Examining a Genomic Test in Predicting Extended Endocrine Benefit and Recurrence Risk in a Diverse Breast Cancer Population
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCI | Breast cancer index |
| EET | Extended endocrine therapy |
| ER | Estrogen receptor |
| HR | Hormone receptor |
| PR | Progesterone receptor |
| SEER | Surveillance, Epidemiology, and End Results |
References
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.; Pan, H.; Godwin, J.; Gray, R.; Arriagada, R.; Raina, V.; Abraham, M.; Medeiros Alencar, V.H.; Badran, A.; Bonfill, X.; et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013, 381, 805–816. [Google Scholar] [CrossRef]
- Goss, P.E.; Ingle, J.N.; Pritchard, K.I.; Robert, N.J.; Muss, H.; Gralow, J.; Gelmon, K.; Whelan, T.; Strasser-Weippl, K.; Rubin, S.; et al. Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years. N. Engl. J. Med. 2016, 375, 209–219. [Google Scholar] [CrossRef]
- Sgroi, D.C.; Chapman, J.-A.W.; Badovinac-Crnjevic, T.; Zarella, E.; Binns, S.; Zhang, Y.; Schnabel, C.A.; Erlander, M.G.; Pritchard, K.I.; Han, L.; et al. Assessment of the prognostic and predictive utility of the Breast Cancer Index (BCI): An NCIC CTG MA.14 study. Breast Cancer Res. 2016, 18, 1. [Google Scholar] [CrossRef]
- Sgroi, D.C.; Sestak, I.; Cuzick, J.; Zhang, Y.; Schnabel, C.A.; Schroeder, B.; Erlander, M.G.; Dunbier, A.; Sidhu, K.; Lopez-Knowles, E.; et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: A prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol. 2013, 14, 1067–1076, Erratum in Lancet Oncol. 2018, 19, e184. [Google Scholar] [CrossRef]
- Zhang, Y.; Schnabel, C.A.; Schroeder, B.E.; Jerevall, P.-L.; Jankowitz, R.C.; Fornander, T.; Stål, O.; Brufsky, A.M.; Sgroi, D.; Erlander, M.G. Breast Cancer Index Identifies Early-Stage Estrogen Receptor–Positive Breast Cancer Patients at Risk for Early- and Late-Distant Recurrence. Clin. Cancer Res. 2013, 19, 4196–4205. [Google Scholar] [CrossRef] [PubMed]
- Sgroi, D.C.; Treuner, K.; Zhang, Y.; Piper, T.; Salunga, R.; Ahmed, I.; Doos, L.; Thornber, S.; Taylor, K.J.; Brachtel, E.; et al. Correlative studies of the Breast Cancer Index (HOXB13/IL17BR) and ER, PR, AR, AR/ER ratio and Ki67 for prediction of extended endocrine therapy benefit: A Trans-aTTom study. Breast Cancer Res. 2022, 24, 90. [Google Scholar] [CrossRef]
- Bartlett, J.M.; Sgroi, D.C.; Treuner, K.; Zhang, Y.; Piper, T.; Salunga, R.C.; Ahmed, I.; Doos, L.; Thornber, S.; Taylor, K.J.; et al. Breast Cancer Index Is a Predictive Biomarker of Treatment Benefit and Outcome from Extended Tamoxifen Therapy: Final Analysis of the Trans-aTTom Study. Clin. Cancer Res. 2022, 28, 1871–1880. [Google Scholar] [CrossRef]
- Zhang, Y.; Schroeder, B.E.; Jerevall, P.-L.; Ly, A.; Nolan, H.; Schnabel, C.A.; Sgroi, D.C. A Novel Breast Cancer Index for Prediction of Distant Recurrence in HR+ Early-Stage Breast Cancer with One to Three Positive Nodes. Clin. Cancer Res. 2017, 23, 7217–7224. [Google Scholar] [CrossRef]
