. Senescent Human Pancreatic Stellate Cells Secrete CXCR2 Agonist CXCLs to Promote Proliferation and Migration of Human Pancreatic Cancer AsPC-1 and MIAPaCa-2 Cell Lines. Int. J. Mol. Sci. 2022, 23, 9275. [Google Scholar] [CrossRef]
- Cheng, N.; Kim, K.-H.; Lau, L.F. Senescent hepatic stellate cells promote liver regeneration through IL-6 and ligands of CXCR2. J. Clin. Investig. 2022, 7, e158207. [Google Scholar] [CrossRef]
- Chambers, E.S.; Vukmanovic-Stejic, M.; Shih, B.B.; Trahair, H.; Subramanian, P.; Devine, O.P.; Glanville, J.; Gilroy, D.; Rustin, M.H.A.; Freeman, T.C.; et al. Recruitment of inflammatory monocytes by senescent fibroblasts inhibits antigen-specific tissue immunity during human aging. Nat. Aging 2021, 1, 101–113. [Google Scholar] [CrossRef]
- Kawagoe, Y.; Kawashima, I.; Sato, Y.; Okamoto, N.; Matsubara, K.; Kawamura, K. CXCL5-CXCR2 signaling is a senescence-associated secretory phenotype in preimplantation embryos. Aging Cell 2020, 19, e13240. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Lee, H.J.; Heo, J.; Lim, J.; Kim, M.; Kim, M.K.; Nam, H.Y.; Hong, G.H.; Cho, Y.S.; Choi, S.J.; et al. Senescence-Associated MCP-1 Secretion Is Dependent on a Decline in BMI1 in Human Mesenchymal Stromal Cells. Antioxid. Redox Signal. 2016, 24, 471–485. [Google Scholar] [CrossRef]
- Mattia, L.; Gossiel, F.; Walsh, J.S.; Eastell, R. Effect of age and gender on serum growth differentiation factor 15 and its relationship to bone density and bone turnover. Bone Rep. 2023, 18, 101676. [Google Scholar] [CrossRef]
- Guo, Y.; Ayers, J.L.; Carter, K.T.; Wang, T.; Maden, S.K.; Edmond, D.; Polly, P.N.; Li, C.; Ulrich, C.; Yu, M.; et al. Senescence-associated tissue microenvironment promotes colon cancer formation through the secretory factor GDF15. Aging Cell 2019, 18, e13013. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Fernandes, A.; Aguilar-Pimentel, J.A.; de Angelis, M.H.; Guedes, J.R.; Brito, M.A.; Ortolano, S.; Pani, G.; Athanasopoulou, S.; Gonos, E.S.; et al. Towards frailty biomarkers: Candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res. Rev. 2018, 47, 214–277. [Google Scholar] [CrossRef]
- Hoare, M.; Ito, Y.; Kang, T.-W.; Weekes, M.; Matheson, N.; Patten, D.; Shetty, S.; Parry, A.; Menon, S.; Salama, R.; et al. NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat. Cell. Biol. 2016, 18, 979–992. [Google Scholar] [CrossRef]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.-W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; de Jong, T.V.; Melov, S.; Guryev, V.; Campisi, J.; DeMaria, M. Unmasking Transcriptional Heterogeneity in Senescent Cells. Curr. Biol. 2017, 27, 2652–2660.e4. [Google Scholar] [CrossRef]
- Braig, M.; Lee, S.; Loddenkemper, C.; Rudolph, C.; Peters, A.H.; Schlegelberger, B.; Stein, H.; Dörken, B.; Jenuwein, T.; Schmitt, C.A. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 2005, 436, 660–665. [Google Scholar] [CrossRef]
- Liu, X.-L.; Ding, J.; Meng, L.-H. Oncogene-induced senescence: A double edged sword in cancer. Acta Pharmacol. Sin. 2018, 39, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Courtois-Cox, S.; Jones, S.L.; Cichowski, K. Many roads lead to oncogene-induced senescence. Oncogene 2008, 27, 2801–2809. [Google Scholar] [CrossRef]
- Chan, A.S.L.; Zhu, H.; Cassidy, L.D.; Young, A.R.J.; Bermejo-Rodriguez, C.; Janowska, A.T.; Chen, H.-C.; Gough, S.; Oshimori, N.; Zender, L.; et al. Titration of RAS alters senescent state and influences tumour initiation. Nature 2024, 633, 678–685. [Google Scholar] [CrossRef]
- Sturmlechner, I.; Zhang, C.; Sine, C.C.; van Deursen, E.-J.; Jeganathan, K.B.; Hamada, N.; Grasic, J.; Friedman, D.; Stutchman, J.T.; Can, I.; et al. p21 produces a bioactive secretome that places stressed cells under immunosurveillance. Science 2021, 374, eabb3420. [Google Scholar] [CrossRef]
- Kang, T.-W.; Yevsa, T.; Woller, N.; Hoenicke, L.; Wuestefeld, T.; Dauch, D.; Hohmeyer, A.; Gereke, M.; Rudalska, R.; Potapova, A.; et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 2011, 479, 547–551. [Google Scholar] [CrossRef]
- Xue, W.; Zender, L.; Miething, C.; Dickins, R.A.; Hernando, E.; Krizhanovsky, V.; Cordon-Cardo, C.; Lowe, S.W. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007, 445, 656–660. [Google Scholar] [CrossRef]
- Lujambio, A.; Akkari, L.; Simon, J.; Grace, D.; Tschaharganeh, D.F.; Bolden, J.E.; Zhao, Z.; Thapar, V.; Joyce, J.A.; Krizhanovsky, V.; et al. Non-Cell-Autonomous Tumor Suppression by p53. Cell 2013, 153, 449–460. [Google Scholar] [CrossRef]
