Crizotinib Inhibits Viability, Migration, and Invasion by Suppressing the c-Met/PI3K/Akt Pathway in the Three-Dimensional Bladder Cancer Spheroid Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Reagents
2.2. Spheroid Formation
2.3. Cell Counting Kit (CCK)-8 Assay
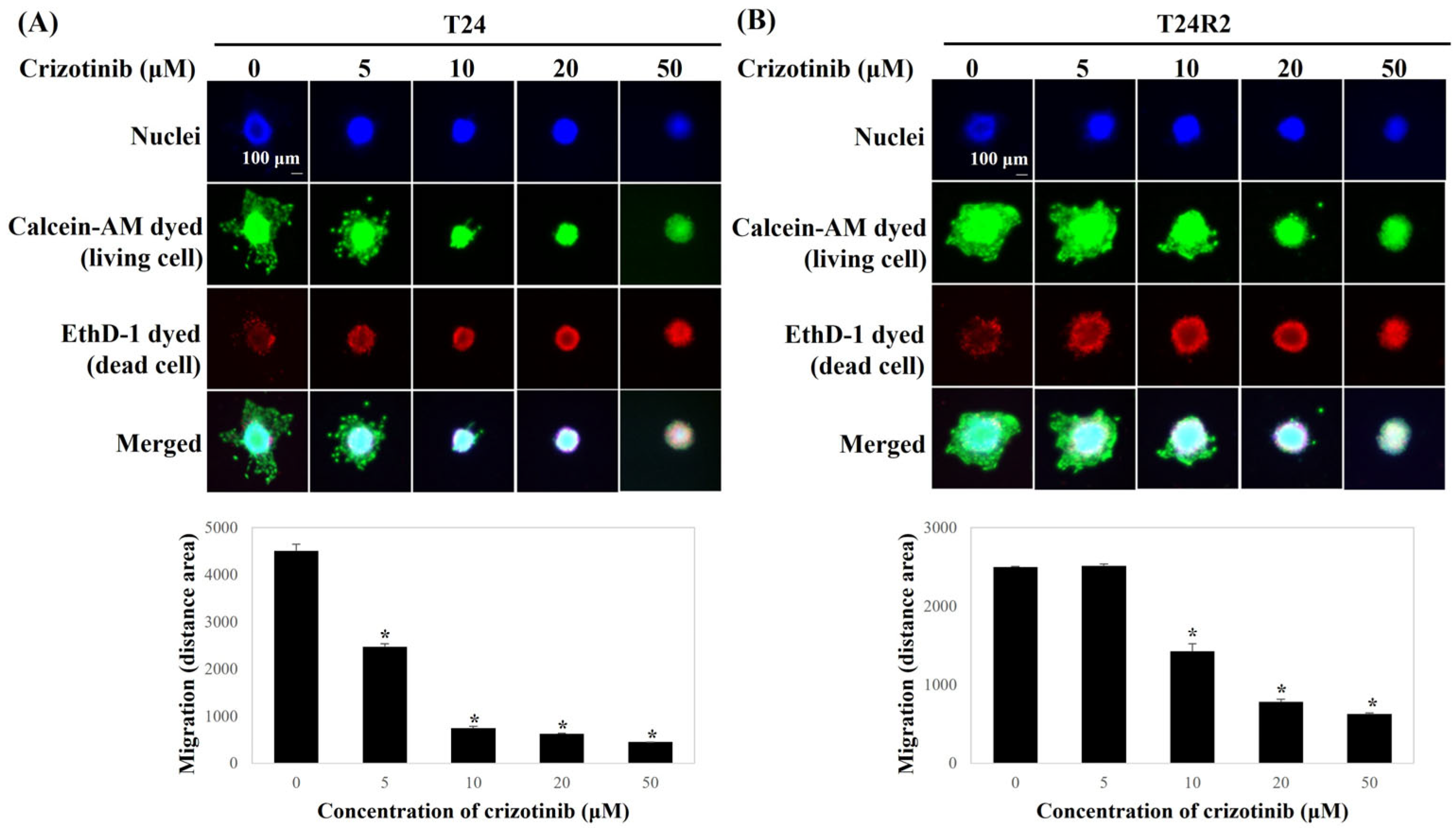
2.4. Live/Dead Staining
2.5. Matrigel Migration Assay
2.6. Collagen Invasion Assay
2.7. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Crizotinib Inhibits the Cell Viability of BC Cells and Spheroids
3.2. Crizotinib Inhibits the Migration of BC Spheroids
3.3. Crizotinib Inhibits the Invasion of BC Spheroids
3.4. Crizotinib Suppresses EMT- and Induces Apoptosis-Related Gene Expression of BC Spheroids
3.5. Crizotinib Inhibits the c-Met/PI3K/Akt Pathway in BC Spheroids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| 2D | Two-dimensional |
| c-Met | Mesenchymal–epithelial transition factor |
| BC | Bladder cancer |
| NMIBC | Non-muscle-invasive bladder cancer |
| MIBC | Muscle-invasive bladder cancer |
| ICI | Immune checkpoint inhibitor |
| FGFR | Fibroblast growth factor receptor |
| ALK | Anaplastic lymphoma kinase |
| HGF | Hepatocyte growth factor |
| FDA | Food and Drug Administration |
| CCK | Cell counting kit |
| qPCR | Quantitative real-time polymerase chain reaction |
| NSCLC | Non-small-cell lung cancer |
| FBS | Fetal bovine serum |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.M.; Hahn, N.M.; Efstathiou, J.A.; Lerner, S.P.; Malmström, P.U.; Choi, W.; Guo, C.C.; Lotan, Y.; Kassouf, W. Bladder cancer. Lancet 2016, 388, 2796–2810. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Capoune, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma In Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Buttigliero, C.; Tucci, M.; Vignani, F.; Scagliotti, G.V.; Di Maio, M. Molecular biomarkers to predict response to neoadjuvant chemotherapy for bladder cancer. Cancer Treat. Rev. 2017, 54, 1–9. [Google Scholar] [CrossRef]
- Li, F.; Zheng, Z.; Chen, W.; Li, D.; Zhang, H.; Zhu, Y.; Mo, Q.; Zhao, X.; Fan, Q.; Deng, F.; et al. Regulation of cisplatin resistance in bladder cancer by epigenetic mechanisms. Drug Resist. Updat. 2023, 68, 100938. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, H.W.; Ha, H.K.; Seo, H.K. Perioperative systemic therapy in muscle invasive bladder cancer: Current standard method, biomarkers and emerging strategies. Investig. Clin. Urol. 2023, 64, 202–218. [Google Scholar] [CrossRef]
- Patel, V.G.; Oh, W.K.; Galsky, M.D. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J. Clin. 2020, 70, 404–423. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Lin, T. Emerging strategies for the improvement of chemotherapy in bladder cancer: Current knowledge and future perspectives. J. Adv. Res. 2022, 39, 187–202. [Google Scholar] [CrossRef]
