Merkel Cell Carcinoma of the Skin: Deducing the Pattern of Spread from an International Aggregated Database of 949 Patients
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
- (a)
- After initial diagnosis, patients with primaries of any size should be referred without delay to experienced specialist(s) for definitive treatment, in a tertiary center if possible, since delay in surgery or postoperative radiotherapy was documented to be associated with worse outcome [31,32]. The Fred Hutchinson Cancer Center in Seattle reported on 124 MCC patients with a median time to initiate postoperative radiotherapy (ttPORT) of 41 days (range: 8–125 days) [31]. The median follow-up was 55 months. In total, 17 (14%) patients experienced a locoregional recurrence (LRR), 14 (82%) of which arose outside the radiation field. LRR at 5 years was increased for ttPORT > 8 weeks vs. ≤ 8 weeks, 28.0% vs. 9.2%, p = 0.006. There was an increase in the cumulative incidence of cancer-specific death with increasing ttPORT (Hazard Ratio = 1.14 per 1-week increase, p = 0.016) [31]. Readers should note that different cutoffs for surgical delays were used in the two above references [28,29,31,32], so currently there is no definite recommended guideline for the optimal time to start treatment. Surgeons are advised to perform wide local excision or Moh’s microsurgery to achieve negative margins but avoid extensive reconstructions that require a longer time to heal and hence delay radiotherapy initiation, as per the NCCN recommendations [21].
- (b)
- Multidisciplinary discussions of the best treatment options should be made on tumor boards. As combination therapy could have more side effects, a consideration of co-morbidities, performance status, and the available social support during and after the treatment for the patient is needed. Fine judgment is necessary to decide if the benefits outweigh the risks in elderly patients.
- (c)
- Clinicians should constantly update their knowledge by looking at websites such as merkelcell.org [33], Fred Hutchison Research Cancer Center [34], Memorial Sloan-Kettering Cancer Center [35], Cleveland Clinic [36], Mayo Clinic [37], the Skin Cancer Foundation [38], and NCCN (National Comprehensive Cancer Network guidelines [21]. More recently, retifanlimab has emerged as a notably effective treatment strategy, leading to US Food and Drug Administration Accelerated Approval for metastatic or recurrent locally advanced MCC based on the phase II POD1UM-201 study [39,40].
- (d)
- Enrollment in clinical trials is strongly encouraged. The results of the recent phase II study ADMEC-O could be confirmed with more trials to encourage clinicians to consider adopting adjuvant nivolumab into their clinical practice. Hypofractionated radiotherapy research is ongoing as well [41].
- (e)
- More research on cancer prevention and the development of treatment resistance should be performed in the future. An example is the study of tumor-associated macrophages (TAMs) in MCC, and the association of S100A8-expressing TAMs with resistance to anti-PD-L1 inhibitors (where PD-L1 stands for programmed death-ligand 1). Silk et al. commented that these data improve our understanding of why some tumors with brisk tumor-infiltrating lymphocytes do not respond to immunotherapy [42]. Another study on MCPyV+ metastatic MCCs treated with an intra-tumoral stimulator of interferon genes (STING) agonist (ADU-S100) plus intravenous anti-PD-1 antibody (spartalizumab) attained a durable objective response with the regression of both injected and non-injected lesions [43]. Eventually, a cancer vaccine may be possible [44].
- (f)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADMEC-O | Adjuvant immunotherapy with nivolumab versus observation |
| ADT | Androgen deprivation therapy |
| CK | Cytokeratin |
| CSS | Cause-specific survival |
| CT | Computerized tomography |
| DFS | Disease-free survival |
| DMs | Distant metastases |
| LNMs | Lymph node metastases |
| LRR | Locoregional recurrence |
| MCC | Merkel cell carcinoma |
| NCDB | National Cancer Database |
| OS | National Comprehensive Cancer Network |
| NCCN | Overall survival |
| PD-L1 | Programmed death-ligand 1 |
| PET | Positron emission tomography |
| PFS | Progression-free survival |
| SEER | Surveillance, Epidemiology, and End Results (SEER) program |
| SLNB | Sentinel lymph node biopsy |
| STING | Stimulator of interferon genes |
| TAMs | Tumor-associated macrophages |
| ttPORT | Time to initiate postoperative radiotherapy |
References
- Tai, P.; Park, S.Y.; Nghiem, P.T. Pathogenesis, clinical features, and diagnosis of Merkel cell (neuroendocrine) carcinoma. In UpToDate; Canellos, G.P., Schnipper, L., Eds.; UpToDate: Waltham, MA, USA, 2025; Available online: www.uptodate.com (accessed on 1 March 2025).