- Foldi, J.; Tsagianni, A.; Salganik, M.; Schnabel, C.A.; Brufsky, A.; van Londen, G.J.; Pusztai, L.; Sanft, T. Persistence to extended adjuvant endocrine therapy following Breast Cancer Index (BCI) testing in women with early-stage hormone receptor-positive (HR +) breast cancer. BMC Cancer 2023, 23, 606. [Google Scholar] [CrossRef] [PubMed]
- Sanft, T.; Aktas, B.; Schroeder, B.; Bossuyt, V.; DiGiovanna, M.; Abu-Khalaf, M.; Chung, G.; Silber, A.; Hofstatter, E.; Mougalian, S.; et al. Prospective assessment of the decision-making impact of the Breast Cancer Index in recommending extended adjuvant endocrine therapy for patients with early-stage ER-positive breast cancer. Breast Cancer Res. Treat. 2015, 154, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Jankowitz, R.C.; Cooper, K.; Erlander, M.G.; Ma, X.-J.; Kesty, N.C.; Li, H.; Chivukula, M.; Brufsky, A. Prognostic utility of the breast cancer index and comparison to Adjuvant! Online in a clinical case series of early breast cancer. Breast Cancer Res. 2011, 13, R98. [Google Scholar] [CrossRef]
- Noordhoek, I.; Treuner, K.; Putter, H.; Zhang, Y.; Wong, J.; Kranenbarg, E.M.-K.; Carpentier, M.D.-D.; van de Velde, C.J.; Schnabel, C.A.; Liefers, G.-J. Breast Cancer Index Predicts Extended Endocrine Benefit to Individualize Selection of Patients with HR+ Early-stage Breast Cancer for 10 Years of Endocrine Therapy. Clin. Cancer Res. 2021, 27, 311–319. [Google Scholar] [CrossRef]
- Ma, X.-J.; Salunga, R.; Dahiya, S.; Wang, W.; Carney, E.; Durbecq, V.; Harris, A.; Goss, P.; Sotiriou, C.; Erlander, M.; et al. A Five-Gene Molecular Grade Index and HOXB13:IL17BR Are Complementary Prognostic Factors in Early Stage Breast Cancer. Clin. Cancer Res. 2008, 14, 2601–2608. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.N.; Ismaila, N.; McShane, L.M.; Andre, F.; Collyar, D.E.; Gonzalez-Angulo, A.M.; Hammond, E.H.; Kuderer, N.M.; Liu, M.C.; Mennel, R.G.; et al. Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women with Early-Stage Invasive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 1134–1150. [Google Scholar] [CrossRef] [PubMed]
- Regan, M.M.; Neven, P.; Giobbie-Hurder, A.; Goldhirsch, A.; Ejlertsen, B.; Mauriac, L.; Forbes, J.F.; Smith, I.; Láng, I.; Wardley, A.; et al. Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: The BIG 1-98 randomised clinical trial at 8·1 years median follow-up. Lancet Oncol. 2011, 12, 1101–1108. [Google Scholar] [CrossRef]
- Solanki, P.A.; Ko, N.Y.; Qato, D.M.; Calip, G.S. Risk of cancer-specific, cardiovascular, and all-cause mortality among Asian and Pacific Islander breast cancer survivors in the United States, 1991–2011. SpringerPlus 2016, 5, 82. [Google Scholar] [CrossRef]
- Yu, A.Y.L.; Thomas, S.M.; DiLalla, G.D.; Greenup, R.A.; Hwang, E.S.; Hyslop, T.; Menendez, C.S.; Plichta, J.K.; Tolnitch, L.A.; Fayanju, O.M. Disease characteristics and mortality among Asian women with breast cancer. Cancer 2021, 128, 1024–1037. [Google Scholar] [CrossRef]
- Cancer at a Glance 2014–2018, Hawai‘i Tumor Registry. 2022. Available online: https://www.uhcancercenter.org/research/shared-resources/hawaii-tumor-registry (accessed on 4 September 2025).