- Motterle, G.; Andrews, J.R.; Morlacco, A.; Karnes, R.J. Predicting Response to Neoadjuvant Chemotherapy in Bladder Cancer. Eur. Urol. Focus. 2020, 6, 642–649. [Google Scholar] [CrossRef]
- Casanova, A.G.; Hernández-Sánchez, M.T.; López-Hernández, F.J.; Martínez-Salgado, C.; Prieto, M.; Vicente-Vicente, L.; Morales, A.I. Systematic review and meta-analysis of the efficacy of clinically tested protectants of cisplatin nephrotoxicity. Eur. J. Clin. Pharmacol. 2020, 76, 23–33. [Google Scholar] [CrossRef]
- Godwin, J.L.; Hoffman-Censits, J.; Plimack, E. Recent developments in the treatment of advanced bladder cancer. Urol. Oncol. 2018, 36, 109–114. [Google Scholar] [CrossRef] [PubMed]
- McHugh, L.A.; Kriajevska, M.; Mellon, J.K.; Griffiths, T.R. Combined treatment of bladder cancer cell lines with lapatinib and varying chemotherapy regimens—Evidence of schedule-dependent synergy. Urology 2007, 69, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Lobo, N.; Afferi, L.; Moschini, M.; Mostafid, H.; Porten, S.; Psutka, S.P.; Gupta, S.; Smith, A.B.; Williams, S.B.; Lotan, Y. Epidemiology, Screening, and Prevention of Bladder Cancer. Eur. Urol. Oncol. 2022, 5, 628–639. [Google Scholar] [CrossRef]
- Peng, M.; Xiao, D.; Bu, Y.; Long, J.; Yang, X.; Lv, S.; Yang, X. Novel Combination Therapies for the Treatment of Bladder Cancer. Front. Oncol. 2021, 10, 539527. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- D’Angelo, A.; Chapman, R.; Sirico, M.; Sobhani, N.; Catalano, M.; Mini, E.; Roviello, G. An update on antibody-drug conjugates in urothelial carcinoma: State of the art strategies and what comes next. Cancer Chemother. Pharmacol. 2022, 90, 191–205. [Google Scholar] [CrossRef]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Vera-Badillo, F.E.; Tannock, I.F.; Booth, C.M. Immunotherapy for Urothelial Cancer: Where Are the Randomized Trials? J. Clin. Oncol. 2019, 37, 2587–2591. [Google Scholar] [CrossRef]
- Nakamura, K.; Smyth, M.J. Targeting cancer-related inflammation in the era of immunotherapy. Immunol. Cell Biol. 2017, 95, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Z.; Xu, X. c-Met: A Promising Therapeutic Target in Bladder Cancer. Cancer Manag. Res. 2022, 14, 2379–2388. [Google Scholar] [CrossRef]
- Wood, G.E.; Hockings, H.; Hilton, D.M.; Kermorgant, S. The role of MET in chemotherapy resistance. Oncogene 2021, 40, 1927–1941. [Google Scholar] [CrossRef]
- Kitowska, K.; Gorska-Arcisz, M.; Antoun, D.; Zarczynska, I.; Czaplinska, D.; Szczepaniak, A.; Skladanowski, A.C.; Wieczorek, M.; Stanczak, A.; Skupinska, M.; et al. MET-Pyk2 Axis Mediates Acquired Resistance to FGFR Inhibition in Cancer Cells. Front. Oncol. 2021, 11, 633410. [Google Scholar] [CrossRef]
