Accelerating Oncology Drug Reimbursement in Canada: Impact of the CDA-AMC Time-Limited Recommendation and pCPA Temporary Access Process
Abstract
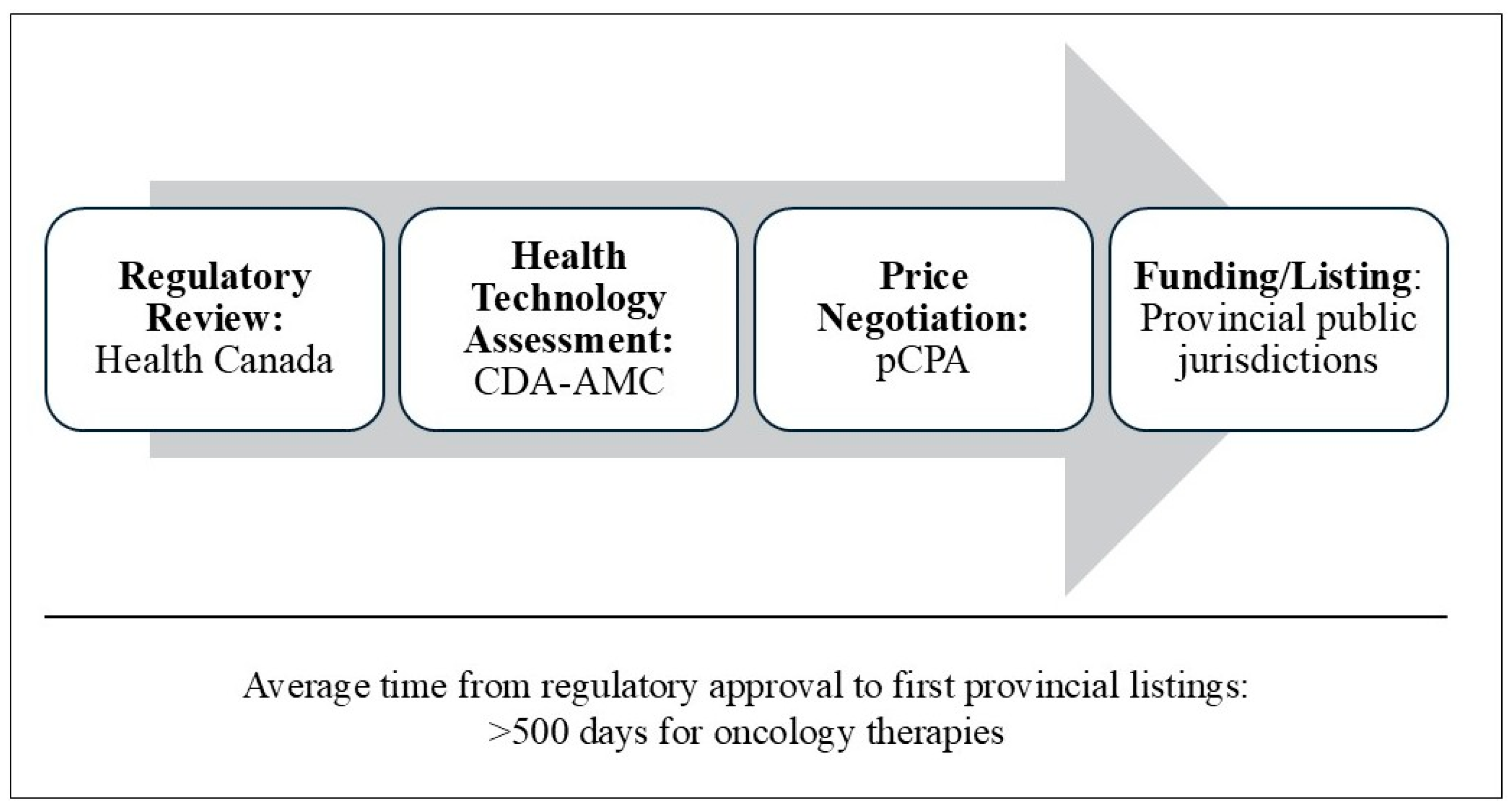
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NOC/c | Notice of Compliance with Conditions |
| CDA-AMC | Canada’s Drug Agency |
| TLR | Time-Limited Recommendation |
| pCPA | pan-Canadian Pharmaceutical Alliance |
| pTAP | pan-Canadian Pharmaceutical Alliance Temporary Access Process |
| QN | Qualifying Notice |
| LoU | Letter of undertaking |
| LoI | Letter of intent |
| SUR | Submission Under Review |
| SBD | Summary Basis of Decision |
| HTA | Health technology assessment |
| OECD | Organisation for Economic Co-operation and Development |
| DLBCL | Diffuse Large B Cell Lymphoma |
References
- Health Canada Guidance Document: Notice of Compliance with Conditions (NOC/c). Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/notice-compliance-conditions.html (accessed on 31 January 2025).