- Rao, M.; Khan, A.J.; Moran, M.S.; Hirshfield, K.M.; Ganesan, S.; Haffty, B.G.; Goyal, S. Clinicopathologic Presentation of Asian-Indian American (AIA) Women with Stage 0, I & II Breast Cancer. J. Immigr. Minor. Health 2010, 13, 42–48. [Google Scholar] [CrossRef]
- Kakarala, M.; Rozek, L.; Cote, M.; Liyanage, S.; Brenner, D.E. Breast cancer histology and receptor status characterization in Asian Indian and Pakistani women in the U.S.—A SEER analysis. BMC Cancer 2010, 10, 191. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Pagan, E.; Viale, G.; Bagnardi, V.; Peruzzotti, G.; De Pas, T.; Bianco, N.; Graffeo, R.; Rocco, E.G.; et al. Endocrine-responsive lobular carcinoma of the breast: Features associated with risk of late distant recurrence. Breast Cancer Res. 2019, 21, 153. [Google Scholar] [CrossRef]
- Pestalozzi, B.C.; Zahrieh, D.; Mallon, E.; Gusterson, B.A.; Price, K.N.; Gelber, R.D.; Holmberg, S.B.; Lindtner, J.; Snyder, R.; Thürlimann, B.; et al. Distinct Clinical and Prognostic Features of Infiltrating Lobular Carcinoma of the Breast: Combined Results of 15 International Breast Cancer Study Group Clinical Trials. J. Clin. Oncol. 2008, 26, 3006–3014. [Google Scholar] [CrossRef]
- Nunes, R.; Sella, T.; Treuner, K.; Atkinson, J.M.; Wong, J.; Zhang, Y.; Exman, P.; Dabbs, D.; Richardson, A.L.; Schnabel, C.A.; et al. Prognostic Utility of Breast Cancer Index to Stratify Distant Recurrence Risk in Invasive Lobular Carcinoma. Clin. Cancer Res. 2021, 27, 5688–5696. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Kelly, E.D.; Abraham, J.; Kruse, M. Invasive lobular breast cancer: A review of pathogenesis, diagnosis, management, and future directions of early stage disease. Semin. Oncol. 2019, 46, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Batra, H.; Mouabbi, J.A.; Ding, Q.; Sahin, A.A.; Raso, M.G. Lobular Carcinoma of the Breast: A Comprehensive Review with Translational Insights. Cancers 2023, 15, 5491. [Google Scholar] [CrossRef] [PubMed]