- Loong, H.H.; Mok, K.; Leung, L.K.; Mok, T.S. Crizotinib in the management of advanced-stage non-small-cell lung cancer. Future Oncol. 2015, 11, 735–745. [Google Scholar] [CrossRef]
- Gambacorti Passerini, C.; Farina, F.; Stasia, A.; Redaelli, S.; Ceccon, M.; Mologni, L.; Messa, C.; Guerra, L.; Giudici, G.; Sala, E.; et al. Crizotinib in advanced, chemoresistant anaplastic lymphoma kinase-positive lymphoma patients. J. Natl. Cancer Inst. 2014, 106, djt378. [Google Scholar] [CrossRef]
- Hou, G.X.; Song, B.B. Gastric cancer patient with c-MET amplification treated with crizotinib after failed multi-line treatment: A case report and literature review. Math. Biosci. Eng. 2019, 16, 5923–5930. [Google Scholar] [CrossRef]
- Varma, D.A.; Tiwari, M. Crizotinib-induced anti-cancer activity in human cervical carcinoma cells via ROS-dependent mitochondrial depolarization and induction of apoptotic pathway. J. Obstet. Gynaecol. Res. 2021, 47, 3923–3930. [Google Scholar] [CrossRef]
- Chen, W.; Li, W.; Bai, B.; Wei, H. Identification of anaplastic lymphoma kinase fusions in clear cell renal cell carcinoma. Oncol. Rep. 2020, 43, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Supko, J.G.; Gray, K.P.; Melnick, Z.J.; Regan, M.M.; Taplin, M.E.; Choudhury, A.D.; Pomerantz, M.M.; Bellmunt, J.; Yu, C.; et al. Dual Blockade of c-MET and the Androgen Receptor in Metastatic Castration-resistant Prostate Cancer: A Phase I Study of Concurrent Enzalutamide and Crizotinib. Clin. Cancer Res. 2020, 26, 6122–6131. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Apolo, A.B.; Agarwal, P.K.; Bottaro, D.P. Characterization of HGF/Met Signaling in Cell Lines Derived from Urothelial Carcinoma of the Bladder. Cancers 2014, 6, 2313–2329. [Google Scholar] [CrossRef] [PubMed]
- Farouk, S.M.; Khafaga, A.F.; Abdellatif, A.M. Bladder cancer: Therapeutic challenges and role of 3D cell culture systems in the screening of novel cancer therapeutics. Cancer Cell Int. 2023, 23, 251. [Google Scholar] [CrossRef]
- Byun, S.S.; Kim, S.W.; Choi, H.; Lee, C.; Lee, E. Augmentation of cisplatin sensitivity in cisplatin-resistant human bladder cancer cells by modulating glutathione concentrations and glutathione-related enzyme activities. BJU Int. 2005, 95, 1086–1090. [Google Scholar] [CrossRef]
- Ho, J.N.; Jeon, J.S.; Kim, D.H.; Ryu, H.; Lee, S. CUDC-907 suppresses epithelial-mesenchymal transition, migration and invasion in a 3D spheroid model of bladder cancer. Oncol. Rep. 2023, 49, 130. [Google Scholar] [CrossRef]
- Bellmunt, J.; Selvarajah, S.; Rodig, S.; Salido, M.; de Muga, S.; Costa, I.; Bellosillo, B.; Werner, L.; Mullane, S.; Fay, A.P.; et al. Identification of ALK gene alterations in urothelial carcinoma. PLoS ONE 2014, 9, e103325. [Google Scholar] [CrossRef]