- Tilling, T.; Moll, I. Which are the cells of origin in Merkel cell carcinoma? J. Skin Cancer 2012, 2012, 680410. [Google Scholar] [CrossRef] [PubMed]
- Tai, P.; Au, J. Skin cancer management—Updates on Merkel cell carcinoma. Ann. Transl. Med. 2018, 6, 282. [Google Scholar] [PubMed]
- Tang, C.K.; Toker, C.; Nedwich, A.; Zaman, A.N. Unusual cutaneous carcinoma with features of small cell (oat cell-like) and squamous cell carcinomas. A variant of malignant Merkel cell neoplasm. Am. J. Dermatopathol. 1982, 4, 537–548. [Google Scholar] [PubMed]
- Raaf, J.H.; Urmacher, C.; Knapper, W.K.; Shiu, M.H.; Cheng, E.W. Trabecular (Merkel cell) carcinoma of the skin. Treatment of primary, recurrent, and metastatic disease. Cancer 1986, 57, 178–182. [Google Scholar] [PubMed]
- Ratner, D.; Nelson, B.R.; Brown, M.D.; Johnson, T.M. Merkel cell carcinoma. J. Am. Acad. Dermatol. 1993, 29 Pt 1, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Sunshine, J.C.; Jahchan, N.S.; Sage, J.; Choi, J. Are there multiple cells of origin of Merkel cell carcinoma? Oncogene 2018, 37, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Nirenberg, A.; Steinman, H.; Dixon, J.; Dixon, A. Merkel cell carcinoma update: The case for two tumours. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Yiengpruksawan, A.; Coit, D.G.; Thaler, H.T.; Urmacher, C.; Knapper, W.K. Merkel cell carcinoma. Prognosis and management. Arch. Surg. 1991, 126, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Victor, N.S.; Morton, B.; Smith, J.W. Merkel cell cancer: Is prophylactic lymph node dissection indicated? Am. Surg. 1996, 62, 879–882. [Google Scholar]
- Queirolo, P.; Gipponi, M.; Peressini, A.; Raposio, E.; Vecchio, S.; Guenzi, M.; Sertoli, M.R.; Santi, P.; Cafiero, F. Merkel cell carcinoma of the skin. Treatment of primary, recurrent and metastatic disease: Review of clinical cases. Anticancer Res. 1997, 17, 2339–2342. [Google Scholar]
- Gillenwater, A.M.; Hessel, A.C.; Morrison, W.H.; Burgess, M.; Silva, E.G.; Roberts, D.; Goepfert, H. Merkel cell carcinoma of the head and neck: Effect of surgical excision and radiation on recurrence and survival. Arch. Otolaryngol. Head Neck Surg. 2001, 127, 149–154. [Google Scholar] [PubMed]
- Gunaratne, D.A.; Howle, J.R.; Veness, M.J. Sentinel lymph node biopsy in Merkel cell carcinoma: A 15-year institutional experience and statistical analysis of 721 reported cases. Br. J. Dermatol. 2016, 174, 273–281. [Google Scholar]
- Santamaria-Barria, J.A.; Boland, G.M.; Yeap, B.Y.; Nardi, V.; Dias-Santagata, D.; Cusack, J.C., Jr. Merkel cell carcinoma: 30-year experience from a single institution. Ann. Surg. Oncol. 2013, 20, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.C.; Ugurel, S.; Leiter, U.; Meier, F.; Gutzmer, R.; Haferkamp, S.; Zimmer, L.; Livingstone, E.; Eigentler, T.K.; Hauschild, A.; et al. Adjuvant immunotherapy with nivolumab versus observation in completely resected Merkel cell carcinoma (ADMEC-O): Disease-free survival results from a randomised, open-label, phase 2 trial. Lancet 2023, 402, 798–808. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Non-parametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–482. [Google Scholar]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. Ser. B 1972, 34, 187–220. [Google Scholar]
- Coggshall, K.; Tello, T.L.; North, J.P.; Yu, S.S. Merkel cell carcinoma: An update and review: Pathogenesis, diagnosis, and staging. J. Am. Acad. Dermatol. 2018, 78, 433–442. [Google Scholar] [PubMed]
- Tai, P.; Park, S.Y.; Nghiem, P.T.; Silk, A. Staging and treatment, and surveillance of locoregional Merkel cell carcinoma. In UpToDate; Canellos, G.P., Schnipper, L., Eds.; UpToDate: Waltham, MA, USA, 2024; Available online: www.uptodate.com (accessed on 15 May 2024).