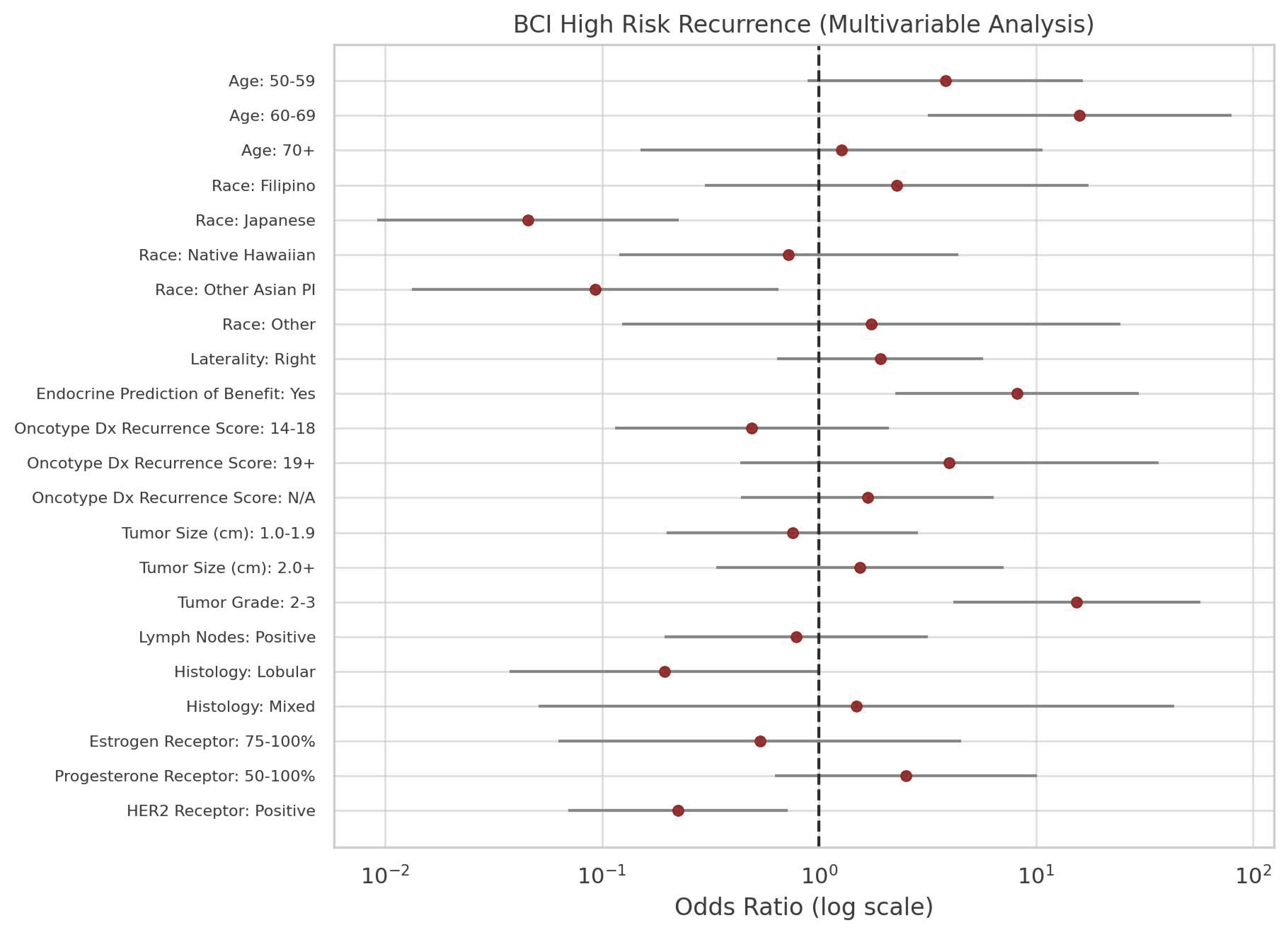
| Variable | Total (n = 159) | Multivariable (EP YES) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Col% | % | OR | LCL | UCL | p | ||
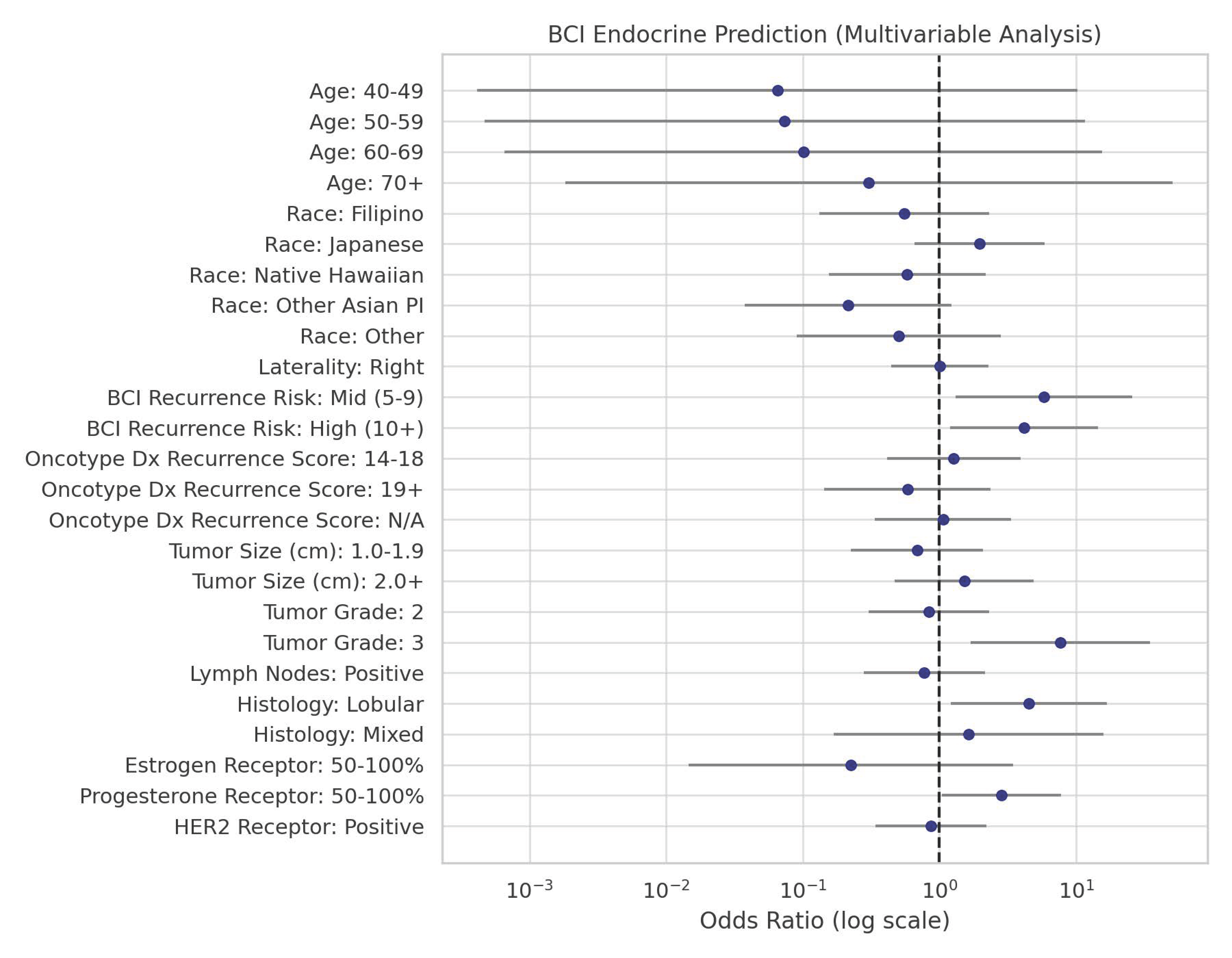
| Age | 30–39 | 4 | 2.5 | 75.0 | 1.00 | |||
| 40–49 | 40 | 25.2 | 16.3 | 0.07 | 0.00 | 10.27 | 0.29 | |
| 50–59 | 52 | 32.7 | 18.1 | 0.07 | 0.00 | 11.58 | 0.31 | |
| 60–69 | 47 | 29.6 | 23.2 | 0.10 | 0.00 | 15.55 | 0.37 | |
| 70+ | 16 | 10.1 | 47.7 | 0.30 | 0.00 | 51.03 | 0.65 | |
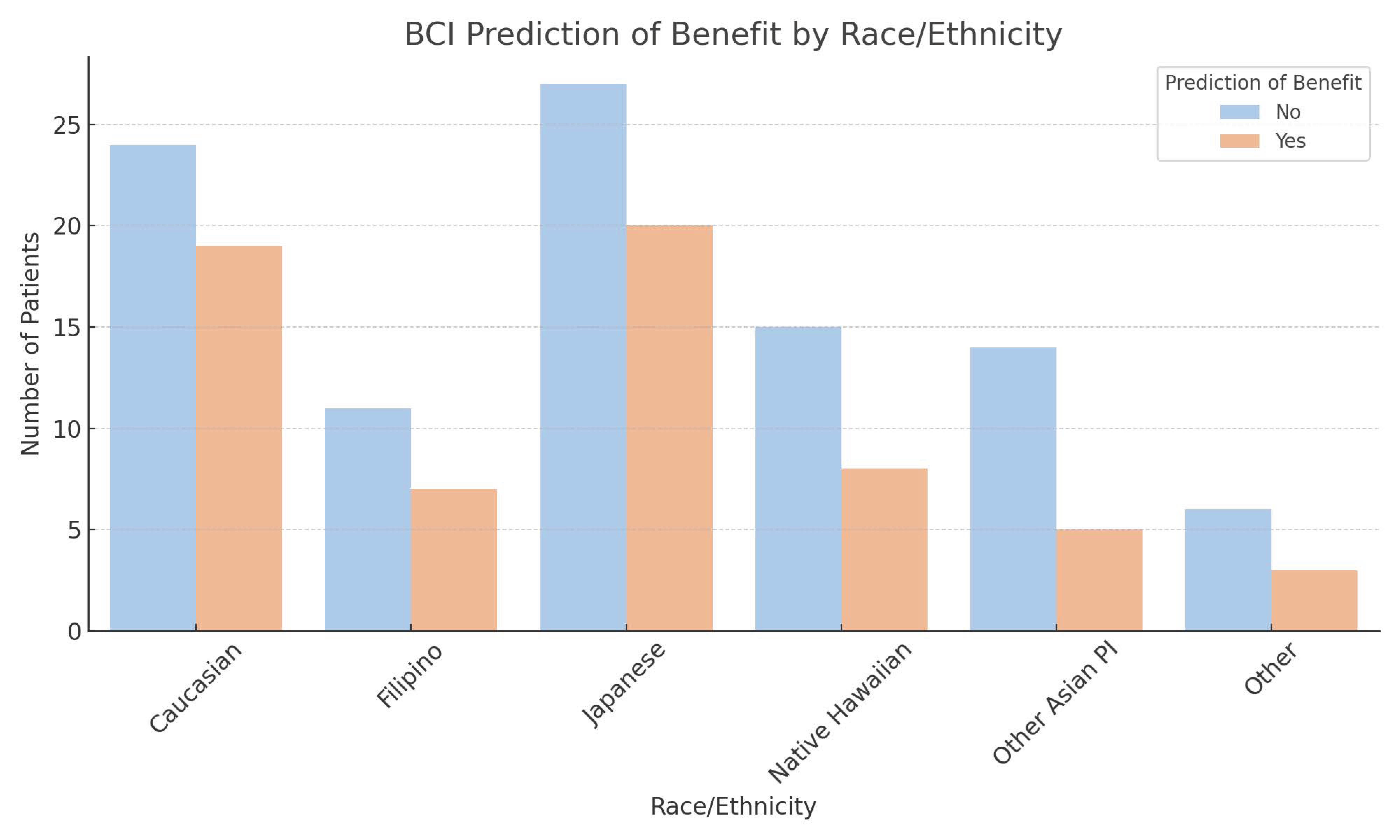
| Race | Caucasian | 43 | 27.0 | 44.2 | 1.00 | |||
| Filipino | 18 | 11.3 | 30.5 | 0.55 | 0.13 | 2.32 | 0.42 | |
| Japanese | 47 | 29.6 | 60.8 | 1.96 | 0.65 | 5.86 | 0.23 | |
| Native Hawaiian | 23 | 14.5 | 31.5 | 0.58 | 0.16 | 2.17 | 0.42 | |
| Other Asian PI | 19 | 11.9 | 14.5 | 0.21 | 0.04 | 1.22 | 0.08 | |
| Other | 9 | 5.7 | 28.5 | 0.50 | 0.09 | 2.82 | 0.44 | |
