Perspectives on Outpatient Delivery of Bispecific T-Cell Engager Therapies for Multiple Myeloma
Abstract
1. Introduction
2. Efficacity and Safety of Bispecific T-Cell Engager Therapies in Multiple Myeloma
2.1. Teclistamab
2.2. Elranatamab
2.3. Talquetamab
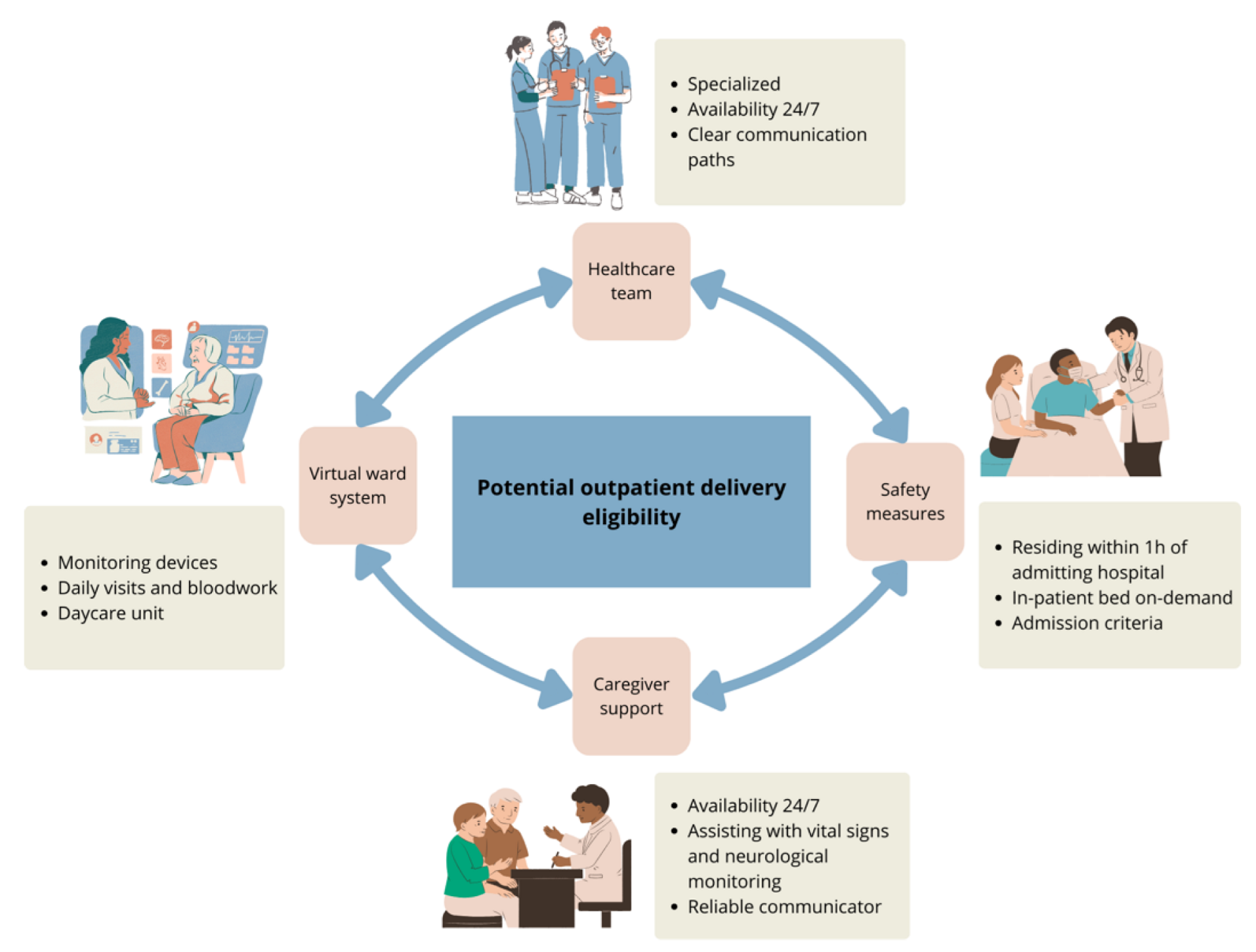
3. Administration of T-Cell Engagers
3.1. Outpatient Bispecific T-Cell Engager Administration
3.2. Mitigation of Cytokine Release Syndrome Risk
4. Toxicity Management
4.1. Cytokine Release Syndrome
4.2. Immune Effector Cell-Associated Neurotoxicity Syndrome
4.3. Infections
4.4. Cytopenias
4.5. Other Complications
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASTCT | American Society for Transplantation and Cellular Therapy |
| CMV | Cytomegalovirus |
| CR | Complete response |
| DoR | Duration of response |
| EBV | Epstein–Barr virus |
| EEG | Electroencephalogram |
| EMA | European Medicines Agency |
| FDA | US Food and Drug Administration |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| HHV6 | Human herpes virus 6 |
| HSV | Herpes simplex virus |
| ICANS | Immune effector cell-associated neurotoxicity syndrome |
| ICE | Immune effector cell-associated encephalopathy |
| IMiD | Immunomodulator |
| IVIG | Intravenous immunoglobulins |
| MM | Multiple myeloma |
| MoAb | Monoclonal antibody |
| MRD | Minimal residual disease |
| ORR | Overall response rate |
| OS | Overall survival |
| PI | Proteasome inhibitor |
| PJP | Pneumocystis jirovecii |
| PR | Partial response |
| RRMM | Relapsed or refractory multiple myeloma |
| TCE | T-cell engager |
| VZV | Herpes zoster virus |
Appendix A
| Eligibility Criteria for the Virtual Ward | ||
|---|---|---|
| Potential User Criteria | ||
| YES | NO | |
| 1. Subjects starting a treatment with a T-cell redirected therapy | ◯ | ◯ |
| 2. Subjects residing or hosted in the territory of the delivering hospital and with the possibility of traveling to the designated site in less than an hour | ◯ | ◯ |
| 3. Presence of a caregiver 24/7 during the step-up dosing, including the stay in virtual care ward | ◯ | ◯ |