- Park, S.Y.; Nghiem, P.T.; Tai, P.; Silk, A. Treatment of recurrent and metastatic Merkel cell carcinoma. In UpToDate; Canellos, G.P., Schnipper, L., Eds.; UpToDate: Waltham, MA, USA, 2024; Available online: www.uptodate.com (accessed on 15 May 2024).
- National Comprehensive Cancer Network (NCCN) Guideline Website. Available online: www.nccn.org (accessed on 15 January 2025).
- Ginsburg, K.B.; Bell, S.; Bukavina, L.; Schober, J.P.; Magee, D.; Kutikov, A. The Phenomenon of “Therapeutic” Nodal Yield at Cystectomy for Bladder Cancer: Do Not Discount the Will Rogers Effect. Eur. Urol. Open Sci. 2022, 47, 43–47. [Google Scholar] [PubMed]
- CASCADE English Meaning—Cambridge Dictionary. Available online: https://dictionary.cambridge.org/us/dictionary/english/cascade (accessed on 14 January 2025).
- Miranda, I.; Jahan, N.; Shevde, L.A. The metastatic cascade through the lens of therapeutic inhibition. Cell Rep. Med. 2025, 6, 1101872. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Russell, J.S.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbé, C.; Milella, M.; Brownell, I.; et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J. Immnother. Cancer 2018, 6, 7. [Google Scholar]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbé, C.; Linette, G.P.; Milella, M.; et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multi-center, single-group, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef]
- Tai, P.T.H.; Yu, E.; Winquist, E.; Hammond, A.; Stitt, L.; Tonita, J.; Gilchrist, J. Chemotherapy in neuroendocrine Merkel cell carcinoma of the skin: Case series and review of 204 cases. J. Clin. Oncol. 2000, 18, 2493–2499. [Google Scholar] [CrossRef]
- Nayak, A.L.; Pickett, A.T.; Delisle, M.; Dingley, B.; Mallick, R.; Hamilton, T.; Stuart, H.; Talbot, M.; McKinnon, G.; Jost, E.; et al. Survival of Patients With Head and Neck Merkel Cell Cancer: Findings From the Pan-Canadian Merkel Cell Cancer Collaborative. JAMA Netw. Open 2023, 6, e2344127. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.B.; Gaskins, J.; Wall, W.; Tennant, P.; Bumpous, J.; Dunlap, N. Immune status and the efficacy of radiotherapy on overall survival for patients with localized Merkel cell carcinoma: An analysis of the National Cancer Database. J. Med. Imaging Radiat. Oncol. 2020, 64, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, N.T.; Ekhator, N.; Rothschild, M.; Wladis, E.J. A SEER program study of survival trends in Merkel cell carcinoma of the eyelid: 2000-2019. Orbit 2024, 43, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Alexander, N.A.; Schaub, S.K.; Goff, P.H.; Hippe, D.S.; Park, S.Y.; Lachance, K.; Bierma, M.; Liao, J.J.; Apisarnthanarax, S.; Bhatia, S.; et al. Increased risk of recurrence and disease-specific death following delayed postoperative radiation for Merkel cell carcinoma. J. Am. Acad. Dermatol. 2024, 90, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.D.; Behbahani, S.; Samie, F.H. Predictors of time to definitive surgery and survival in Merkel cell carcinoma: Analysis of the US National Cancer Database. Clin. Exp. Dermatol. 2022, 47, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Clinical trials for Merkel Cell Carcinoma. Available online: https://merkelcell.org/treatment/clinical-trials/ (accessed on 14 January 2025).