- Ruscetti, M.; Morris, J.P.; Mezzadra, R.; Russell, J.; Leibold, J.; Romesser, P.B.; Simon, J.; Kulick, A.; Ho, Y.J.; Fennell, M.; et al. Senescence-Induced Vascular Remodeling Creates Therapeutic Vulnerabilities in Pancreas Cancer. Cell 2021, 184, 4838–4839. [Google Scholar] [CrossRef]
- Ruscetti, M.; Leibold, J.; Bott, M.J.; Fennell, M.; Kulick, A.; Salgado, N.R.; Chen, C.-C.; Ho, Y.-J.; Sanchez-Rivera, F.J.; Feucht, J.; et al. NK cell–mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science 2018, 362, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, C.; Raigel, M.; Limberger, T.; Trachtová, K.; Schlederer, M.; Lindner, D.; Kodajova, P.; Yang, J.; Ziegler, R.; Kalla, J.; et al. Cell-autonomous IL6ST activation suppresses prostate cancer development via STAT3/ARF/p53-driven senescence and confers an immune-active tumor microenvironment. Mol. Cancer 2024, 23, 245. [Google Scholar] [CrossRef] [PubMed]
- Colucci, M.; Zumerle, S.; Bressan, S.; Gianfanti, F.; Troiani, M.; Valdata, A.; D’Ambrosio, M.; Pasquini, E.; Varesi, A.; Cogo, F.; et al. Retinoic acid receptor activation reprograms senescence response and enhances anti-tumor activity of natural killer cells. Cancer Cell 2024, 42, 646–661.e9. [Google Scholar] [CrossRef]
- Romesser, P.B.; Lowe, S.W. The Potent and Paradoxical Biology of Cellular Senescence in Cancer. Annu. Rev. Cancer Biol. 2023, 7, 207–228. [Google Scholar] [CrossRef]
- Goel, S.; DeCristo, M.J.; Watt, A.C.; BrinJones, H.; Sceneay, J.; Li, B.B.; Khan, N.; Ubellacker, J.M.; Xie, S.; Metzger-Filho, O.; et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017, 548, 471–475. [Google Scholar] [CrossRef]
- Chen, H.A.; Ho, Y.J.; Mezzadra, R.; Adrover, J.M.; Smolkin, R.; Zhu, C.; Woess, K.; Bernstein, N.; Schmitt, G.; Fong, L.; et al. Senescence Rewires Microenvironment Sensing to Facilitate Antitumor Immunity. Cancer Discov. 2023, 13, 432–453. [Google Scholar] [CrossRef]
- Sagiv, A.; Burton, D.G.A.; Moshayev, Z.; Vadai, E.; Wensveen, F.; Ben-Dor, S.; Golani, O.; Polic, B.; Krizhanovsky, V. NKG2D ligands mediate immunosurveillance of senescent cells. Aging 2016, 8, 328–344. [Google Scholar] [CrossRef]
- Ruhland, M.K.; Loza, A.J.; Capietto, A.-H.; Luo, X.; Knolhoff, B.L.; Flanagan, K.C.; Belt, B.A.; Alspach, E.; Leahy, K.; Luo, J.; et al. Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat. Commun. 2016, 7, 11762. [Google Scholar] [CrossRef]
- Eggert, T.; Wolter, K.; Ji, J.; Ma, C.; Yevsa, T.; Klotz, S.; Medina-Echeverz, J.; Longerich, T.; Forgues, M.; Reisinger, F.; et al. Distinct Functions of Senescence-Associated Immune Responses in Liver Tumor Surveillance and Tumor Progression. Cancer Cell 2016, 30, 533–547. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Myeloid-derived suppressor cells (MDSC): An important partner in cellular/tissue senescence. Biogerontology 2018, 19, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Toso, A.; Revandkar, A.; Di Mitri, D.; Guccini, I.; Proietti, M.; Sarti, M.; Pinton, S.; Zhang, J.; Kalathur, M.; Civenni, G.; et al. Enhancing Chemotherapy Efficacy in Pten-Deficient Prostate Tumors by Activating the Senescence-Associated Antitumor Immunity. Cell Rep. 2014, 9, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Montero, P.; Londoño-Vallejo, A.; Vernot, J.-P. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun. Signal. 2017, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Fu, Y.; Loza, A.J.; Murali, B.; Leahy, K.M.; Ruhland, M.K.; Gang, M.; Su, X.; Zamani, A.; Shi, Y.; et al. Stromal-Initiated Changes in the Bone Promote Metastatic Niche Development. Cell Rep. 2016, 14, 82–92. [Google Scholar] [CrossRef]
- Ancrile, B.; Lim, K.-H.; Counter, C.M. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 2007, 21, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Yang, X.; Zhang, Y.; Dong, H.; Liu, X.; Xu, X.; Zhang, X.; Shi, Y.; Hou, M.; Song, B.; et al. Palbociclib Enhances Migration and Invasion of Cancer Cells via Senescence-Associated Secretory Phenotype-Related CCL5 in Non-Small-Cell Lung Cancer. J. Oncol. 2022, 2022, 2260625. [Google Scholar] [CrossRef] [PubMed]
- Eyman, D.; Damodarasamy, M.; Plymate, S.; Reed, M. CCL5 secreted by senescent aged fibroblasts induces proliferation of prostate epithelial cells and expression of genes that modulate angiogenesis. J. Cell. Physiol. 2009, 220, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hornsby, P.J. Senescent Human Fibroblasts Increase the Early Growth of Xenograft Tumors via Matrix Metalloproteinase Secretion. Cancer Res. 2007, 67, 3117–3126. [Google Scholar] [CrossRef]