| 4. User is able to move independently by walking without support or with an assistive device | ◯ | ◯ |
| 5. User and/or the caregiver reads and understands language used for communication | ◯ | ◯ |
| 6. If needed, the caregiver is able to assist with communication between the user and the healthcare team | ◯ | ◯ |
| 7. User is able to follow and adhere to the healthcare team’s instructions, including self-administration of medications | ◯ | ◯ |
| 8. User and caregiver’s proficiency with devices enabling home hospitalization (including cellphones, tablets, and punctual monitoring device) | ◯ | ◯ |
| 9. No anticipated infections or severe complications during the medical course or assessment | ◯ | ◯ |
| 10. No drug or alcohol abuse by the user and the caregiver | ◯ | ◯ |
| 11. User has given their consent | ◯ | ◯ |
| Criteria for Admission to Hospital From Virtual Ward | ||
|---|---|---|
| Criteria for Admission | ||
| YES | NO | |
| General condition deteriorating (clinical judgment) | ◯ | ◯ |
| New episode of fever | ◯ | ◯ |
| Hypotension or desaturation | ◯ | ◯ |
| Side effects of a medication requiring readmission | ◯ | ◯ |
| Neurotoxicity | ◯ | ◯ |
| Biological blood abnormalities | ◯ | ◯ |
| Transfusion needs, IV electrolytes, medical evaluation | ◯ | ◯ |
| Other: | ◯ | ◯ |
| Criteria for Virtual Ward Discharge | ||
|---|---|---|
| Criteria for Discharge | ||
| YES | NO | |
| 48 h post-administration of day 8 cycle 1 | ◯ | ◯ |
| Afebrile for at least 48 h | ◯ | ◯ |
| No hypotension or desaturation or confusion | ◯ | ◯ |
| No need for blood test within 72 h | ◯ | ◯ |
| No anticipated need for transfusion for 7 days | ◯ | ◯ |
Appendix B
| Orientation | 4 points |
| Knows the year | 1 point |
| Knows the month | 1 point |
| Knows the city | 1 point |
| Knows the name of the hospital | 1 point |
| Obeys a simple command | 1 point |
| Can write a legible sentence | 1 point |
| Can identify three objects | 3 points (1 point each) |
| Can count backwards from 100 by 10 s | 1 point |
References
- Ferlay, J.E.M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.who.int/today (accessed on 29 March 2025).
