Feasibility of a Physiatry Assessment Clinic to Address Physical Impairment in Head and Neck Cancer Patients Following Neck Resection and Free Flap Reconstruction
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Ethical Approval
2.3. Participants and Sample Size
2.4. Physiatry Assessment Clinic (PAC)
2.5. Variables and Data Management
2.5.1. Measures of Feasibility
2.5.2. Demographic and Clinical Characteristics
2.5.3. Physical Function
2.5.4. Short Physical Performance Battery (SPPB)
2.5.5. Patient-Reported Outcomes (PROs)
Shoulder and Arm Function Outcome Measure
Shoulder and Neck Function Outcome Measure
Swallowing Function
2.6. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
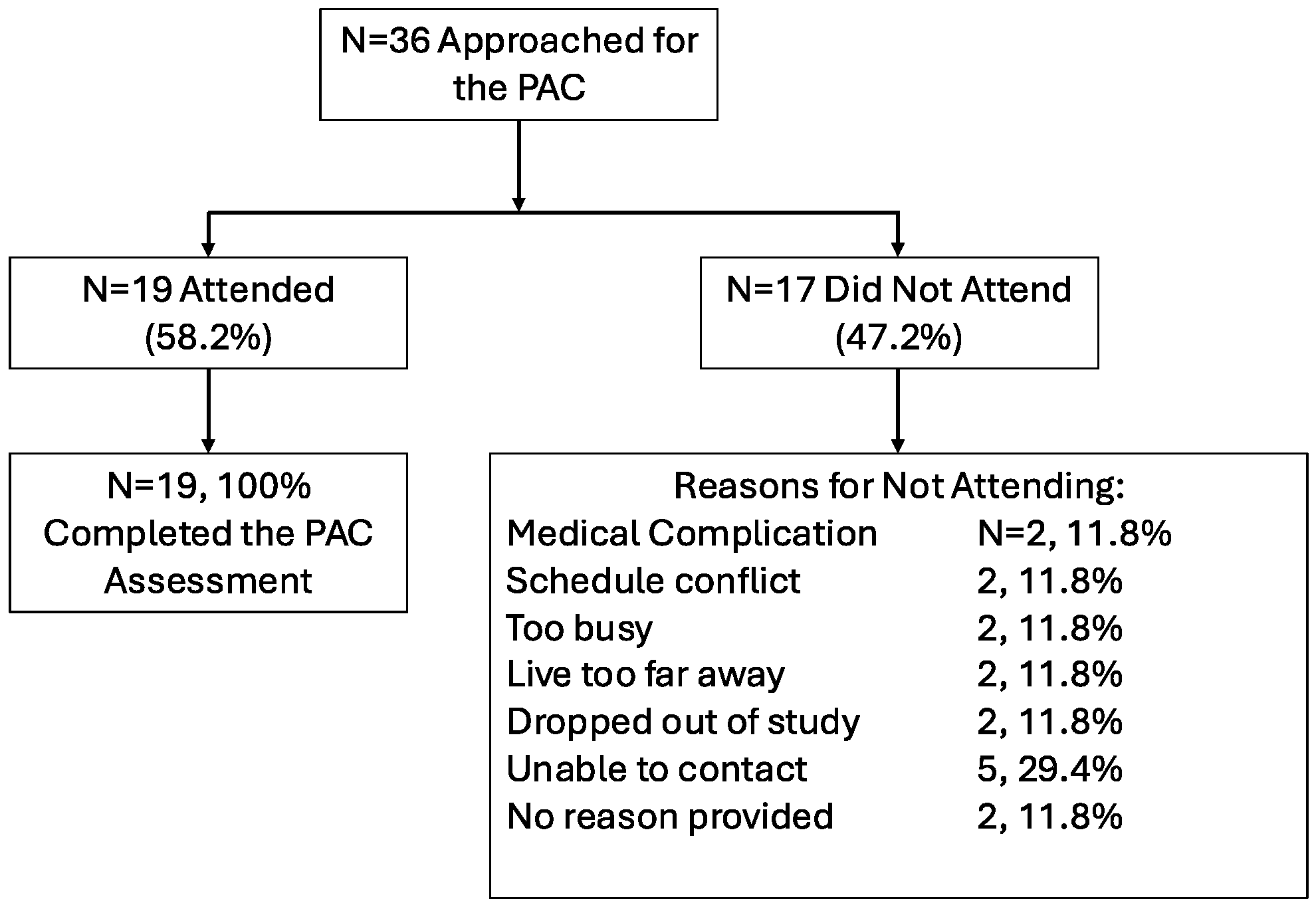
3.2. Feasibility
3.3. Shoulder and Neck Function
3.4. Shoulder and Neck Function Across Timepoints and Between Groups
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSEP-CEP | Canadian Society for Exercise Physiology Clinical Exercise Physiologist |
| PAC | Physiatry Assessment Clinic |
| SPPB | Short Physical Performance Battery |
| PROs | Patient-Reported Outcomes |
| NDII | Neck Dissection Impairment Index |
| HNC | Head and Neck Cancer |
| QOL | Quality of Life |
| HREBA-CC | Health Research Ethics Board of Alberta—Cancer Committee |
| REDCap | Research Electronic Data Capture |
| MRC | Medical Research Council |
| SD | Standard Deviation |
| CND | Canadian |
| BMI | Body Mass Index |
| PROMIS | Patient-Reported Outcome Measure Information System |
| CREST | Cancer Rehabilitation and Exercise Screening Tool |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef]
- Gane, E.M.; McPhail, S.M.; Hatton, A.L.; Panizza, B.J.; O’Leary, S.P. Neck and Shoulder Motor Function following Neck Dissection: A Comparison with Healthy Control Subjects. Otolaryngol. Head Neck Surg. 2019, 160, 1009–1018. [Google Scholar] [CrossRef]
- Lahtinen, S.; Koivunen, P.; Ala-Kokko, T.; Laurila, P.; Kaarela, O.; Liisanantti, J.H. Quality of life after free flap surgery for cancer of the head and neck in patients with or without postoperative complications. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 2575–2584. [Google Scholar] [CrossRef] [PubMed]
- Vosler, P.S.; Orsini, M.; Enepekides, D.J.; Higgins, K.M. Predicting complications of major head and neck oncological surgery: An evaluation of the ACS NSQIP surgical risk calculator. J. Otolaryngol. Head Neck Surg. 2018, 47, 21. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, S.; Koivunen, P.; Ala-Kokko, T.; Kaarela, O.; Ohtonen, P.; Laurila, P.; Liisanantti, J.H. Complications and outcome after free flap surgery for cancer of the head and neck. Br. J. Oral Maxillofac. Surg. 2018, 56, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Mascarella, M.A.; Richardson, K.; Mlynarek, A.; Forest, V.I.; Hier, M.; Sadeghi, N.; Mayo, N. Evaluation of a Preoperative Adverse Event Risk Index for Patients Undergoing Head and Neck Cancer Surgery. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 345–351. [Google Scholar] [CrossRef]
- Gane, E.M.; McPhail, S.M.; Hatton, A.L.; Panizza, B.J.; O’Leary, S.P. Predictors of health-related quality of life in patients treated with neck dissection for head and neck cancer. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 4183–4193. [Google Scholar] [CrossRef]
- Gane, E.M.; McPhail, S.M.; Hatton, A.L.; Panizza, B.J.; O’Leary, S.P. The relationship between physical impairments, quality of life and disability of the neck and upper limb in patients following neck dissection. J. Cancer Surviv. 2018, 12, 619–631. [Google Scholar] [CrossRef]
- Silver, J.K.; Baima, J. Cancer prehabilitation: An opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am. J. Phys. Med. Rehabil. 2013, 92, 715–727. [Google Scholar] [CrossRef]
- Capozzi, L.C.; Daun, J.T.; Francis, G.J.; de Guzman Wilding, M.; Urgoiti, G.R.; Langelier, D.; Culos-Reed, N. Feasibility and Implementation of an Oncology Rehabilitation Triage Clinic: Assessing Rehabilitation, Exercise Need, and Triage Pathways within the Alberta Cancer Exercise–Neuro–Oncology Study. Curr. Oncol. 2023, 30, 6220–6245. [Google Scholar] [CrossRef]
- Wagoner, C.W.; Daun, J.T.; Danyluk, J.; Twomey, R.; Murphy, L.; Peterson, M.; Gentleman, E.; Capozzi, L.C.; Francis, G.J.; Chandarana, S.P.; et al. Multiphasic exercise prehabilitation for patients undergoing surgery for head and neck cancer: A hybrid effectiveness-implementation study protocol. Support. Care Cancer 2023, 31, 726. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Daun, J.T.; Twomey, R.; Capozzi, L.C.; Crump, T.; Francis, G.J.; Matthews, T.W.; Chandarana, S.; Hart, R.D.; Schrag, C.; Matthews, J.; et al. The feasibility of patient-reported outcomes, physical function, and mobilization in the care pathway for head and neck cancer surgical patients. Pilot Feasibility Stud. 2022, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Compston, A. Aids to the investigation of peripheral nerve injuries. Medical Research Council: Nerve Injuries Research Committee. His Majesty’s Stationery Office: 1942; pp. 48 (iii) and 74 figures and 7 diagrams; with aids to the examination of the peripheral nervous system. By Michael O’Brien for the Guarantors of Brain. Saunders Elsevier: 2010; pp. [8] 64 and 94 Figures. Brain 2010, 133, 2838–2844. [Google Scholar] [CrossRef]