- Guccini, I.; Revandkar, A.; D’AMbrosio, M.; Colucci, M.; Pasquini, E.; Mosole, S.; Troiani, M.; Brina, D.; Sheibani-Tezerji, R.; Elia, A.R.; et al. Senescence Reprogramming by TIMP1 Deficiency Promotes Prostate Cancer Metastasis. Cancer Cell 2021, 39, 68–82.e9. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Komoda, K.; Mikawa, R.; Asai, A.; Sugimoto, M. Cellular senescence promotes cancer metastasis by enhancing soluble E-cadherin production. iScience 2021, 24, 103022. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, Y.W.; Lee, J.; Soh, E.Y.; Kim, J.-H.; Park, T.J. Senescent tumor cells lead the collective invasion in thyroid cancer. Nat. Commun. 2017, 8, 15208. [Google Scholar] [CrossRef]
- Wang, T.-W.; Nakanishi, M. Immune surveillance of senescence: Potential application to age-related diseases. Trends Cell Biol. 2025, 35, 248–257. [Google Scholar] [CrossRef]
- Majewska, J.; Agrawal, A.; Mayo, A.; Roitman, L.; Chatterjee, R.; Kralova, J.S.; Landsberger, T.; Katzenelenbogen, Y.; Meir-Salame, T.; Hagai, E.; et al. p16-dependent increase of PD-L1 stability regulates immunosurveillance of senescent cells. Nat. Cell Biol. 2024, 26, 1336–1345. [Google Scholar] [CrossRef]
- Wang, T.-W.; Johmura, Y.; Suzuki, N.; Omori, S.; Migita, T.; Yamaguchi, K.; Hatakeyama, S.; Yamazaki, S.; Shimizu, E.; Imoto, S.; et al. Blocking PD-L1–PD-1 improves senescence surveillance and ageing phenotypes. Nature 2022, 611, 358–364. [Google Scholar] [CrossRef]
- Onorati, A.; Havas, A.P.; Lin, B.; Rajagopal, J.; Sen, P.; Adams, P.D.; Dou, Z. Upregulation of PD-L1 in Senescence and Aging. Mol. Cell. Biol. 2022, 42, e0017122. [Google Scholar] [CrossRef]
- Reimann, M.; Schrezenmeier, J.F.; Richter-Pechanska, P.; Dolnik, A.; Hick, T.P.; Schleich, K.; Cai, X.; Fan, D.N.Y.; Lohneis, P.; Masswig, S.; et al. Adaptive T-cell immunity controls senescence-prone MyD88- or CARD11-mutant B-cell lymphomas. Blood 2021, 137, 2785–2799. [Google Scholar] [CrossRef] [PubMed]
- Shahbandi, A.; Chiu, F.-Y.; Ungerleider, N.A.; Kvadas, R.; Mheidly, Z.; Sun, M.J.S.; Tian, D.; Waizman, D.A.; Anderson, A.Y.; Machado, H.L.; et al. Breast cancer cells survive chemotherapy by activating targetable immune-modulatory programs characterized by PD-L1 or CD80. Nat. Cancer 2022, 3, 1513–1533. [Google Scholar] [CrossRef] [PubMed]
- Chaib, S.; López-Domínguez, J.A.; Lalinde-Gutiérrez, M.; Prats, N.; Marin, I.; Boix, O.; García-Garijo, A.; Meyer, K.; Muñoz, M.I.; Aguilera, M.; et al. The efficacy of chemotherapy is limited by intratumoral senescent cells expressing PD-L2. Nat. Cancer 2024, 5, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.I.; Devine, O.P.; Vukmanovic-Stejic, M.; Chambers, E.S.; Subramanian, P.; Patel, N.; Virasami, A.; Sebire, N.J.; Kinsler, V.; Valdovinos, A.; et al. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8+ T cell inhibition. Nat. Commun. 2019, 10, 2387. [Google Scholar] [CrossRef]
- Muñoz, D.P.; Yannone, S.M.; Daemen, A.; Sun, Y.; Vakar-Lopez, F.; Kawahara, M.; Freund, A.M.; Rodier, F.; Wu, J.D.; Desprez, P.-Y.; et al. Targetable mechanisms driving immunoevasion of persistent senescent cells link chemotherapy-resistant cancer to aging. J. Clin. Investig. 2019, 5, e124716. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Liu, L.; Huang, H.; Cheng, B.; Xie, H.; Peng, W.; Cui, H.; Liang, J.; Cao, M.; Yang, Y.; Chen, W.; et al. Revealing the role of cancer-associated fibroblast senescence in prognosis and immune landscape in pancreatic cancer. iScience 2025, 28, 111612. [Google Scholar] [CrossRef]
- Assouline, B.; Kahn, R.; Hodali, L.; Condiotti, R.; Engel, Y.; Elyada, E.; Mordechai-Heyn, T.; Pitarresi, J.R.; Atias, D.; Steinberg, E.; et al. Senescent cancer-associated fibroblasts in pancreatic adenocarcinoma restrict CD8+ T cell activation and limit responsiveness to immunotherapy in mice. Nat. Commun. 2024, 15, 6162. [Google Scholar] [CrossRef]
- Belle, J.I.; Sen, D.; Baer, J.M.; Liu, X.; Lander, V.E.; Ye, J.; Sells, B.E.; Knolhoff, B.L.; Faiz, A.; Kang, L.-I.; et al. Senescence Defines a Distinct Subset of Myofibroblasts That Orchestrates Immunosuppression in Pancreatic Cancer. Cancer Discov. 2024, 14, 1324–1355. [Google Scholar] [CrossRef]
- Ye, J.; Baer, J.M.; Faget, D.V.; Morikis, V.A.; Ren, Q.; Melam, A.; Delgado, A.P.; Luo, X.; Bagchi, S.M.; Belle, J.I.; et al. Senescent CAFs Mediate Immunosuppression and Drive Breast Cancer Progression. Cancer Discov. 2024, 14, 1302–1323. [Google Scholar] [CrossRef]