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019, 33, 2266–2275. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Weisel, K.; De Stefano, V.; Goldschmidt, H.; Delforge, M.; Mohty, M.; Cavo, M.; Vij, R.; Lindsey-Hill, J.; Dytfeld, D.; et al. LocoMMotion: A prospective, non-interventional, multinational study of real-life current standards of care in patients with relapsed and/or refractory multiple myeloma. Leukemia 2022, 36, 1371–1376. [Google Scholar] [CrossRef]
- Visram, A.; De La Torre, A.; White, D.; Kardjadj, M.; Masih-Khan, E.; Chu, M.P.; Jimenez-Zepeda, V.H.; McCurdy, A.; Leblanc, R.; Song, K.; et al. Real World Data on Outcomes of Anti-CD38 Antibody Refractory, Including Triple Class Refractory, Patients with Multiple Myeloma: A Multi-Institutional Report from the Canadian Myeloma Research Group (CMRG) Database. Blood 2022, 140, 4287–4289. [Google Scholar] [CrossRef]
- Hosny, M.; Verkleij, C.P.M.; Van Der Schans, J.; Frerichs, K.A.; Mutis, T.; Zweegman, S.; Van De Donk, N.W.C.J. Current State of the Art and Prospects of T Cell-Redirecting Bispecific Antibodies in Multiple Myeloma. J. Clin. Med. 2021, 10, 4593. [Google Scholar] [CrossRef]
- Cho, S.-F.; Yeh, T.-J.; Anderson, K.C.; Tai, Y.-T. Bispecific antibodies in multiple myeloma treatment: A journey in progress. Front. Oncol. 2022, 12, 1032775. [Google Scholar] [CrossRef] [PubMed]
- Caraccio, C.; Krishna, S.; Phillips, D.J.; Schürch, C.M. Bispecific Antibodies for Multiple Myeloma: A Review of Targets, Drugs, Clinical Trials, and Future Directions. Front. Immunol. 2020, 11, 501. [Google Scholar] [CrossRef]
- Lancman, G.; Parsa, K.; Kotlarz, K.; Avery, L.; Lurie, A.; Lieberman-Cribbin, A.; Cho, H.J.; Parekh, S.S.; Richard, S.; Richter, J.; et al. IVIg Use Associated with Ten-Fold Reduction of Serious Infections in Multiple Myeloma Patients Treated with Anti-BCMA Bispecific Antibodies. Blood Cancer Discov. 2023, 4, 440–451. [Google Scholar] [CrossRef]
- Lee, L.; Bounds, D.; Paterson, J.; Herledan, G.; Sully, K.; Seestaller-Wehr, L.M.; Fieles, W.E.; Tunstead, J.; McCahon, L.; Germaschewski, F.M.; et al. Evaluation of B cell maturation antigen as a target for antibody drug conjugate mediated cytotoxicity in multiple myeloma. Br. J. Haematol. 2016, 174, 911–922. [Google Scholar] [CrossRef]
- Pillarisetti, K.; Edavettal, S.; Mendonça, M.; Li, Y.; Tornetta, M.; Babich, A.; Majewski, N.; Husovsky, M.; Reeves, D.; Walsh, E.; et al. A T-cell–redirecting bispecific G-protein–coupled receptor class 5 member D x CD3 antibody to treat multiple myeloma. Blood 2020, 135, 1232–1243. [Google Scholar] [CrossRef]
- Elkins, K.; Zheng, B.; Go, M.; Slaga, D.; Du, C.; Scales, S.J.; Yu, S.-F.; McBride, J.; De Tute, R.; Rawstron, A.; et al. FcRL5 as a Target of Antibody–Drug Conjugates for the Treatment of Multiple Myeloma. Mol. Cancer Ther. 2012, 11, 2222–2232. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.-R.; Young, K.H. New agents and regimens for diffuse large B cell lymphoma. J. Hematol. Oncol. 2020, 13, 175. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Garfall, A.L.; Van De Donk, N.W.C.J.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Tomasson, M.H.; Iida, S.; Niesvizky, R.; Mohty, M.; Bahlis, N.J.; Martinez-Lopez, J.; Koehne, G.; Rodriguez-Otero, P.; Miles Prince, H.; Viqueira, A.; et al. Long-term survival and safety of elranatamab in patients with relapsed or refractory multiple myeloma: Update from the MagnetisMM-3 study. HemaSphere 2024, 8, e136. [Google Scholar] [CrossRef]