- Beaton, D.E.; Wright, J.G.; Katz, J.N. Development of the QuickDASH: Comparison of three item-reduction approaches. J. Bone Jt. Surg. Am. 2005, 87, 1038–1046. [Google Scholar]
- LeBlanc, M.; Stineman, M.; DeMichele, A.; Stricker, C.; Mao, J.J. Validation of QuickDASH outcome measure in breast cancer survivors for upper extremity disability. Arch. Phys. Med. Rehabil. 2014, 95, 493–498. [Google Scholar] [CrossRef]
- Taylor, I.W. The Relative Contribution of Vitamin D Receptor (VDR), Collagen Type 1, α-1 (COL1A1), Tumor Necrosis Factor Receptor 2 (TNFR2), Polymorphisms, Physical Activity, and Bone Mineral-Free Lean Mass to Bone Parameters in Children. Master’s Thesis, University of British Columbia, Vancouver, BC, Canada, 2002. [Google Scholar]
- Belafsky, P.C.; Mouadeb, D.A.; Rees, C.J.; Pryor, J.C.; Postma, G.N.; Allen, J.; Leonard, R.J. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann. Otol. Rhinol. Laryngol. 2008, 117, 919–924. [Google Scholar] [CrossRef]
- Crevenna, R.; Maehr, B.; Fialka-Moser, V.; Keilani, M. Strength of skeletal muscle and quality of life in patients suffering from "typical male" carcinomas. Support. Care Cancer 2009, 17, 1325–1328. [Google Scholar] [CrossRef]
- Cheng, J.T.; Ramos Emos, M.; Leite, V.; Capozzi, L.; Woodrow, L.E.; Gutierrez, C.; Ngo-Huang, A.; Krause, K.J.; Parke, S.C.; Langelier, D.M. Rehabilitation Interventions in Head and Neck Cancer: A Scoping Review. Am. J. Phys. Med. Rehabil. 2024, 103 (Suppl. S1), S62–S71. [Google Scholar]
- Silver, J.K.; Baima, J.; Mayer, R.S. Impairment-driven cancer rehabilitation: An essential component of quality care and survivorship. CA Cancer J. Clin. 2013, 63, 295–317. [Google Scholar] [CrossRef]
- Stout, N.L.; Santa Mina, D.; Lyons, K.D.; Robb, K.; Silver, J.K. A systematic review of rehabilitation and exercise recommendations in oncology guidelines. CA Cancer J. Clin. 2021, 71, 149–175. [Google Scholar] [CrossRef]
- Alfano, C.M.; Cheville, A.L.; Mustian, K. Developing High-Quality Cancer Rehabilitation Programs: A Timely Need. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, C.; Nelson, M.B. Physical Medicine and Rehabilitation. Cancer Treat Res. 2021, 182, 255–271. [Google Scholar] [PubMed]