- Giroux-Leprieur, E.; Fallet, V.; Cadranel, J.; Wislez, M. Spotlight on crizotinib in the first-line treatment of ALK-positive advanced non-small-cell lung cancer: Patients selection and perspectives. Lung Cancer 2016, 7, 83–90. [Google Scholar]
- Amaral, R.L.F.; Miranda, M.; Marcato, P.D.; Swiech, K. Comparative Analysis of 3D Bladder Tumor Spheroids Obtained by Forced Floating and Hanging Drop Methods for Drug Screening. Front. Physiol. 2017, 8, 605. [Google Scholar] [CrossRef]
- Nunes, A.S.; Costa, E.C.; Barros, A.S.; de Melo-Diogo, D.; Correia, I.J. Establishment of 2D Cell Cultures Derived From 3D MCF-7 Spheroids Displaying a Doxorubicin Resistant Profile. Biotechnol. J. 2019, 14, e1800268. [Google Scholar] [CrossRef]
- Muguruma, M.; Teraoka, S.; Miyahara, K.; Ueda, A.; Asaoka, M.; Okazaki, M.; Kawate, T.; Kuroda, M.; Miyagi, Y.; Ishikawa, T. Differences in drug sensitivity between two-dimensional and three-dimensional culture systems in triple-negative breast cancer cell lines. Biochem. Biophys. Res. Commun. 2020, 533, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Balmaña, M.; Diniz, F.; Feijão, T.; Barrias, C.C.; Mereiter, S.; Reis, C.A. Analysis of the Effect of Increased α2,3-Sialylation on RTK Activation in MKN45 Gastric Cancer Spheroids Treated with Crizotinib. Int. J. Mol. Sci. 2020, 21, 722. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, R.D.; Choi, W.; Hong, D.S.; McConkey, D.J. Autophagy is required for crizotinib-induced apoptosis in MET-amplified gastric cancer cells. Oncotarget 2017, 8, 51675–51687. [Google Scholar] [CrossRef] [PubMed]
- Berrouet, C.; Dorilas, N.; Rejniak, K.A.; Tuncer, N. Comparison of Drug Inhibitory Effects (IC50) in Monolayer and Spheroid Cultures. Bull. Math. Biol. 2020, 82, 68. [Google Scholar] [CrossRef]
- Singh, R.; Ansari, J.A.; Maurya, N.; Mandhani, A.; Agrawal, V.; Garg, M. Epithelial-To-Mesenchymal Transition and Its Correlation with Clinicopathologic Features in Patients with Urothelial Carcinoma of the Bladder. Clin. Genitourin. Cancer 2017, 15, e187–e197. [Google Scholar] [CrossRef]
- Linehan, W.M.; Walther, M.M.; Zbar, B. The genetic basis of cancer of the kidney. J. Urol. 2003, 170, 2163–2172. [Google Scholar] [CrossRef]
- Oltvai, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Debatin, K.M. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol. Immunother. 2004, 53, 153–159. [Google Scholar] [CrossRef]
- MacKenzie, S.H.; Clark, A.C. Targeting cell death in tumors by activating caspases. Curr. Cancer Drug Targets 2008, 8, 98–109. [Google Scholar]
- Organ, S.L.; Tsao, M.S. An overview of the c-MET signaling pathway. Ther. Adv. Med. Oncol. 2011, 3, S7–S19. [Google Scholar] [CrossRef]
- Sathe, A.; Nawroth, R. Targeting the PI3K/AKT/mTOR Pathway in Bladder Cancer. Methods Mol. Biol. 2018, 1655, 335–350. [Google Scholar] [PubMed]