- Bahlis, N.J.; Costello, C.L.; Raje, N.S.; Levy, M.Y.; Dholaria, B.; Solh, M.; Tomasson, M.H.; Damore, M.A.; Jiang, S.; Basu, C.; et al. Elranatamab in relapsed or refractory multiple myeloma: The MagnetisMM-1 phase 1 trial. Nat. Med. 2023, 29, 2570–2576. [Google Scholar] [CrossRef] [PubMed]
- Lesokhin, A.M.; Tomasson, M.H.; Arnulf, B.; Bahlis, N.J.; Miles Prince, H.; Niesvizky, R.; Rodrίguez-Otero, P.; Martinez-Lopez, J.; Koehne, G.; Touzeau, C.; et al. Elranatamab in relapsed or refractory multiple myeloma: Phase 2 MagnetisMM-3 trial results. Nat. Med. 2023, 29, 2259–2267. [Google Scholar] [CrossRef]
- Chari, A.; Minnema, M.C.; Berdeja, J.G.; Oriol, A.; Van De Donk, N.W.C.J.; Rodríguez-Otero, P.; Askari, E.; Mateos, M.-V.; Costa, L.J.; Caers, J.; et al. Talquetamab, a T-Cell–Redirecting GPRC5D Bispecific Antibody for Multiple Myeloma. N. Engl. J. Med. 2022, 387, 2232–2244. [Google Scholar] [CrossRef]
- Schinke, C.D.; Touzeau, C.; Minnema, M.C.; Van De Donk, N.W.C.J.; Rodríguez-Otero, P.; Mateos, M.-V.; Rasche, L.; Ye, J.C.; Vishwamitra, D.; Ma, X.; et al. Pivotal phase 2 MonumenTAL-1 results of talquetamab (tal), a GPRC5DxCD3 bispecific antibody (BsAb), for relapsed/refractory multiple myeloma (RRMM). J. Clin. Oncol. 2023, 41, 8036. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Karlin, L.; Benboubker, L.; Nahi, H.; San-Miguel, J.; Trancucci, D.; Qi, K.; Stephenson, T.; Perales-Puchalt, A.; Chastain, K.; et al. Durability of responses with biweekly dosing of teclistamab in patients with relapsed/refractory multiple myeloma achieving a clinical response in the majesTEC-1 study. J. Clin. Oncol. 2023, 41, 8034. [Google Scholar] [CrossRef]
- Sandahl, T.B.; Soefje, S.A.; Fonseca, R.; Ailawadhi, S.; Parrondo, R.; Lin, D.; Wu, B.; Calay, E.S.; Silvert, E.; Kim, N.; et al. Real-World Safety and Health Care Resource Utilization of Teclistamab Under an Outpatient Model for Step-Up Dosing Administration. JCO Oncol. Pract. 2024, OP-24-00489. [Google Scholar] [CrossRef]
- Korst, C.; Groen, K.; Bosman, P.W.C.; van der Valk, F.; Verkleij, C.P.M.; Kruyswijk, S.; de Ruijter, M.E.M.; Heijink, D.M.; Kuipers, M.T.; Zweegman, S.; et al. Prophylactic tocilizumab reduces the incidence of cytokine release syndrome in relapsed/refractory myeloma patients treated with teclistamab: Implications for outpatient step-up dosing. Hemasphere 2024, 8, e132. [Google Scholar] [CrossRef]
- Shimabukuro-Vornhagen, A.; Gödel, P.; Subklewe, M.; Stemmler, H.J.; Schlößer, H.A.; Schlaak, M.; Kochanek, M.; Böll, B.; Von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.G.; Mateos, M.V.; Nooka, A.; Banerjee, A.; Kobos, R.; Pei, L.; Qi, M.; Verona, R.; Doyle, M.; Smit, J.; et al. Detailed overview of incidence and management of cytokine release syndrome observed with teclistamab in the MajesTEC-1 study of patients with relapsed/refractory multiple myeloma. Cancer 2023, 129, 2035–2046. [Google Scholar] [CrossRef]