- Martin, A.; Hunt, M.; Blommaert, S.; Udayakumar, S.; Lu, B.; Chatterjee, S.; Sathiyabalan, G.; Brun, J.; Kampman, M.; Lambert, L.; et al. Oncology drug approvals under Health Canada’s Notice of Compliance with Conditions policy: A retrospective cohort analysis. Can. J. Health Technol. 2024, 4, 1–41. [Google Scholar] [CrossRef]
- Lau, C.Y.; Rawson, N.S.B. Is Canada Moving towards a More Agile Regulatory Approval and Reimbursement Process with a Shifting Role for Real-World Evidence (RWE) for Oncology Drugs? Curr. Oncol. 2024, 31, 5599–5607. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Dranitsaris, G. Impact of Regulatory Approval Status on CADTH Reimbursement of Oncology Drugs and Role of Real-World Evidence on Conditional Approvals from 2019 to 2021. Curr. Oncol. 2022, 29, 8031–8042. [Google Scholar] [CrossRef] [PubMed]
- Provincial and Territorial Public Drug Benefit Programs. Available online: https://www.canada.ca/en/health-canada/services/health-care-system/pharmaceuticals/access-insurance-coverage-prescription-medicines/provincial-territorial-public-drug-benefit-programs.html (accessed on 19 February 2025).
- Explaining Public Reimbursement Delays for New Medicines for Canadian Patients. Available online: https://innovativemedicines.ca/wp-content/uploads/2020/07/CADTH-TTL-8.5x11-EN-Final.pdf (accessed on 25 February 2025).
- CDA-AMC Time Limited Recommendations Consultation. Available online: https://www.cda-amc.ca/news/new-consultation-additional-improvements-drug-reimbursement-reviews (accessed on 31 January 2025).
- CDA-AMC Time Limited Recommendations Procedures. Available online: https://www.cda-amc.ca/news/our-time-limited-recommendation-category-aims-support-earlier-access-promising-drugs (accessed on 31 January 2025).
- pCPA Temporary Access Process (pTAP). Available online: https://www.pcpacanada.ca/ptap (accessed on 31 January 2025).
- Wills, A. Expanding the eligibility criteria for drugs in Canada’s time-limited health technology assessment and temporary drug access processes will further accelerate access to new medicines. J. Pharm. Pharm. Sci. 2024, 27, 13694. [Google Scholar] [CrossRef] [PubMed]
- Salek, S.; Hoskyn, S.L.; Johns, J.R.; Allen, N.; Sehgal, C. Factors Influencing Delays in Patient Access to New Medicines in Canada: A Retrospective Study of Reimbursement Processes in Public Drug Plans. Front Pharmacol. 2019, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- AbbVie’s EPKINLY™ Receives First-Ever Time-Limited Reimbursement Recommendation by Canada’s Drug Agency. Available online: https://finance.yahoo.com/news/abbvies-epkinly-receives-first-ever-110200465.html (accessed on 31 January 2025).
- Enhertu CDA-AMC TLR Review. Available online: https://www.cda-amc.ca/trastuzumab-deruxtecan-2 (accessed on 31 January 2025).
- Enhertu Accepted for pTAP Negotiation. Available online: https://www.pcpacanada.ca/negotiation/23037 (accessed on 31 January 2025).
- Health Canada Submission Under Review (SUR). Available online: https://www.canada.ca/en/health-canada/services/drug-health-product-review-approval/submissions-under-review.html (accessed on 31 January 2025).
- Health Canada Summary Basis of Decision. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/summary-basis-decision.html (accessed on 4 February 2025).
- Canada’s Drug Agency (CDA-AMC) Reimbursement Review Reports. Available online: https://www.cda-amc.ca/find-reports (accessed on 31 January 2025).
- Pan-Canadian Pharmaceutical Alliance. Available online: https://www.pcpacanada.ca/ (accessed on 31 January 2025).
- Sources Used for the First 4 Provincial Listing Dates Used in the Analysis. Available online: https://www.ramq.gouv.qc.ca/sites/default/files/documents/non_indexes/liste-med-etab-2024-08-14-fr.pdf (accessed on 2 February 2025).
- Sources Used for the First 4 Provincial Listing Dates. Available online: https://www.ramq.gouv.qc.ca/sites/default/files/documents/non_indexes/liste-med-etab-2024-12-12-fr.pdf (accessed on 2 February 2025).