- Stout, N.L.; Harrington, S.E.; Perry, A.; Alappattu, M.J.; Pfab, V.; Stewart, B.; Manes, M.R. Implementation of a Cancer Rehabilitation Navigation Program: A qualitative analysis of implementation determinants and strategies. J. Cancer Surviv. 2024, 18, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Campbell, A.M.; Stuiver, M.M.; Pinto, B.M.; Schwartz, A.L.; Morris, G.S.; Ligibel, J.A.; Cheville, A.; Galvão, D.A.; Alfano, C.M.; et al. Exercise is medicine in oncology: Engaging clinicians to help patients move through cancer. CA Cancer J. Clin. 2019, 69, 468–484. [Google Scholar] [CrossRef]
- Cheville, A.L.; Beck, L.A.; Petersen, T.L.; Marks, R.S.; Gamble, G.L. The detection and treatment of cancer-related functional problems in an outpatient setting. Support. Care Cancer 2009, 17, 61–67. [Google Scholar] [CrossRef]
- Smith, S.R.; Zheng, J.Y.; Silver, J.; Haig, A.J.; Cheville, A. Cancer rehabilitation as an essential component of quality care and survivorship from an international perspective. Disabil. Rehabil. 2020, 42, 8–13. [Google Scholar] [CrossRef]
- Jensen, R.E.; Potosky, A.L.; Reeve, B.B.; Hahn, E.; Cella, D.; Fries, J.; Smith, A.W.; Keegan, T.H.; Wu, X.C.; Paddock, L.; et al. Validation of the PROMIS physical function measures in a diverse US population-based cohort of cancer patients. Qual. Life Res. 2015, 24, 2333–2344. [Google Scholar] [CrossRef]
- Covington, K.R.; Marshall, T.; Campbell, G.; Williams, G.R.; Fu, J.B.; Kendig, T.D.; Howe, N.; Alfano, C.M.; Pergolotti, M. Development of the Exercise in Cancer Evaluation and Decision Support (EXCEEDS) algorithm. Support. Care Cancer 2021, 29, 6469–6480. [Google Scholar] [CrossRef]
- Taylor, R.J.; Chepeha, J.C.; Teknos, T.N.; Bradford, C.R.; Sharma, P.K.; Terrell, J.E.; Hogikyan, N.D.; Wolf, G.T.; Chepeha, D.B. Development and validation of the neck dissection impairment index: A quality of life measure. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 44–49. [Google Scholar] [CrossRef]
- List, M.A.; D’Antonio, L.L.; Cella, D.F.; Siston, A.; Mumby, P.; Haraf, D.; Vokes, E. The Performance Status Scale for Head and Neck Cancer Patients and the Functional Assessment of Cancer Therapy-Head and Neck Scale. A study of utility and validity. Cancer 1996, 77, 2294–2301. [Google Scholar] [CrossRef]
- Mewes, J.C.; Steuten, L.M.; Ijzerman, M.J.; van Harten, W.H. Effectiveness of multidimensional cancer survivor rehabilitation and cost-effectiveness of cancer rehabilitation in general: A systematic review. Oncologist 2012, 17, 1581–1593. [Google Scholar] [CrossRef]
- Stout, N.L.; Brown, J.C.; Schwartz, A.L.; Marshall, T.F.; Campbell, A.M.; Nekhlyudov, L.; Zucker, D.S.; Basen-Engquist, K.M.; Campbell, G.; Meyerhardt, J.; et al. An exercise oncology clinical pathway: Screening and referral for personalized interventions. Cancer 2020, 126, 2750–2758. [Google Scholar] [CrossRef]