- Khanam, R.; Faiman, B.; Batool, S.; Najmuddin, M.M.; Usman, R.; Kuriakose, K.; Ahmed, A.; Rehman, M.E.U.; Roksana, Z.; Syed, Z.; et al. Management of Adverse Reactions for BCMA-Directed Therapy in Relapsed Multiple Myeloma: A Focused Review. J. Clin. Med. 2023, 12, 5539. [Google Scholar] [CrossRef]
- Rodriguez-Otero, P.; Usmani, S.; Cohen, A.D.; Van De Donk, N.W.C.J.; Leleu, X.; Pérez-Larraya, J.G.; Manier, S.; Nooka, A.K.; Mateos, M.V.; Einsele, H.; et al. International Myeloma Working Group immunotherapy committee consensus guidelines and recommendations for optimal use of T-cell-engaging bispecific antibodies in multiple myeloma. Lancet Oncol. 2024, 25, e205–e216. [Google Scholar] [CrossRef] [PubMed]
- Gazeau, N.; Liang, E.C.; Wu, Q.V.; Voutsinas, J.M.; Barba, P.; Iacoboni, G.; Kwon, M.; Ortega, J.L.R.; López-Corral, L.; Hernani, R.; et al. Anakinra for Refractory Cytokine Release Syndrome or Immune Effector Cell-Associated Neurotoxicity Syndrome after Chimeric Antigen Receptor T Cell Therapy. Transpl. Cell Ther. 2023, 29, 430–437. [Google Scholar] [CrossRef]
- Jatiani, S.S.; Aleman, A.; Madduri, D.; Chari, A.; Cho, H.J.; Richard, S.; Richter, J.; Brody, J.; Jagannath, S.; Parekh, S. Myeloma CAR-T CRS Management With IL-1R Antagonist Anakinra. Clin. Lymphoma Myeloma Leuk. 2020, 20, 632–636.e1. [Google Scholar] [CrossRef]
- Frerichs, K.A.; Verkleij, C.P.M.; Mateos, M.V.; Martin, T.G.; Rodriguez, C.; Nooka, A.; Banerjee, A.; Chastain, K.; Perales-Puchalt, A.; Stephenson, T.; et al. Teclistamab impairs humoral immunity in patients with heavily pretreated myeloma: Importance of immunoglobulin supplementation. Blood Adv. 2024, 8, 194–206. [Google Scholar] [CrossRef]
- Nooka, A.K.; Rodriguez, C.; Mateos, M.V.; Manier, S.; Chastain, K.; Banerjee, A.; Kobos, R.; Qi, K.; Verona, R.; Doyle, M.; et al. Incidence, timing, and management of infections in patients receiving teclistamab for the treatment of relapsed/refractory multiple myeloma in the MajesTEC-1 study. Cancer 2024, 130, 886–900. [Google Scholar] [CrossRef]
- Raje, N.; Anderson, K.; Einsele, H.; Efebera, Y.; Gay, F.; Hammond, S.P.; Lesokhin, A.M.; Lonial, S.; Ludwig, H.; Moreau, P.; et al. Monitoring, prophylaxis, and treatment of infections in patients with MM receiving bispecific antibody therapy: Consensus recommendations from an expert panel. Blood Cancer J. 2023, 13, 116. [Google Scholar] [CrossRef]
- Ludwig, H.; Terpos, E.; Van De Donk, N.; Mateos, M.-V.; Moreau, P.; Dimopoulos, M.-A.; Delforge, M.; Rodriguez-Otero, P.; San-Miguel, J.; Yong, K.; et al. Prevention and management of adverse events during treatment with bispecific antibodies and CAR T cells in multiple myeloma: A consensus report of the European Myeloma Network. Lancet Oncol. 2023, 24, e255–e269. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, M.; Bohlke, K.; Baptiste, D.M.; Dunleavy, K.; Fueger, A.; Jones, L.; Kelkar, A.H.; Law, L.Y.; Lefebvre, K.B.; Ljungman, P.; et al. Vaccination of Adults With Cancer: ASCO Guideline. J. Clin. Oncol. 2024, 42, 1699–1721. [Google Scholar] [CrossRef]