- Leone, P.; Malerba, E.; Susca, N.; Favoino, E.; Perosa, F.; Brunori, G.; Prete, M.; Racanelli, V. Endothelial cells in tumor micro-environment: Insights and perspectives. Front. Immunol. 2024, 15, 1367875. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; He, X.; Fu, Y.; Ge, S.; Yang, Z. Senescent endothelial cells promote liver metastasis of uveal melanoma in single-cell resolution. J. Transl. Med. 2024, 22, 605. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Lee, Y.R.; Kang, D.; Lee, H.C.; Seo, H.R.; Ryu, J.K.; Kim, Y.N.; Ko, Y.G.; Park, H.J.; Lee, J.S. Endothelial cells under therapy-induced senescence secrete CXCL11, which increases aggressiveness of breast cancer cells. Cancer Lett. 2020, 490, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xiao, F.; Feng, Z.; Li, M.; Kong, L.; Huang, L.; Wei, Y.; Li, H.; Liu, F.; Zhang, H.; et al. Sunitinib facilitates metastatic breast cancer spreading by inducing endothelial cell senescence. Breast Cancer Res. 2020, 22, 103. [Google Scholar] [CrossRef]
- Wieland, E.; Rodriguez-Vita, J.; Liebler, S.S.; Mogler, C.; Moll, I.; Herberich, S.E.; Espinet, E.; Herpel, E.; Menuchin, A.; Chang-Claude, J.; et al. Endothelial Notch1 Activity Facilitates Metastasis. Cancer Cell 2017, 31, 355–367. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular mechanisms and diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef]
- Fane, M.; Weeraratna, A.T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer 2020, 20, 89–106. [Google Scholar] [CrossRef]
- Sanoff, H.K.; Deal, A.M.; Krishnamurthy, J.; Torrice, C.; Dillon, P.; Sorrentino, J.; Ibrahim, J.G.; Jolly, T.A.; Williams, G.; Carey, L.A.; et al. Effect of Cytotoxic Chemotherapy on Markers of Molecular Age in Patients with Breast Cancer. J. Natl. Cancer Inst. 2014, 106, dju057. [Google Scholar] [CrossRef]
- Amundson, S.A.; Grace, M.B.; McLeland, C.B.; Epperly, M.W.; Yeager, A.; Zhan, Q.; Greenberger, J.S.; Fornace, A.J., Jr. Human in vivo radiation-induced biomarkers: Gene expression changes in radiotherapy patients. Cancer Res. 2004, 64, 6368–6371. [Google Scholar] [CrossRef]
- Schwartz, G.K.; Shah, M.A. Targeting the Cell Cycle: A New Approach to Cancer Therapy. J. Clin. Oncol. 2005, 23, 9408–9421. [Google Scholar] [CrossRef]
- Zhou, B.-B.S.; Elledge, S.J. The DNA damage response: Putting checkpoints in perspective. Nature 2000, 408, 433–439. [Google Scholar] [CrossRef]
- Faheem, M.M.; Seligson, N.D.; Ahmad, S.M.; Rasool, R.U.; Gandhi, S.G.; Bhagat, M.; Goswami, A. Convergence of therapy-induced senescence (TIS) and EMT in multistep carcinogenesis: Current opinions and emerging perspectives. Cell Death Discov. 2020, 6, 51. [Google Scholar] [CrossRef]
- Wang, M.; Morsbach, F.; Sander, D.; Gheorghiu, L.; Nanda, A.; Benes, C.; Kriegs, M.; Krause, M.; Dikomey, E.; Baumann, M.; et al. EGF receptor inhibition radiosensitizes NSCLC cells by inducing senescence in cells sustaining DNA double-strand breaks. Cancer Res. 2011, 71, 6261–6269. [Google Scholar] [CrossRef]
- Hasan, M.R.; Ho, S.H.Y.; Owen, D.A.; Tai, I.T. Inhibition of VEGF induces cellular senescence in colorectal cancer cells. Int. J. Cancer 2011, 129, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Amatori, S.; Bagaloni, I.; Viti, D.; Fanelli, M. Premature senescence induced by DNA demethylating agent (Decitabine) as therapeutic option for malignant pleural mesothelioma. Lung Cancer 2011, 71, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Turchick, A.; Zimmermann, A.; Chiu, L.Y.; Dahmen, H.; Elenbaas, B.; Zenke, F.T.; Blaukat, A.; Vassilev, L.T. Selective Inhibition of ATM-Dependent Double-Strand Break Repair and Checkpoint Control Synergistically Enhances the Efficacy of ATR Inhibitors. Mol. Cancer Ther. 2023, 22, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Dobler, C.; Jost, T.; Hecht, M.; Fietkau, R.; Distel, L. Senescence Induction by Combined Ionizing Radiation and DNA Damage Response Inhibitors in Head and Neck Squamous Cell Carcinoma Cells. Cells 2020, 9, 2012. [Google Scholar] [CrossRef]
- Vendetti, F.P.; Lau, A.; Schamus, S.; Conrads, T.P.; O’Connor, M.J.; Bakkenist, C.J. The orally active and bioavailable ATR kinase inhibitor AZD6738 potentiates the anti-tumor effects of cisplatin to resolve ATM-deficient non-small cell lung cancer in vivo. Oncotarget 2015, 6, 44289–44305. [Google Scholar] [CrossRef] [PubMed]
- Tesei, A.; Arienti, C.; Bossi, G.; Santi, S.; De Santis, I.; Bevilacqua, A.; Zanoni, M.; Pignatta, S.; Cortesi, M.; Zamagni, A.; et al. TP53 drives abscopal effect by secretion of senescence-associated molecular signals in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2021, 40, 89. [Google Scholar] [CrossRef] [PubMed]
- Kansara, M.; Leong, H.S.; Lin, D.M.; Popkiss, S.; Pang, P.; Garsed, D.W.; Walkley, C.R.; Cullinane, C.; Ellul, J.; Haynes, N.M.; et al. Immune response to RB1-regulated senescence limits radiation-induced osteosarcoma formation. J. Clin. Investig. 2013, 123, 5351–5360. [Google Scholar] [CrossRef]