- AbbVie Announces Ontario and Quebec are First Provinces to Reimburse Subcutaneous EPKINLY™ (Epcoritamab) for the Treatment of Diffuse Large B-Cell Lymphoma Under New Early Access Process. Available online: https://www.newswire.ca/news-releases/abbvie-announces-ontario-and-quebec-are-first-provinces-to-reimburse-subcutaneous-epkinly-tm-epcoritamab-for-the-treatment-of-diffuse-large-b-cell-lymphoma-under-new-early-access-process-820720865.html (accessed on 2 February 2025).
- pCPA Temporary Access Process (pTAP). Available online: https://www.pcpacanada.ca/sites/default/files/eng/2025/pCPA_pTAP_flowchart.pdf (accessed on 2 February 2025).
- Summary Basis of Decision for Carvykti. Available online: https://dhpp.hpfb-dgpsa.ca/review-documents/resource/SBD1684955300002 (accessed on 2 February 2025).
- Ciltacabtagene Autoleucel. Available online: https://www.cda-amc.ca/ciltacabtagene-autoleucel-0 (accessed on 2 February 2025).
- Carvykti (Ciltacabtagene Autoleucel). Available online: https://www.pcpacanada.ca/negotiation/22363 (accessed on 2 February 2025).
- Summary Basis of Decision for Columvi. Available online: https://dhpp.hpfb-dgpsa.ca/review-documents/resource/SBD1685634038615 (accessed on 2 February 2025).
- Glofitamab. Available online: https://www.cadth.ca/glofitamab (accessed on 2 February 2025).
- Columvi (Glofitamab). Available online: https://www.pcpacanada.ca/negotiation/22587 (accessed on 2 February 2025).
- Abiraterone Acetate, Niraparib. Available online: https://pdf.hres.ca/dpd_pm/00071216.PDF (accessed on 2 February 2025).
- Regulatory Decision Summary for Akeega, Abiraterone Acetate. Available online: https://dhpp.hpfb-dgpsa.ca/review-documents/resource/RDS1694526985734 (accessed on 2 February 2025).
- Niraparib Abiraterone Acetate. Available online: https://www.cda-amc.ca/niraparib-abiraterone-acetate (accessed on 2 February 2025).
- Summary Basis of Decision for Tecvayli. Available online: https://dhpp.hpfb-dgpsa.ca/review-documents/resource/SBD1695302358644 (accessed on 4 February 2025).
- Teclistama. Available online: https://www.cda-amc.ca/teclistamab (accessed on 4 February 2025).
- Tecvayli (Teclistama). Available online: https://www.pcpacanada.ca/negotiation/22654 (accessed on 4 February 2025).
- Summary Basis of Decision (SBD) for Epkinly. Available online: https://dhpp.hpfb-dgpsa.ca/review-documents/resource/SBD1709128914848#SummaryBasisDecision (accessed on 4 February 2025).
- Epcoritamab. Available online: https://www.cadth.ca/epcoritamab (accessed on 4 February 2025).
- Epkinly (Concluded with pTAP LOI) (Epcoritamab). Available online: https://www.pcpacanada.ca/negotiation/22708 (accessed on 4 February 2025).
- Summary Basis of Decision for Elrexfio. Available online: https://dhpp.hpfb-dgpsa.ca/review-documents/resource/SBD1722283649339 (accessed on 4 February 2025).
- Elranatamab. Available online: https://www.cda-amc.ca/elranatamab (accessed on 4 February 2025).
- Elrexfio (Elranatamab). Available online: https://www.pcpacanada.ca/negotiation/22724 (accessed on 4 February 2025).
- Summary Basis of Decision for Talvey. Available online: https://dhpp.hpfb-dgpsa.ca/review-documents/resource/SBD1732553847408 (accessed on 4 February 2025).
- Talquetamab. Available online: https://www.cda-amc.ca/talquetamab (accessed on 2 February 2025).
- Tarlatamab. Notice of Compliance Information. Available online: https://health-products.canada.ca/noc-ac/nocInfo?no=34010 (accessed on 2 February 2025).
- Regulatory Decision Summary for Imdelltra. Available online: https://dhpp.hpfb-dgpsa.ca/review-documents/resource/RDS1726763640809 (accessed on 2 February 2025).
- Tarlatamab. Available online: https://www.cda-amc.ca/tarlatamab (accessed on 2 February 2025).
- Olaparib. Available online: https://www.cda-amc.ca/olaparib-1 (accessed on 2 February 2025).
- Health Canada. Guidance Document NOCc. Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/dhp-mps/alt_formats/pdf/prodpharma/applic-demande/guide-ld/compli-conform/noccg_accd-eng.pdf (accessed on 6 February 2025).