- Available online: https://www.fredhutch.org (accessed on 20 January 2025).
- Available online: https://www.mskcc.org (accessed on 20 January 2025).
- Available online: https://my.clevelandclinic.org (accessed on 20 January 2025).
- Available online: https://www.mayoclinic.org (accessed on 20 January 2025).
- Available online: https://www.skincancer.org (accessed on 20 January 2025).
- touchONCOLOGY-D-23-00021.pdf. Available online: https://touchoncology.com/wp-content/uploads/sites/2/2024/03/touchONCOLOGY-D-23-00021.pdf (accessed on 15 January 2025).
- Grignani, G.; Rutkowski, P.; Lebbe, C.; Guida, M.; Marqueste, C.G.; De Braud, F.G.M.; Spagnolo, F.; Burgess, M.; Montaudie, H.; Depenni, R.; et al. 1146P Updated results from POD1UM-201: A phase II study of retifanlimab in patients with advanced or metastatic Merkel cell carcinoma (MCC). Ann. Oncol. 2023, 34, S686. [Google Scholar] [CrossRef]
- Treatment Clinical Trials for Merkel Cell Cancer—NCI. Available online: https://www.cancer.gov/research/participate/clinical-trials/disease/merkel-cell/treatment (accessed on 20 January 2025).
- Silk, A.W.; Davar, D. Tumor-Associated Macrophages in Merkel Cell Carcinoma: Old Balances, New Checks. Clin. Cancer Res. 2024, 30, 1076–1078. [Google Scholar] [CrossRef] [PubMed]
- Pulliam, T.; Jani, S.; Goff, P.H.; Bhakuni, R.; Tabachnick-Cherny, S.; Smythe, K.; Seaton, B.W.; Tachiki, L.; Kulikauskas, R.; Church, C.; et al. Intratumoral STING agonist reverses immune evasion in PD-(L)1-refractory Merkel cell carcinoma: Mechanistic insights from detailed biomarker analyses. J. Immunother. Cancer 2024, 12, e009803. [Google Scholar] [CrossRef] [PubMed]