- Brick, R.; Natori, A.; Moreno, P.I.; Molinares, D.; Koru-Sengul, T.; Penedo, F.J. Predictors of cancer rehabilitation medicine referral and utilization based on the Moving Through Cancer physical activity screening assessment. Support. Care Cancer 2023, 31, 216. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Chongaway, A.; Saeed, A.; Fontana, T.; Wood, K.; Gibson, S.; Trilk, J.; Adsul, P.; Baker, S. An initiative to implement a triage and referral system to make exercise and rehabilitation referrals standard of care in oncology. Support. Care Cancer 2024, 32, 259. [Google Scholar] [CrossRef]
| Demographic Variable | Total (n = 36) | Attended (n = 19, %) | Not Attended (n = 17, %) |
|---|---|---|---|
| Sex Male Female | 26 10 | 15, 78.9% 4, 21.0% | 11, 64.7% 6, 35.3% |
| Self-Identified Gender Man Woman | 26 10 | 15, 78.9% 4, 21.0% | 11, 64.7% 6, 35.3% |
| Age: Mean ± SD, years | 62.5 ± 11.8 | 61.0 ± 14.2 | 64.5 ± 8.7 |
| Highest Level of Education Some Secondary Education Completed High School Some University/College, No Degree Completed University/College Completed Graduate School Prefer Not to Answer | 1 6 12 11 5 1 | 0 2, 10.5% 7, 36.8% 6, 31.6% 3, 15.8% 1, 5.26% | 1, 5.88% 4, 21.1% 5, 29.4% 5, 29.4% 2, 10.5%0 0 |
| Annual Family Income, CAD <CAD 20,000 CAD 20,000-39,999 CAD 40,000-59,999 CAD 60,000-79,999 CAD 80,000-99,999 >CAD 100,000 Prefer not to answer | 2 3 6 5 3 11 6 | 1, 5.26% 2, 10.5% 3, 15.8% 4, 21.0% 1, 5.26% 5, 26.3% 3, 15.8% | 1, 5.88% 1, 5.88% 3, 17.6% 1, 5.88% 2, 10.5% 6, 35.3% 3, 17.6% |
| Employment Status Unable to Work Due to Cancer Retired Part Time Homemaker Full Time | 6 18 2 2 8 | 3, 15.8% 9, 47.4% 1, 5.26% 1, 5.26% 5, 26.3% | 3, 17.6% 9, 52.9% 1, 5.88% 1, 5.88% 3, 17.6% |
| Distance From Clinic Distance in KM Distance in Minutes Travelled | 44.9 + 59.5 37.3 + 34.6 | 90.9 + 113.2 64.8 + 67.9 | |
| Self-Described Racial Background White South Asian East Asian | 32 3 1 | 17, 89.5% 1, 5.26% 1, 5.26% | 15, 88.2% 2, 10.5% 0 |
| Smoking Status Never Smoked Ex-Smoker Current Smoker Not Stated | 14 13 7 2 | 6, 31.6% 8, 42.1% 4, 21.0% 1, 5.26% | 8, 47.1% 5, 29.4% 3, 17.6% 1, 5.88% |
| Alcohol Use Never Consumed Alcohol Light Drinker Moderate Drinker Heavy Drinker Not Stated | 8 10 2 10 6 | 3, 15.8% 5, 26.3% 2, 10.5% 6, 31.6% 3, 15.8% | 5, 29.4% 5, 29.4% 0 4, 21.1% 3, 17.6% |
| Clinical Characteristic | Total (n = 36) | Attended (n = 19, %) | Not Attended (n = 17, %) |
|---|---|---|---|
| Time Since Surgery: Mean ± SD, weeks | 8.6 ± 3.6 | ||