- Dong, Q.; Fu, L.; Zhao, Y.; Tan, S.; Wang, E. Derlin-1 overexpression confers poor prognosis in muscle invasive bladder cancer and contributes to chemoresistance and invasion through PI3K/AKT and ERK/MMP signaling. Oncotarget 2017, 8, 17059–17069. [Google Scholar] [CrossRef] [PubMed]
- Van Der Steen, N.; Deben, C.; Deschoolmeester, V.; Wouters, A.; Lardon, F.; Rolfo, C.; Germonpré, P.; Giovannetti, E.; Peters, G.J.; Pauwels, P. Better to be alone than in bad company: The antagonistic effect of cisplatin and crizotinib combination therapy in non-small cell lung cancer. World J. Clin. Oncol. 2016, 7, 425–432. [Google Scholar] [CrossRef] [PubMed]





| Gene | Primer 5′→3′ |
|---|---|
| ALK (F) | AAA GAA ACC CAC AGC TGC AG |
| ALK (R) | TAA ACC AGG AGC CGT ACG TT |
| c-Met (F) | ATA CGG TCC TAT GGC TGG TG |
| c-Met (R) | TTG AAA TGG TTT GGG CTG GG |
| E-cadherin (F) | CAG CAC GTA CAC AGC CCT AA |
| E-cadherin (R) | ACC TGA GGC TTT GGA TTC CT |
| Vimentin (F) | GTT TCC AAG CCT GAC CTC AC |
| Vimentin (R) | GCT TCA ACG GCA AAG TTC TC |
| Bax (F) | AGA CAG GGG CCT TTT TGC TA |
| Bax (R) | AAT TCG CCG GAG ACA CTC G |
| Bcl-2 (F) | CTT TGA GTT CGG TGG GGT CA |
| Bcl-2 (R) | AGT TCC ACA AAG GCA TCC CA |
| Caspase-3 (F) | AAG ATA CCG GTG GAG GCT GA |
| Caspase-3 (R) | AAG GGA CTG GAT GAA CCA CG |
| Caspase-8 (F) | TCT GGA GCA TCT GCT GTC TG |
| Caspase-8 (R) | CCT GCC TGG TGT CTG AAG TT |
| Caspase-9 (F) | GGC TGT CTA CGG CAC AGA TGG A |
| Caspase-9 (R) | CTG GCT CGG GGT TAC TGC CAG |
| GAPDH (F) | TGC ACC ACC AAC TGC TTA G |
| GAPDH (R) | AGA GGC AGG GAT GAT GTT C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, B.; Kim, D.; Ho, J.-N.; Le, V.-H.; Lee, S. Crizotinib Inhibits Viability, Migration, and Invasion by Suppressing the c-Met/PI3K/Akt Pathway in the Three-Dimensional Bladder Cancer Spheroid Model. Curr. Oncol. 2025, 32, 236. https://doi.org/10.3390/curroncol32040236
Song B, Kim D, Ho J-N, Le V-H, Lee S. Crizotinib Inhibits Viability, Migration, and Invasion by Suppressing the c-Met/PI3K/Akt Pathway in the Three-Dimensional Bladder Cancer Spheroid Model. Current Oncology. 2025; 32(4):236. https://doi.org/10.3390/curroncol32040236
Chicago/Turabian StyleSong, Byeongdo, Danhyo Kim, Jin-Nyoung Ho, Van-Hung Le, and Sangchul Lee. 2025. "Crizotinib Inhibits Viability, Migration, and Invasion by Suppressing the c-Met/PI3K/Akt Pathway in the Three-Dimensional Bladder Cancer Spheroid Model" Current Oncology 32, no. 4: 236. https://doi.org/10.3390/curroncol32040236
APA StyleSong, B., Kim, D., Ho, J.-N., Le, V.-H., & Lee, S. (2025). Crizotinib Inhibits Viability, Migration, and Invasion by Suppressing the c-Met/PI3K/Akt Pathway in the Three-Dimensional Bladder Cancer Spheroid Model. Current Oncology, 32(4), 236. https://doi.org/10.3390/curroncol32040236