- Goldsmith, R.; Cornax, I.; Ma, J.Y.; Yao, X.; Peng, P.; Carreira, V. P-095: Normal human tissue expression of G-protein coupled receptor 5D (GPRC5D), a promising novel target for Multiple Myeloma, is restricted to plasma cells and hard keratinized tissues. Clin. Lymphoma Myeloma Leuk. 2021, 21, S91. [Google Scholar] [CrossRef]
- Lery, M.; Perrot, A.; Ortiz-Brugués, A.; Vigarios, E.; Anghel, D.; Bories, P.; Sibaud, V. Dermatological toxicities induced by T-cell-redirecting G protein-coupled receptor family C class 5 member D bispecific antibody talquetamab. J. Am. Acad. Dermatol. 2024, 90, 376–377. [Google Scholar] [CrossRef]
- Narayan, N.; Williams, B.; Lipe, B.; De Benedetto, A. Onychomadesis and palmoplantar keratoderma associated with talquetamab therapy for relapsed and refractory multiple myeloma. JAAD Case Rep. 2023, 31, 66–68. [Google Scholar] [CrossRef]
- Laheij, A.M.G.A.; Van De Donk, N.W.C.J. Characterization of dysgeusia and xerostomia in patients with multiple myeloma treated with the T-cell redirecting GPRC5D bispecific antibody talquetamab. Support. Care Cancer 2024, 32, 20. [Google Scholar] [CrossRef]
- Chari, A.; Oriol, A.; Krishnan, A.; Martinez Chamorro, M.D.C.; Costa, L.; Mateos, M.V.; Minnema, M.C.; Campagna, M.; Masterson, T.J.; Hilder, B.W.; et al. Efficacy and Safety of Less Frequent/Lower Intensity Dosing of Talquetamab in Patients with Relapsed/Refractory Multiple Myeloma: Results from the Phase 1/2 MonumenTAL-1 Study. Blood 2023, 142, 1010. [Google Scholar] [CrossRef]
| Cycle, Day | Teclistamab (mg/kg) | Elranatamab (mg) | Talquetamab (mg/kg) |
|---|---|---|---|
| C1D1 † | 0.06 | 12 | 0.01 |
| C1D3 † | 0.3 | 32 | 0.06 |
| C1D5 | 1.5 | - | 0.4 |
| C1D7 | - | 76 | - |
| Cycle 2+ | 1.5 q 1 week * | C2-C24: 76 q 1 weeks C25 et +: 76 q 2 weeks ** | 0.4 q 1 week OR D10 onwards: 0.8 q 2 weeks |
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| Criteria | Fever ≥ 38 °C WITHOUT hypotension AND hypoxia | Fever ≥ 38 °C WITH hypotension not requiring vasopressors AND/OR hypoxia requiring ≤6 L/min O2 | Fever ≥ 38 °C WITH hypotension requiring a vasopressor AND/OR hypoxia requiring >6 L/min O2 | Fever ≥ 38 °C WITH hypotension requiring multiple vasopressors AND/OR hypoxia requiring positive pressure (CPAP, BiPAP, or mechanical ventilation) |
| Investigations and non-pharmaceutical | Blood cultures, chest X-ray, and urinalysis If outpatient, consider patient admission | Consider intensive care unit transfer Blood cultures, chest X-ray, and urine analysis Close monitoring of ferritin, fibrinogen, and INR Vital signs q 2–4 h Continuous cardiac monitoring | Intensive care unit transfer Hemocultures, chest X-ray, and urine analysis Close monitoring of ferritin, fibrinogen, and INR Serial vital signs Continuous cardiac monitoring | Intensive care unit transfer Hemocultures, pulmonary X-ray, and urine analysis Close monitoring of ferritin, fibrinogen, and INR Serial vital signs Continuous cardiac monitoring |