| Primary Site of Head and Neck Tumor Oral Cavity Oropharynx Paranasal Sinus Skin Salivary Gland Larynx | 28 2 1 3 1 1 | 14, 73.7% 2, 10.5% 1, 5.3% 1, 5.3% 1, 5.3% 0 | 14, 73.7% 0 0 2, 10.5% 0 1, 5.9% |
| Histology Squamous Cell Carcinoma Sarcoma Mucoepidermoid Carcinoma Adenoid Cystic Carcinoma Basal Cell Carcinoma Benign Not stated | 28 2 2 1 1 1 1 | 15, 78.9% 2, 10.5% 1, 5.3% 1, 5.3% 0 0 0 | 13, 76.4% 0 1, 5.9% 0 1, 5.9% 1, 5.9% 1, 5.9% |
| Stage * (Using the American Joint Committee on Cancer 8, AJCC8) 0 I II III IV A or B Not Stated | 1 3 6 5 19 2 | 0 1, 5.3% 5, 26.3% 5, 26.3% 7, 36.8% 1, 5.3% | 1, 5.9% 2, 10.5% 1, 5.9% 0 12, 70.6% 1, 5.9% |
| pT Classification T0 T1 T2 T3 T4a Not Stated | 2 4 9 9 11 1 | 1, 5.3% 2, 10.5% 6, 31.6% 8, 42.1% 2, 10.5% 0 | 1, 5.9% 2, 10.5% 3, 17.6% 1, 5.9% 9, 52.9% 1, 5.9% |
| pN Classification N0 N1 N2b N3 Not Stated | 16 4 2 13 1 | 10, 52.6% 3, 15.8% 1, 5.3% 5, 26.3% 0 | 6, 35.3% 1, 5.9% 1, 5.9% 8, 47.1% 1, 5.9% |
| Surgical Side Left Right Bilateral | 8 10 17 | 6, 31.6% 7, 36.8% 6, 31.6% | 2, 10.5% 3, 17.6% 11, 64.7% |
| Adjuvant Treatment Radiation Only Concurrent Chemoradiation No Adjuvant | 18 9 9 | 13, 72.2% 3, 33.3% 3, 33.3% | 5, 27.8% 6, 66.7% 6, 66.7% |
| Exam Component | Result (Mean ± SD) |
|---|---|
| Resting Heart Rate, bpm | 77.4 ± 8.7 |
| Resting Blood Pressure, mm Hg Systolic Blood Pressure Diastolic Blood Pressure | 123/79 123.0 ± 9.4 78.6 ± 7.1 |
| Height, kg | 174.6 ± 11.3 |
| Weight, cm | 80.8 ± 14.9 |
| BMI, kg/m2 | 26.5 ± 3.9 |
| SPPB Balance Score, out of 4 Gait Speed Score, out of 4 Gait Aids: walker (n = 4), cane (n = 5), none (n = 44) Chair Stand Test Score, out of 4 Total Score, out of 12 | 3.8 ± 0.7 3.9 ± 0.4 3.4 ± 1.0 11.0 ± 1.6 |
| Category | Surgical Side (Mean ± SD) | Non-Surgical Side (Mean ± SD) |
|---|---|---|
| Spinal Accessory Nerve (CN XI)-Innervated Muscles (MRC scale, /5 ± SD) | ||
| Sternocleidomastoid | 5.0 ± 0 | 5.0 ± 0 |
| Trapezius | 5.0 ± 0.2 | 5.0 ± 0 |
| Upper Extremity Strength (myotome) (MRC scale, /5 ± SD) | ||
| Deltoids/Shoulder Abduction (C5) | 4.9 ± 0.3 | 5.0 ± 0 |
| Biceps/Elbow Flexion (C6) | 5.0 ± 0 | 5.0 ± 0 |
| Wrist Extension (C7) | 5.0 ± 0 | 5.0 ± 0 |
| Triceps/Elbow Extension (C8) | 5.0 ± 0 | 5.0 ± 0 |
| Intrinsics/Finger Abduction (T1) | 5.0 ± 0 | 5.0 ± 0 |
| Neck Extension | 5.0 ± 0 affected vs. unaffected side N/A | |
| Neck Flexion | 4.9 ± 0.2 affected vs. unaffected side N/A | |
| Shoulder Active Range of Motion (mean degrees ± SD) | ||
| Abduction | 154.2 ± 26.7 | 151.6 ± 32.1 |
| Flexion | 158.2 ± 12.3 | 160.1 ± 11.7 |