- Tabachnick-Cherny, S.; Pulliam, T.; Church, C.; Koelle, D.M.; Nghiem, P. Polyomavirus-driven Merkel cell carcinoma: Prospects for therapeutic vaccine development. Mol. Carcinog. 2020, 59, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Joseph, K.; Wong, J.; Abraham, A.; Zebak, J.; Patel, A.; Jones Thachuthara, A.; Iqbal, U.; Pham, T.M.; Menon, A.; Ghosh, S.; et al. Patterns and predictors of relapse in Merkel cell carcinoma: Results from a population-based study. Radiother. Oncol. 2022, 166, 110–117. [Google Scholar] [PubMed]
- Park, S.Y.; Tai, P.; Assouline, A.; Koul, R.; Dubey, A.; Lian, J.; Yu, E.; Veness, M.; Joseph, K. Merkel cell carcinoma (MCC) of the skin: Comprehensive analysis of radiation (RT) doses of aggregate patient data from literature. Radiother. Oncol. 2024, 198 (Suppl. S1), S74–S75. [Google Scholar]
- Joseph, K.; Tai, P.; Veness, M.; Lian, J.; Assouline, A.; Koul, R.; Dubey, A.; Park, S.Y.; Yu, E. Merkel cell carcinoma (MCC) of the skin: When is local radiotherapy (RT) without nodal coverage adequate? Comprehensive analysis of aggregate patient data from literature. Radiother. Oncol. 2024, 198 (Suppl. S1), S75. [Google Scholar]

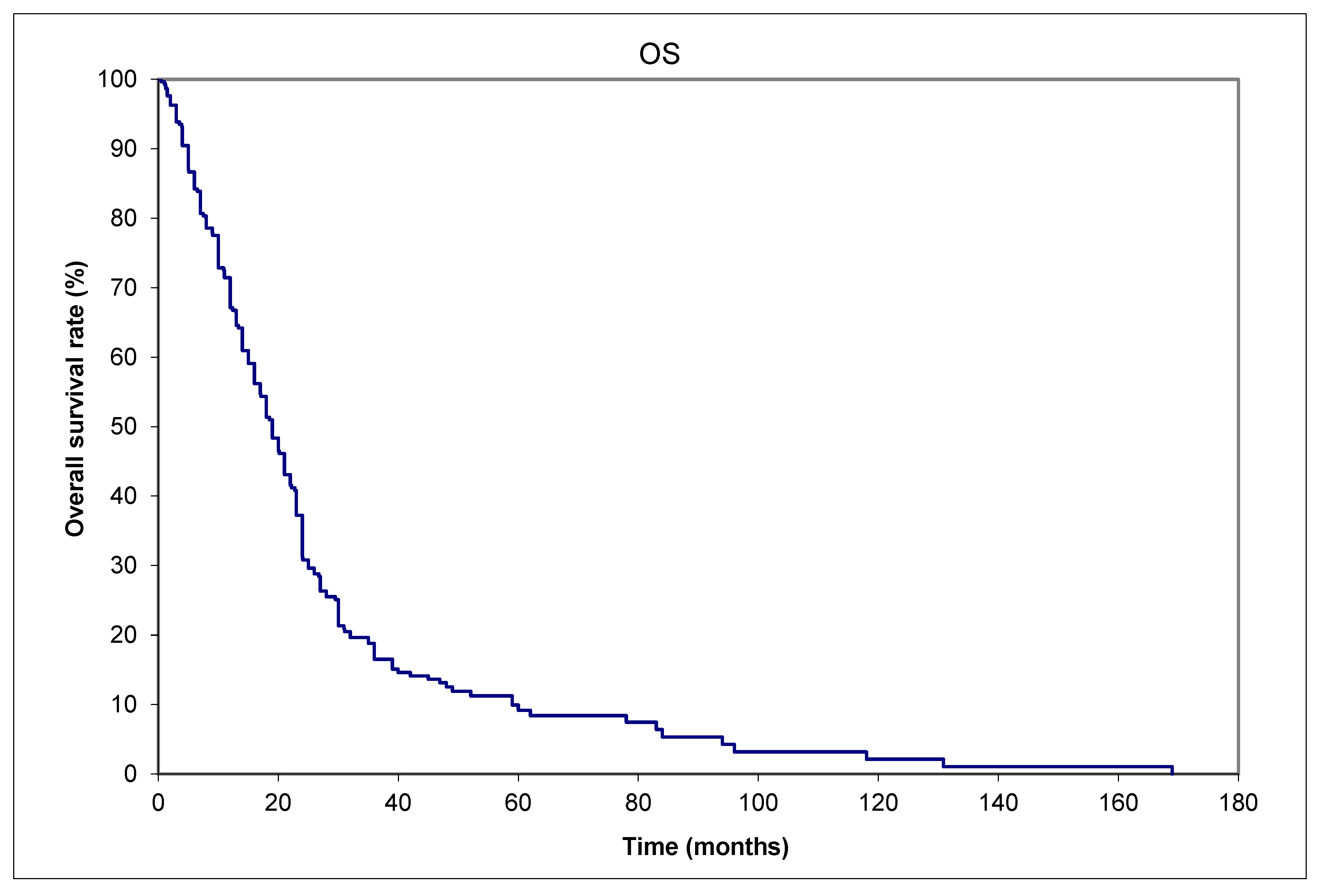
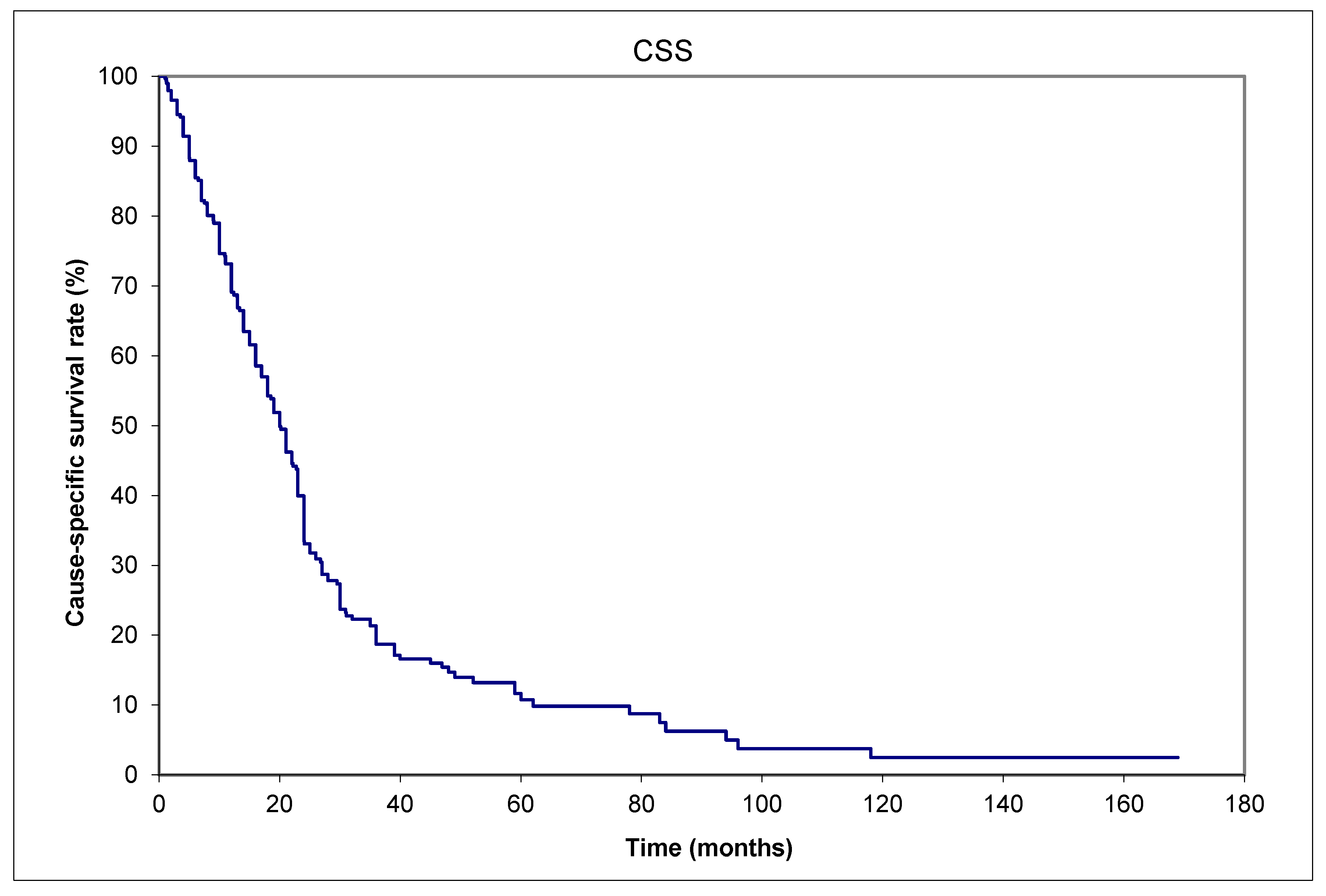
| Aggregated data from | Saskatchewan (Canada) | 13 (16%) | |||
| Alberta (Canada) | 17 (22%) | ||||
| London, Ontario (Canada) | 15 (19%) | ||||
| Windsor/Ontario, Canada | 5 (6%) | ||||
| Amiens (France) | 9 (11%) | ||||
| Westmead, New South Wales (Australia) | 20 (25%) | ||||
| Baseline characteristics | Age: | median 78 (range: 47–95) years | |||
| Sex: | 29 males and 50 females | ||||
| Size of primary tumor: | median 2.5 (range: 0.2–17) cm | ||||
| Initial stages | Local | Nodal | Distant metastases | Unknown | |
| Clinical | 42 (53%) | 28 (35%) | 8 (10%) | 1 (1%) | |