- Liu, Y.; Pagacz, J.; Wolfgeher, D.J.; Bromerg, K.D.; Gorman, J.V.; Kron, S.J. Senescent cancer cell vaccines induce cytotoxic T cell responses targeting primary tumors and disseminated tumor cells. J. Immunother. Cancer 2023, 11, e005862. [Google Scholar] [CrossRef]
- Meng, Y.; Efimova, E.V.; Hamzeh, K.W.; Darga, T.E.; Mauceri, H.J.; Fu, Y.-X.; Kron, S.J.; Weichselbaum, R.R. Radiation-inducible Immunotherapy for Cancer: Senescent Tumor Cells as a Cancer Vaccine. Mol. Ther. 2012, 20, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Uceda-Castro, R.; Margarido, A.S.; Cornet, L.; Vegna, S.; Hahn, K.; Song, J.-Y.; Putavet, D.A.; van Geldorp, M.; Çitirikkaya, C.H.; de Keizer, P.L.; et al. Re-purposing the pro-senescence properties of doxorubicin to introduce immunotherapy in breast cancer brain metastasis. Cell Rep. Med. 2022, 3, 100821. [Google Scholar] [CrossRef]
- Marin, I.; Boix, O.; Garcia-Garijo, A.; Sirois, I.; Caballe, A.; Zarzuela, E.; Ruano, I.; Attolini, C.S.; Prats, N.; Lopez-Dominguez, J.A.; et al. Cellular Senescence Is Immunogenic and Promotes Antitumor Immunity. Cancer Discov. 2023, 13, 410–431. [Google Scholar] [CrossRef]
- Soriani, A.; Iannitto, M.L.; Ricci, B.; Fionda, C.; Malgarini, G.; Morrone, S.; Peruzzi, G.; Ricciardi, M.R.; Petrucci, M.T.; Cippitelli, M.; et al. Reactive Oxygen Species– and DNA Damage Response–Dependent NK Cell Activating Ligand Upregulation Occurs at Transcriptional Levels and Requires the Transcriptional Factor E2F1. J. Immunol. 2014, 193, 950–960. [Google Scholar] [CrossRef]
- Hao, X.; Zhao, B.; Zhou, W.; Liu, H.; Fukumoto, T.; Gabrilovich, D.; Zhang, R. Sensitization of ovarian tumor to immune checkpoint blockade by boosting senescence-associated secretory phenotype. iScience 2021, 24, 102016. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kang, D.; Shin, J.; Jung, J.; Ko, S.; Jung, K.H.; Hong, S.-S.; Park, J.E.; Oh, M.J.; An, H.J.; et al. Therapy-induced senescent cancer cells contribute to cancer progression by promoting ribophorin 1-dependent PD-L1 upregulation. Nat. Commun. 2025, 16, 353. [Google Scholar] [CrossRef]
- Chembukavu, S.N.; Lindsay, A.J. Therapy-induced senescence in breast cancer: An overview. Explor. Target. Anti-Tumor Ther. 2024, 5, 902–920. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Shi, B.; Zheng, H.; Min, L.; Yang, J.; Li, X.; Liao, X.; Huang, W.; Zhang, M.; Xu, S.; et al. Senescence-associated secretory factors induced by cisplatin in melanoma cells promote non-senescent melanoma cell growth through activation of the ERK1/2-RSK1 pathway. Cell Death Dis. 2018, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.G.; Pant, V.; Li, Q.; Chang, L.L.; Quintás-Cardama, A.; Garza, D.; Tavana, O.; Yang, P.; Manshouri, T.; Li, Y.; et al. p53-Mediated Senescence Impairs the Apoptotic Response to Chemotherapy and Clinical Outcome in Breast Cancer. Cancer Cell 2012, 21, 793–806. [Google Scholar] [CrossRef]
- Wang, L.; Lankhorst, L.; Bernards, R. Exploiting senescence for the treatment of cancer. Nat. Rev. Cancer 2022, 22, 340–355. [Google Scholar] [CrossRef]
- Zhu, Y.I.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Thadathil, N.; Selvarani, R.; Mohammed, S.; Nicklas, E.H.; Tran, A.L.; Kamal, M.; Luo, W.; Brown, J.L.; Lawrence, M.M.; Borowik, A.K.; et al. Senolytic treatment reduces cell senescence and necroptosis in Sod1 knockout mice that is associated with reduced inflammation and hepatocellular carcinoma. Aging Cell 2022, 21, e13676. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, B.; Lu, W.; Cheng, Y.; Zhu, J.; Ai, G.; Zhang, X.; Liu, X.; Cheng, Z. Senolytic drugs dasatinib and quercetin combined with Carboplatin or Olaparib reduced the peritoneal and adipose tissue metastasis of ovarian cancer. Biomed. Pharmacother. 2024, 174, 116474. [Google Scholar] [CrossRef]
- Saleh, T.; Carpenter, V.J.; Tyutyunyk-Massey, L.; Murray, G.; Leverson, J.D.; Souers, A.J.; Alotaibi, M.R.; Faber, A.C.; Reed, J.; Harada, H.; et al. Clearance of therapy-induced senescent tumor cells by the senolytic ABT-263 via interference with BCL-X(L) -BAX interaction. Mol. Oncol. 2020, 14, 2504–2519. [Google Scholar] [CrossRef] [PubMed]
- Fleury, H.; Malaquin, N.; Tu, V.; Gilbert, S.; Martinez, A.; Olivier, M.-A.; Sauriol, S.A.; Communal, L.; Leclerc-Desaulniers, K.; Carmona, E.; et al. Exploiting interconnected synthetic lethal interactions between PARP inhibition and cancer cell reversible senescence. Nat. Commun. 2019, 10, 2556. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gualda, E.; Paez-Ribes, M.; Lozano-Torres, B.; Macias, D.; Wilson, J.R., III; Gonzalez-Lopez, C.; Ou, H.L.; Miron-Barroso, S.; Zhang, Z.; Lerida-Viso, A.; et al. Galacto-conjugation of Navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity. Aging Cell 2020, 19, e13142. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Vegna, S.; Jin, H.; Benedict, B.; Lieftink, C.; Ramirez, C.; de Oliveira, R.L.; Morris, B.; Gadiot, J.; Wang, W.; et al. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature 2019, 574, 268–272. [Google Scholar] [CrossRef]