| External Rotation | 72.9 ± 18.1 | 80.8 ± 10.5 |
| Internal Rotation | 51.4 ± 19.6 | 48.1 ± 23.2 |
| Scapular Winging—number (%) | ||
| Yes | 8, 32.0% | 1, 7.7% |
| No | 17, 68.0% | 12, 92.3% |
| Neck Active Range of Motion (mean degrees ± SD) | ||
| Flexion | 43.8 ± 14.2 surgical vs. non-surgical side N/A | |
| Extension | 45.7 ± 12.2 surgical vs. non-surgical side N/A | |
| Lateral Rotation | 65.4 ± 23.8 | 57.4 ± 11.8 |
| Lateral Flexion | 33.6 ± 13.1 | 31.8 ± 11.4 |
| Baseline | 6 Months | 12 Months | ||||
|---|---|---|---|---|---|---|
| Questionnaire | Attended (n = 16) | Not Attended (n = 14) | Attended (n = 13) | Not Attended (n = 12) | Attended (n = 13) | Not Attended (n = 8) |
| Shoulder Function (QuickDASH) | 17.4 ± 8.4 | 20.9 ± 9.0 | 14.9 ± 3.9 * | 19.2 ± 4.5 | 14.0 ± 4.0 | 18.9 ± 10.8 |
| Neck Function (NDII) | 18.7 ± 10.8 | 17.7 ± 6.4 | 14.3 ± 5.8 | 19.1 ± 7.0 | 14.0 ± 5.3 | 17.1 ± 10.6 |
| Swallow Function (EAT-10) | 12.1 ± 11.0 ** | 12.1 ± 10.4 | 4.6 ± 6.0 ** | 12.8 ± 12.5 | 6.3 ± 10.2 | 8.8 ± 11.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capozzi, L.C.; Wagoner, C.; Daun, J.T.; Murphy, L.; Nakoneshny, S.C.; Francis, G.J.; Dort, J.C.; Sauro, K.; Culos-Reed, S.N. Feasibility of a Physiatry Assessment Clinic to Address Physical Impairment in Head and Neck Cancer Patients Following Neck Resection and Free Flap Reconstruction. Curr. Oncol. 2025, 32, 562. https://doi.org/10.3390/curroncol32100562
Capozzi LC, Wagoner C, Daun JT, Murphy L, Nakoneshny SC, Francis GJ, Dort JC, Sauro K, Culos-Reed SN. Feasibility of a Physiatry Assessment Clinic to Address Physical Impairment in Head and Neck Cancer Patients Following Neck Resection and Free Flap Reconstruction. Current Oncology. 2025; 32(10):562. https://doi.org/10.3390/curroncol32100562
Chicago/Turabian StyleCapozzi, Lauren C., Chad Wagoner, Julia T. Daun, Lisa Murphy, Steven C. Nakoneshny, George J. Francis, Joseph C. Dort, Khara Sauro, and S. Nicole Culos-Reed. 2025. "Feasibility of a Physiatry Assessment Clinic to Address Physical Impairment in Head and Neck Cancer Patients Following Neck Resection and Free Flap Reconstruction" Current Oncology 32, no. 10: 562. https://doi.org/10.3390/curroncol32100562
APA StyleCapozzi, L. C., Wagoner, C., Daun, J. T., Murphy, L., Nakoneshny, S. C., Francis, G. J., Dort, J. C., Sauro, K., & Culos-Reed, S. N. (2025). Feasibility of a Physiatry Assessment Clinic to Address Physical Impairment in Head and Neck Cancer Patients Following Neck Resection and Free Flap Reconstruction. Current Oncology, 32(10), 562. https://doi.org/10.3390/curroncol32100562