- CAR T-Cell Therapy. Available online: https://my.clevelandclinic.org/health/treatments/17726-car-t-cell-therapy (accessed on 25 February 2025).
- CDA-AMC Drug Review Process. Available online: https://www.cda-amc.ca/sites/default/files/attachments/2022-02/Rapid%20Review%20Process_0.pdf (accessed on 8 February 2025).
- pCPA Guidance. Available online: https://www.pcpacanada.ca/sites/default/files/eng/pCPA_Brand_Process_Guidelines.pdf (accessed on 6 February 2025).
- Cowling, T.; Nayakarathna, R.; Wills, A.L.; Tankala, D.; Roc, N.P.; Barakat, S. Early access for innovative oncology medicines: A different story in each nation. J. Med. Econ. 2023, 26, 944–953. [Google Scholar] [CrossRef] [PubMed]
- New Target Zero Initiative Aims to Help Improve Access to New Drugs. Available online: https://www.cda-amc.ca/news/new-target-zero-initiative-aims-help-improve-access-new-drugs (accessed on 23 February 2025).
- Product Monograph Columvi (Glofitamab). Available online: https://pdf.hres.ca/dpd_pm/00070059.PDF (accessed on 8 February 2025).
- Product Monograph Epkinly (Epcortimab). Available online: https://www.abbvie.ca/content/dam/abbvie-dotcom/ca/en/documents/products/EPKINLY_PM_EN.pdf (accessed on 8 February 2025).
- Priority Review of Drug Submissions (Therapeutic Products). Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/dhp-mps/alt_formats/hpfb-dgpsa/pdf/prodpharma/prfs_tpfd-eng.pdf (accessed on 23 February 2025).

| Drugs (Generic Name) Approved with NOC/c From 1 January 2023 to 31 December 2024 | Time From NOC/c Issuance to First Provincial Listings | Health Canada Time from File Accepted for Review to NOC/c QN Issuance | Time from NOC/c Issuance to CDA-AMC Acceptance for Review | Time from CDA-AMC Acceptance for Review to Draft Recommendation | Time from CDA-AMC Draft to Final Recommendation | Time for pCPA Initiation and Consideration | Time for pCPA Negotiation | Time from End of pCPA Negotiation to First Provincial Listing |
|---|---|---|---|---|---|---|---|---|
| Columvi (glofitamab) | Completed 509 | 199 | 130 | 141 | 63 | 42 | 110 | 23 |
| Akeega (niraparib/abiraterone) | Completed 549 | 297 | 16 | 173 | 64 | 140 | 114 | 42 |
| Epkinly (epcoritamab) TLR-pTAP | Completed 306 | 200 | 46 | 148 | 55 | 49 a | 98 | 26 |
| Carvykti (ciltacabtagene autoleucel) | Active >722 | 287 | −125 | 165 | 57 | 177 | Active | NA |
| Tecvayli (teclistamab) | Active >555 | 200 | 70 | 147 | 56 | 167 | Active | NA |
| Elrexfio (elranatamab) | Active >422 | 199 | −12 | 151 | 56 | 112 | Active | NA |
| Imdelltra (tarlatamab) | Active >142 | 198 | −14 | 146 | NA | NA | Active | NA |
| Talvey (talquetamab) | NA | NA b | 15 | 160 | NA (CDA-AMC DNR, no submission to pCPA) | |||
| Lynparza (olaparib) supplemental | NA | 307 | NA (No submission to CDA-AMC or pCPA) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, C.Y.; Mitha, A.; Wills, A. Accelerating Oncology Drug Reimbursement in Canada: Impact of the CDA-AMC Time-Limited Recommendation and pCPA Temporary Access Process. Curr. Oncol. 2025, 32, 235. https://doi.org/10.3390/curroncol32040235
Lau CY, Mitha A, Wills A. Accelerating Oncology Drug Reimbursement in Canada: Impact of the CDA-AMC Time-Limited Recommendation and pCPA Temporary Access Process. Current Oncology. 2025; 32(4):235. https://doi.org/10.3390/curroncol32040235
Chicago/Turabian StyleLau, Catherine Y., Arif Mitha, and Allison Wills. 2025. "Accelerating Oncology Drug Reimbursement in Canada: Impact of the CDA-AMC Time-Limited Recommendation and pCPA Temporary Access Process" Current Oncology 32, no. 4: 235. https://doi.org/10.3390/curroncol32040235
APA StyleLau, C. Y., Mitha, A., & Wills, A. (2025). Accelerating Oncology Drug Reimbursement in Canada: Impact of the CDA-AMC Time-Limited Recommendation and pCPA Temporary Access Process. Current Oncology, 32(4), 235. https://doi.org/10.3390/curroncol32040235