- Wakita, M.; Takahashi, A.; Sano, O.; Loo, T.M.; Imai, Y.; Narukawa, M.; Iwata, H.; Matsudaira, T.; Kawamoto, S.; Ohtani, N.; et al. A BET family protein degrader provokes senolysis by targeting NHEJ and autophagy in senescent cells. Nat. Commun. 2020, 11, 1935. [Google Scholar] [CrossRef]
- Samaraweera, L.; Adomako, A.; Rodriguez-Gabin, A.; McDaid, H.M. A Novel Indication for Panobinostat as a Senolytic Drug in NSCLC and HNSCC. Sci. Rep. 2017, 7, 1900. [Google Scholar] [CrossRef]
- Johmura, Y.; Yamanaka, T.; Omori, S.; Wang, T.-W.; Sugiura, Y.; Matsumoto, M.; Suzuki, N.; Kumamoto, S.; Yamaguchi, K.; Hatakeyama, S.; et al. Senolysis by glutaminolysis inhibition ameliorates various age-associated disorders. Science 2021, 371, 265–270. [Google Scholar] [CrossRef]
- Kovacovicova, K.; Skolnaja, M.; Heinmaa, M.; Mistrik, M.; Pata, P.; Pata, I.; Bartek, J.; Vinciguerra, M. Senolytic Cocktail Dasatinib + Quercetin (D + Q) Does Not Enhance the Efficacy of Senescence-Inducing Chemotherapy in Liver Cancer. Front. Oncol. 2018, 8, 459. [Google Scholar] [CrossRef] [PubMed]
- Raffaele, M.; Kovacovicova, K.; Frohlich, J.; Re, O.L.; Giallongo, S.; Oben, J.A.; Faldyna, M.; Leva, L.; Giannone, A.G.; Cabibi, D.; et al. Mild exacerbation of obesity- and age-dependent liver disease progression by senolytic cocktail dasatinib + quercetin. Cell Commun. Signal. 2021, 19, 44. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, J.D.; Infante, J.R.; Spigel, D.R.; Arrowsmith, E.R.; Boccia, R.V.; Burris, H.A. A phase II trial of panobinostat, a histone deacetylase inhibitor, in the treatment of patients with refractory metastatic renal cell carcinoma. Cancer Investig. 2011, 29, 451–455. [Google Scholar] [CrossRef]
- Rathkopf, D.E.; Picus, J.; Hussain, A.; Ellard, S.; Chi, K.N.; Nydam, T.; Allen-Freda, E.; Mishra, K.K.; Porro, M.G.; Scher, H.I.; et al. A phase 2 study of intravenous panobinostat in patients with castration-resistant prostate cancer. Cancer Chemother. Pharmacol. 2013, 72, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Murali, B.; Ren, Q.; Luo, X.; Faget, D.V.; Wang, C.; Johnson, R.M.; Gruosso, T.; Flanagan, K.C.; Fu, Y.; Leahy, K.; et al. Inhibition of the Stromal p38MAPK/MK2 Pathway Limits Breast Cancer Metastases and Chemotherapy-Induced Bone Loss. Cancer Res. 2018, 78, 5618–5630. [Google Scholar] [CrossRef]
- Jerby-Arnon, L.; Shah, P.; Cuoco, M.S.; Rodman, C.; Su, M.-J.; Melms, J.C.; Leeson, R.; Kanodia, A.; Mei, S.; Lin, J.-R.; et al. A Cancer Cell Program Promotes T Cell Exclusion and Resistance to Checkpoint Blockade. Cell 2018, 175, 984–997.e24. [Google Scholar] [CrossRef]
- Amor, C.; Feucht, J.; Leibold, J.; Ho, Y.-J.; Zhu, C.; Alonso-Curbelo, D.; Mansilla-Soto, J.; Boyer, J.A.; Li, X.; Giavridis, T.; et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 2020, 583, 127–132. [Google Scholar] [CrossRef]
- Tolcher, A.W.; LoRusso, P.; Arzt, J.; Busman, T.A.; Lian, G.; Rudersdorf, N.S.; Vanderwal, C.A.; Kirschbrown, W.; Holen, K.D.; Rosen, L.S. Safety, efficacy, and pharmacokinetics of navitoclax (ABT-263) in combination with erlotinib in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2015, 76, 1025–1032. [Google Scholar] [CrossRef]
- Vlahovic, G.; Karantza, V.; Wang, D.; Cosgrove, D.; Rudersdorf, N.; Yang, J.; Xiong, H.; Busman, T.; Mabry, M. A phase I safety and pharmacokinetic study of ABT-263 in combination with carboplatin/paclitaxel in the treatment of patients with solid tumors. Investig. New Drugs 2014, 32, 976–984. [Google Scholar] [CrossRef]
- Cleary, J.M.; Lima, C.M.S.R.; Hurwitz, H.I.; Montero, A.J.; Franklin, C.; Yang, J.; Graham, A.; Busman, T.; Mabry, M.; Holen, K.; et al. A phase I clinical trial of navitoclax, a targeted high-affinity Bcl-2 family inhibitor, in combination with gemcitabine in patients with solid tumors. Investig. New Drugs 2014, 32, 937–945. [Google Scholar] [CrossRef]
- Puglisi, M.; Molife, L.R.; de Jonge, M.J.; Khan, K.H.; Doorn, L.V.; Forster, M.D.; Blanco, M.; Gutierrez, M.; Franklin, C.; Busman, T.; et al. A Phase I study of the safety, pharmacokinetics and efficacy of navitoclax plus docetaxel in patients with advanced solid tumors. Future Oncol. 2021, 17, 2747–2758. [Google Scholar] [CrossRef]
- Pietanza, M.C.; Rudin, C.M. Novel Therapeutic Approaches for Small Cell Lung Cancer: The Future has Arrived. Curr. Probl. Cancer 2012, 36, 156–173. [Google Scholar] [CrossRef]