| Treatment | Supportive care (acetaminophen, broad spectrum antibiotics if neutropenic, IV fluids) Consider one dose of Tocilizumab 8 mg/kg IV or dexamethasone 10 mg if grade 1 or if fever persists for >24–48 h | Supportive care (acetaminophen, antibiotics, IV fluids) Tocilizumab 8 mg/kg IV and repeat q 8 h if no improvement (max. three doses) If no improvement, consider adding dexamethasone 10 mg IV q 6 h and/or anakinra 100 mg SC/IV q 12 h | Supportive care (acetaminophen, antibiotics, IV fluids, vasopressor) Tocilizumab 8 mg/kg IV and repeat q 8 h if (max. three doses) AND dexamethasone 10 mg IV q 6 h with anakinra 100 mg SC/IV q 12 h | Supportive care (acetaminophen, antibiotics, IV fluids, vasopressors) Tocilizumab 8 mg/kg IV and repeat q 8 h if (max. three doses) AND methylprednisolone 1000 mg IV DIE with anakinra 100 mg SC/IV q 12 h |
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| Criteria | ICE 7–9 Spontaneous awakening | ICE 3–6 Awakening on verbal stimulation | ICE 0–2 OR any clinical seizures, focal or generalized, that resolve rapidly OR non-convulsive seizures on EEG that resolve with intervention OR focal/local edema on neuroimaging | Unconscious patient OR life-threatening prolonged seizures (>5 min) OR status epilepticus OR deep focal motor weakness OR diffuse cerebral edema on neuroimaging or clinical sign of elevated intracranial pressure |
| Investigations and non-pharmaceutical | ICE score and neuro signs q 4 h Evaluate and treat for other causes of AMS Delirium precautions | ICE score and neuro signs q 2–4 h Consider neurology consultation Evaluate and treat for other causes of AMS Delirium precautions Perform CT and consider MRI if not done in previous 24 h Consider EEG | ICE score and neuro signs q 2 h Intensive care unit transfer Evaluate and treat for other causes of AMS Delirium precautions Neurology consultation Perform CT and MRI imaging if not done in previous 24 h Consider EEG Consider lumbar puncture with pressure measurement | ICE score and neuro signs q 1 h Intensive care unit transfer Evaluate and treat for other causes of AMS Delirium precautions Neurology consultation Perform CT and MRI imaging if not done in previous 24 h Consider continuous EEG Consider lumbar puncture with pressure measurement |
| Treatment | Consider dexamethasone 10 mg IV × 1 Consider adding levetiracetam 500 mg PO BID for prophylaxis | Dexamethasone 10 mg IV q 6–12 h Levetiracetam 500 mg PO BID | Dexamethasone 10 mg IV q 6 h, if no improvement after 24 h, consider 20 mg IV q 6 h or methylprednisolone 1000 mg/kg IV q 12–24 h with anakinra 100 mg SC/IV q 12 h Levetiracetam 500 mg PO BID | Dexamethasone 10 mg IV q 6 h, if no improvement, consider high dose methylprednisolone 1000–2000 mg/kg IV q 12–24 h with anakinra 100 mg SC/IV q 12 h Levetiracetam 500 mg PO BID |
| Indication | Agent | Duration | |
|---|---|---|---|
| Herpes simplex virus and varicella zoster virus | All patients | Valacyclovir 500 mg PO BID | Through treatment and until 6–12 months after the end |