| Pathological | 35 (44%) | 35 (44%) | 8 (10%) | 1 (1%) | |
| Primary site | Head and neck | 35 (44%) | |||
| Limb (upper or lower) | 23 (29%) | ||||
| Trunk | 13 (16%) | ||||
| Unknown primary, presented with nodes only | 8 (10%) | ||||
| Timing of nodal metastases (patient number = 47) | Before distant metastases diagnosis | 31/47 (66%) | |||
| Within 1 month of distant metastases diagnosis | 10/47 (21%) | ||||
| After distant metastases diagnosis | 1/47 (2%) | ||||
| Unknown time relative to distant metastases | 5/47 (11%) | ||||
| Treatment of localized disease at presentation (patient number = 43) | Surgery | 24/43 (56%) |
| Surgery + Radiotherapy | 11/43 (26%) | |
| Surgery + Chemotherapy | 1/43 (2%) | |
| Radiotherapy alone | 4/43 (9%) | |
| Radiotherapy + Chemotherapy | 1/43 (2%) | |
| None | 2/43 (5%) | |
| Treatment of nodal metastases at presentation (patient number = 28) | Surgery | 6/28 (21%) |
| Surgery + Radiotherapy | 7/28 (25%) | |
| Surgery + Radiotherapy + Chemotherapy | 2/28 (7%) | |
| Radiotherapy alone | 13/28 (46%) | |
| Treatment of distant metastases at presentation (patient number = 8) | Radiotherapy + Chemotherapy | 3/8 (38%) |
| Chemotherapy alone | 2/8 (25%) | |
| None | 3/8 (38%) | |
| Final vital status | Alive | 8/79 (10%) |
| Dead | 71/79 (90%) | |
| Cause of death among those expired (patient number = 71) | Merkel cell carcinoma | 65/71 (92%) |
| Intercurrent disease | 6/71 (8%) |
| Variable | Hazard Ratio | (95% Confidence Interval) | p Values | |
|---|---|---|---|---|
| Age: | 60 | Reference variable | ||
| 70 | 0.90 | (0.64–1.26) | 0.50 | |
| 80 | 1.06 | (0.66–1.68) | 0.82 | |
| 90 | 1.75 | (0.89–3.46) | 0.11 | |
| Sex: | Male | 0.87 | (0.54–1.42) | 0.59 |
| Female | Reference variable | |||