- Goldberg, J.; Sulis, M.L.; Bender, J.; Jeha, S.; Gardner, R.; Pollard, J.; Aquino, V.; Laetsch, T.; Winick, N.; Fu, C.; et al. A phase I study of panobinostat in children with relapsed and refractory hematologic malignancies. Pediatr. Hematol. Oncol. 2020, 37, 465–474. [Google Scholar] [CrossRef]
- Berenson, J.R.; Hilger, J.D.; Yellin, O.; Boccia, R.V.; Matous, J.; Dressler, K.; Ghazal, H.H.; Jamshed, S.; Kingsley, E.C.; Harb, W.A.; et al. A phase 1/2 study of oral panobinostat combined with melphalan for patients with relapsed or refractory multiple myeloma. Ann. Hematol. 2014, 93, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Drappatz, J.; Lee, E.Q.; Hammond, S.; Grimm, S.A.; Norden, A.D.; Beroukhim, R.; Gerard, M.; Schiff, D.; Chi, A.S.; Batchelor, T.T.; et al. Phase I study of panobinostat in combination with bevacizumab for recurrent high-grade glioma. J. Neuro-Oncol. 2012, 107, 133–138. [Google Scholar] [CrossRef]
- Lee, E.Q.; Reardon, D.A.; Schiff, D.; Drappatz, J.; Muzikansky, A.; Grimm, S.A.; Norden, A.D.; Nayak, L.; Beroukhim, R.; Rinne, M.L.; et al. Phase II study of panobinostat in combination with bevacizumab for recurrent glioblastoma and anaplastic glioma. Neuro-Oncology 2015, 17, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manero, G.; Sekeres, M.A.; Egyed, M.; Breccia, M.; Graux, C.; Cavenagh, J.D.; Salman, H.; Illes, A.; Fenaux, P.; DeAngelo, D.J.; et al. A phase 1b/2b multicenter study of oral panobinostat plus azacitidine in adults with MDS, CMML or AML with ≤30% blasts. Leukemia 2017, 31, 2799–2806. [Google Scholar] [CrossRef] [PubMed]
- Uy, G.L.; Duncavage, E.J.; Chang, G.S.; Jacoby, M.A.; Miller, C.A.; Shao, J.; Heath, S.; Elliott, K.; Reineck, T.; Fulton, R.S.; et al. Dynamic changes in the clonal structure of MDS and AML in response to epigenetic therapy. Leukemia 2017, 31, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Aggarwal, R.; Jahan, T.; Ryan, C.; Troung, T.; Cripps, A.M.; Raha, P.; Thurn, K.T.; Chen, S.; Grabowsky, J.A.; et al. A phase I trial of panobinostat and epirubicin in solid tumors with a dose expansion in patients with sarcoma. Ann. Oncol. 2016, 27, 947–952. [Google Scholar] [CrossRef] [PubMed]

| Identifier | Phase | Type of Cancer | Senescence Inducer (First Punch) | Senolytics (Second Punch) | Status |
|---|---|---|---|---|---|
| NCT06355037 | 2 | Triple negative breast cancer | Taxane, Anthracycline, Eribulin, Mesylate, Vinorelbine, Capecitabine, Carboplatin, UTD1, Platinum | Dasatinib and Quercetin | Recruiting |
| NCT05724329 | 2 | Head and neck squamous carcinomas | - | Dasatinib and Quercetin, Immune checkpoint inhibitor (Tislelizumab) | Active |
| NCT06940297 | 2 | Elapsed or refractory multiple myeloma | Cyclophosphamide, Fludarabine | Dasatinib and Quercetin, CAR-T therapy | Not yet recruiting |
| Identifier | Phase | Type of Cancer | Senescence Inducer (First Punch) | Senolytics (Second Punch) | Status | Reference | Adverse Events | Antitumor Effect |
|---|---|---|---|---|---|---|---|---|
| NCT05455294 | 1 | Acute myeloid leukemia, myeloid malignancy, myeloproliferative neoplasm | Decitabine | ABT-263 | Active | |||
| NCT05222984 | 1 | Recurrent, refractory acute myeloid leukemia | Decitabine | ABT-263 | Active | |||
| NCT05192889 | 1, 2 | Refractory, relapsed acute lymphoblastic leukemia | Vincristine, Calaspargase Pegol, Cytarabine, Methotrexate, Mercaptopurine, Cyclophosphamide, Etoposide, Pegaspargase, Erwinia Asparaginase | ABT-263 | Active | |||
| NCT03181126 | 1 | Acute lymphoblastic leukemia (ALL), lymphoblastic lymphoma | Vincristine, Pegaspargase | ABT-263 | Completed | |||
| NCT02143401 | 1 | metastatic, recurrent malignant solid neoplasm, recurrent hepatocellular carcinoma, refractory malignant neoplasm, Stage IV hepatocellular carcinoma AJCC v7, unresectable solid neoplasm | Sorafenib | ABT-263 | Completed | |||
| NCT02079740 | 1, 2 | Metastatic, refractory, unresectable malignant solid neoplasm | Trametinib | ABT-263 | Active | |||
| NCT01989585 | 1, 2 | Clinical Stage III, IV cutaneous melanoma AJCC v8, malignant solid neoplasm, metastatic, unresectable melanoma | Dabrafenib, Trametinib | ABT-263 | Active | |||
| NCT01009073 | 1 | Solid tumors | Erlotinib, Irinotecan | ABT-263 | Completed | [121] | Diarrhea, Nausea, Vomiting, Decreased appetite | 27% of disease control rate |
| NCT00891605 | 1 | Solid tumors | Paclitaxel | ABT-263 | Completed | [122] | Alopecia, Anemia, Nausea, Constipation, Diarrhea, Fatigue, Neutropenia, Thrombocytopenia, Vomiting, Decreased appetite, Dehydration, Hypomagnesaemia | Modest antitumor activity |