| Pneumocystis jirovecii | All patients | Trimethoprim-sulfamethoxazole 800–160 mg one CO three times a week OR Atovaquone 1500 mg PO daily OR Pentamidine 300 mg inhaled q 4 weeks | Through treatment and until 6–12 months after the end |
| Bacterial | Optional, recommended if prolonged neutropenia, high infectious risk, or history of recurrent bacterial infections | Levofloxacin 500 mg PO daily OR Moxifloxacin 400 mg PO daily OR Doxycycline 200 mg PO daily | At least for the first 3 months of treatment and consider if persistent neutropenia or prolonged glucocorticoid use |
| Fungal | Consider in all patients Initiate if prolonged neutropenia | Fluconazole 400 mg PO daily | Until resolution of neutropenia |
| Immunoglobulins | Patients with IgG levels < 4 g/L | 400 mg/kg every 2–4 weeks | Through treatment |
| G-CSF | Patients with neutrophils < 1.0 × 109/L | Filgrastim 300–480 mcg SC daily or weekly | Target neutrophils > 1.0 × 109/L |
| Grade | Intervention | |
|---|---|---|
| Anemia | Grade 3 (hemoglobin < 80 g/L) or symptoms | Continue treatment Consider transfusion |
| Neutropenia | Grade 3 (ANC 0.5–1.0 × 109/L) without fever | Continue treatment Consider G-CSF use until ANC > 1.0 × 109/L |
| Grade 4 (ANC < 0.5 × 109/L) or febrile neutropenia | Hold treatment until ANC > 1.0 × 109/L Use G-CSF until ANC > 1.0 × 109/L Consider extending dosing interval if desired response is achieved and myeloma is in good control Consider prophylactic G-CSF when restarting medication | |
| Thrombocytopenia | Grade 4 (platelets < 25,000) | Hold treatment until platelets > 50,000 |
| Grade 3 (platelets 25,000–50,000) with bleeding |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelland, A.-A.; Dumas, M.; Lemieux-Blanchard, É.; LeBlanc, R.; Côté, J.; Boudreault, J.-S.; Duquette, D.; Kaedbey, R.; Lalancette, M.; Larose, F.; et al. Perspectives on Outpatient Delivery of Bispecific T-Cell Engager Therapies for Multiple Myeloma. Curr. Oncol. 2025, 32, 238. https://doi.org/10.3390/curroncol32040238
Pelland A-A, Dumas M, Lemieux-Blanchard É, LeBlanc R, Côté J, Boudreault J-S, Duquette D, Kaedbey R, Lalancette M, Larose F, et al. Perspectives on Outpatient Delivery of Bispecific T-Cell Engager Therapies for Multiple Myeloma. Current Oncology. 2025; 32(4):238. https://doi.org/10.3390/curroncol32040238
Chicago/Turabian StylePelland, Andrée-Anne, Mathilde Dumas, Émilie Lemieux-Blanchard, Richard LeBlanc, Julie Côté, Jean-Samuel Boudreault, Dominic Duquette, Rayan Kaedbey, Marc Lalancette, Frédéric Larose, and et al. 2025. "Perspectives on Outpatient Delivery of Bispecific T-Cell Engager Therapies for Multiple Myeloma" Current Oncology 32, no. 4: 238. https://doi.org/10.3390/curroncol32040238
APA StylePelland, A.-A., Dumas, M., Lemieux-Blanchard, É., LeBlanc, R., Côté, J., Boudreault, J.-S., Duquette, D., Kaedbey, R., Lalancette, M., Larose, F., Nikonova, A., Pavic, M., Shamy, A., Roy, J., Sebag, M., Trudel, S., & Claveau, J.-S. (2025). Perspectives on Outpatient Delivery of Bispecific T-Cell Engager Therapies for Multiple Myeloma. Current Oncology, 32(4), 238. https://doi.org/10.3390/curroncol32040238