| Laterality | Left | 77 | 48.4 | 40.3 | 1.00 | |||
| Right | 82 | 51.6 | 40.4 | 1.01 | 0.45 | 2.28 | 0.99 | |
| BCI Recurrence Risk | Low (0–4) | 48 | 30.2 | 18.8 | 1.00 | |||
| Mid (5–9) | 24 | 15.1 | 57.4 | 5.84 | 1.32 | 25.85 | 0.02 | |
| High (10+) | 87 | 54.7 | 49.0 | 4.16 | 1.19 | 14.46 | 0.03 | |
| Recurrence Score | 0–13 | 40 | 25.2 | 32.5 | 1.00 | |||
| 14–18 | 37 | 23.3 | 38.0 | 1.27 | 0.41 | 3.93 | 0.67 | |
| 19+ | 28 | 17.6 | 21.9 | 0.58 | 0.14 | 2.38 | 0.45 | |
| N/A | 54 | 34.0 | 33.8 | 1.06 | 0.34 | 3.33 | 0.92 | |
| Tumor Size (cm) | 0.0–0.9 | 38 | 23.9 | 34.2 | 1.00 | |||
| 1.0–1.9 | 70 | 44.0 | 26.3 | 0.69 | 0.23 | 2.09 | 0.51 | |
| 2.0+ | 51 | 32.1 | 44.1 | 1.52 | 0.47 | 4.90 | 0.49 | |
| Tumor Grade | 1 | 55 | 34.6 | 27.3 | 1.00 | |||
| 2 | 74 | 46.5 | 23.9 | 0.84 | 0.30 | 2.32 | 0.73 | |
| 3 | 30 | 18.9 | 74.1 | 7.64 | 1.68 | 34.68 | 0.008 | |
| Lymph Nodes | Negative | 122 | 76.7 | 41.0 | 1.00 | |||