| Chemotherapy: | Yes | 0.56 | (0.19–1.62) | 0.29 |
| No | Reference variable | |||
| Clinical stage: | Localized disease | 2.53 | (1.21–5.28) | 0.013 |
| Primary ≤1 cm | Reference variable | |||
| Primary >1 cm | 1.32 | (0.61–2.89) | 0.49 | |
| Nodal metastases | 3.27 | (1.85–5.78) | <0.001 | |
| Distant metastases | 21.42 | (7.15–64.21) | <0.001 | |
| Previous irradiation: | Yes | 2.95 | (0.90–9.61) | 0.073 |
| No | Reference variable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tai, P.; Joseph, K.; Prajapati, V.H.; Jones Thachuthara, A.; Lian, J.; Assouline, A.; Yu, E.; Veness, M. Merkel Cell Carcinoma of the Skin: Deducing the Pattern of Spread from an International Aggregated Database of 949 Patients. Curr. Oncol. 2025, 32, 211. https://doi.org/10.3390/curroncol32040211
Tai P, Joseph K, Prajapati VH, Jones Thachuthara A, Lian J, Assouline A, Yu E, Veness M. Merkel Cell Carcinoma of the Skin: Deducing the Pattern of Spread from an International Aggregated Database of 949 Patients. Current Oncology. 2025; 32(4):211. https://doi.org/10.3390/curroncol32040211
Chicago/Turabian StyleTai, Patricia, Kurian Joseph, Vimal H. Prajapati, Aoife Jones Thachuthara, Jidong Lian, Avi Assouline, Edward Yu, and Michael Veness. 2025. "Merkel Cell Carcinoma of the Skin: Deducing the Pattern of Spread from an International Aggregated Database of 949 Patients" Current Oncology 32, no. 4: 211. https://doi.org/10.3390/curroncol32040211
APA StyleTai, P., Joseph, K., Prajapati, V. H., Jones Thachuthara, A., Lian, J., Assouline, A., Yu, E., & Veness, M. (2025). Merkel Cell Carcinoma of the Skin: Deducing the Pattern of Spread from an International Aggregated Database of 949 Patients. Current Oncology, 32(4), 211. https://doi.org/10.3390/curroncol32040211