| NCT00887757 | 1 | Solid tumors | Gemcitabine | ABT-263 | Completed | [123] | Hematologic abnormalities (thrombocytopenia, neutropenia, and anemia), Liver enzyme elevations (ALT and AST), Gastrointestinal disturbances (diarrhea, nausea, and vomiting) | 54% of stable disease |
| NCT00888108 | 1 | Solid tumors | Docetaxel | ABT-263 | Completed | [124] | Thrombocytopenia, Fatigue, Nausea, Neutropenia | 10% of partial responses |
| NCT00878449 | 1 | Solid tumors | Etoposide, Cisplatin | ABT-263 | Completed | [125] | ||
| NCT00868413 | 1 | Chronic lymphocytic leukemia | Fludarabine/Cyclophosphamide/Rituximab, Bendamustine/Rituximab | ABT-263 | Completed |
| Identifier | Phase | Type of Cancer | Senescence Inducer (First Punch) | Senolytics (Second Punch) | Status | Reference | Adverse Events | Antitumor Effect |
|---|---|---|---|---|---|---|---|---|
| NCT01321346 | 1 | Childhood lymphoblastic/myelogenous leukemia, Hodgkin’s disease, Non-Hodgkin’s disease | Cytarabine | Panobinostat | Completed | [126] | Gastrointestinal effects | No response |
| NCT00743288 | 1, 2 | Multiple myeloma | Melphalan | Panobinostat | Completed | [127] | Neutropenia, Thrombocytopenia | 7.5% of partial response |
| NCT01005797 | 1 | Renal cancer, Non-small cell lung cancer, Soft tissue sarcoma | Sorafenib | Panobinostat | Completed | |||
| NCT01336842 | 1 | Solid tumors, Non-small cell lung cancer | Cisplatin, Pemetrexed | Panobinostat | Completed | |||
| NCT01463046 | 1 | Acute myeloid leukemia, Advanced myelodysplastic syndrome | Cytarabine, Daunorubicin | Panobinostat | Completed | |||
| NCT00859222 | 1, 2 | Malignant glioma | Bevacizumab | Panobinostat | Completed | [128,129] | Thrombocytopenia, Hypophosphatemia, Esophageal hemorrhage, Deep venous thrombosis | 25% of partial response, 58% of stable disease (Phase1); No significant improvement of 6-month progression-free survival compared with bevacizumab monotherapy (Phase2) |
| NCT00738751 | 1 | Lung cancer, Head and neck cancer | Erlotinib | Panobinostat | Completed | |||
| NCT00632489 | 1 | Breast cancer | Capecitabine, Lapatinib | Panobinostat | Completed | |||
| NCT00788931 | 1 | HER-2 positive breast cancer, Metastatic breast cancer | Paclitaxel | Panobinostat | Completed | |||
| NCT00946647 | 1, 2 | Myelodysplastic syndromes, Chronic myelomonocytic leukemia, Acute myeloid leukemia | 5-Azacytidine | Panobinostat | Completed | [130] | Nausea, Diarrhea, Fatigue, Thrombocytopenia, Vomiting, Constipation | 28% of composite complete response |
| NCT00691938 | 1, 2 | Acute myeloid leukemia, Myelodysplastic syndromes | Decitabine | Panobinostat | Completed | [131] | Fatigue, Febrile neutropenia, Diarrhea, Nausea | 5% of complete response/cytogenic complete response |
| NCT01169636 | 1, 2 | Hodgkin’s lymphoma | Ifosfamide, Carboplatin, Etoposide | Panobinostat | Completed | |||
| NCT01055795 | 1 | Advanced solid tumors | Bevacizumab | Panobinostat | Completed | |||
| NCT00556088 | 1 | Solid tumors | Paclitaxel, Carboplatin, Bevacizumab | Panobinostat | Completed | |||
| NCT00878904 | 1 | Unspecified adult solid tumor | Epirubicin hydrochloride | Panobinostat | Completed | [132] | Thrombocytopenia, Febrile neutropenia, Fatigue | 11% of response |
| NCT02506959 | 2 | Plasma cell leukemia, Plasmacytoma, Recurrent plasma cell myeloma, Refractory plasma cell myeloma | Busulfan, Gemcitabine hydrochloride, Melphalan | Panobinostat | Completed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meguro, S.; Makabe, S.; Yaginuma, K.; Onagi, A.; Tanji, R.; Matsuoka, K.; Hoshi, S.; Koguchi, T.; Kayama, E.; Hata, J.; et al. Targeting Senescence in Oncology: An Emerging Therapeutic Avenue for Cancer. Curr. Oncol. 2025, 32, 467. https://doi.org/10.3390/curroncol32080467
Meguro S, Makabe S, Yaginuma K, Onagi A, Tanji R, Matsuoka K, Hoshi S, Koguchi T, Kayama E, Hata J, et al. Targeting Senescence in Oncology: An Emerging Therapeutic Avenue for Cancer. Current Oncology. 2025; 32(8):467. https://doi.org/10.3390/curroncol32080467
Chicago/Turabian StyleMeguro, Satoru, Syunta Makabe, Kei Yaginuma, Akifumi Onagi, Ryo Tanji, Kanako Matsuoka, Seiji Hoshi, Tomoyuki Koguchi, Emina Kayama, Junya Hata, and et al. 2025. "Targeting Senescence in Oncology: An Emerging Therapeutic Avenue for Cancer" Current Oncology 32, no. 8: 467. https://doi.org/10.3390/curroncol32080467
APA StyleMeguro, S., Makabe, S., Yaginuma, K., Onagi, A., Tanji, R., Matsuoka, K., Hoshi, S., Koguchi, T., Kayama, E., Hata, J., Sato, Y., Akaihata, H., Kataoka, M., Ogawa, S., Uemura, M., & Kojima, Y. (2025). Targeting Senescence in Oncology: An Emerging Therapeutic Avenue for Cancer. Current Oncology, 32(8), 467. https://doi.org/10.3390/curroncol32080467