| Positive | 37 | 23.3 | 35.0 | 0.77 | 0.28 | 2.15 | 0.62 | |
| Histology | Ductal | 138 | 86.8 | 37.0 | 1.00 | |||
| Lobular | 17 | 10.7 | 72.6 | 4.52 | 1.21 | 16.85 | 0.02 | |
| Mixed | 4 | 2.5 | 48.9 | 1.63 | 0.17 | 15.82 | 0.67 | |
| ER | 0–49 | 5 | 3.1 | 80.0 | 1.00 | |||
| 50–100 | 154 | 96.9 | 47.3 | 0.22 | 0.01 | 3.48 | 0.29 | |
| PR | 0–49 | 47 | 29.6 | 36.2 | 1.00 | |||
| 50–100 | 112 | 70.4 | 61.7 | 2.85 | 1.04 | 7.79 | 0.04 | |
| HER2 | Negative | 96 | 60.4 | 38.5 | 1.00 | |||
| Positive | 63 | 39.6 | 35.2 | 0.86 | 0.34 | 2.20 | 0.76 | |
| Variable | Low | Mid | High | ||||||
|---|---|---|---|---|---|---|---|---|---|
| % | OR | % | OR | p | % | OR | p | ||
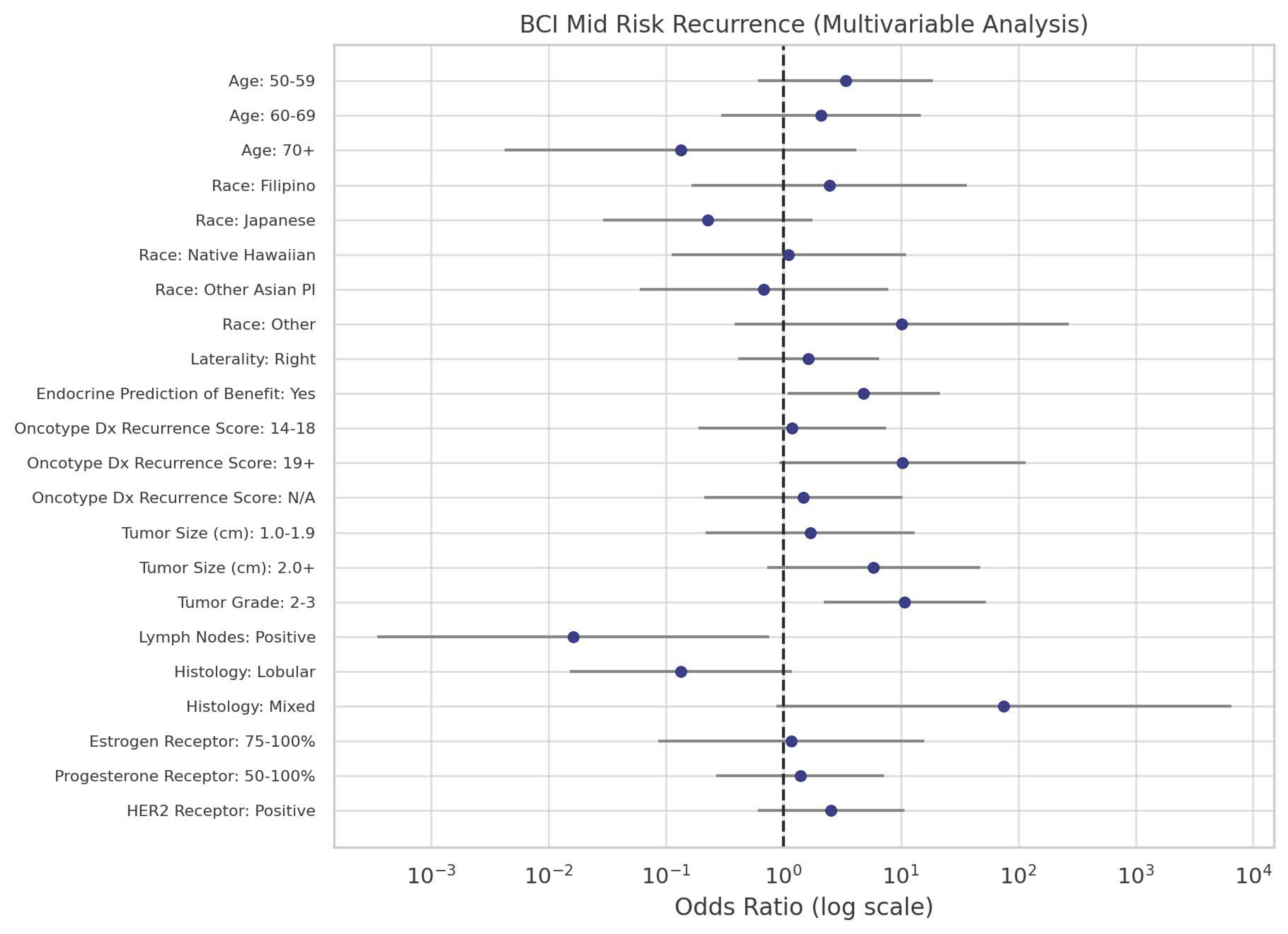
| Age | 30–49 | 38.6 | 1.00 | 20.5 | 1.00 | 40.9 | 1.00 | ||
| 50–59 | 14.6 | 1.00 | 26.1 | 3.37 | 0.16 | 59.3 | 3.83 | 0.07 | |
| 60–69 | 5.3 | 1.00 | 5.8 | 2.09 | 0.46 | 88.9 | 15.92 | 0.0008 | |
| 70+ | 41.4 | 1.00 | 2.9 | 0.13 | 0.25 | 55.7 | 1.27 | 0.83 | |
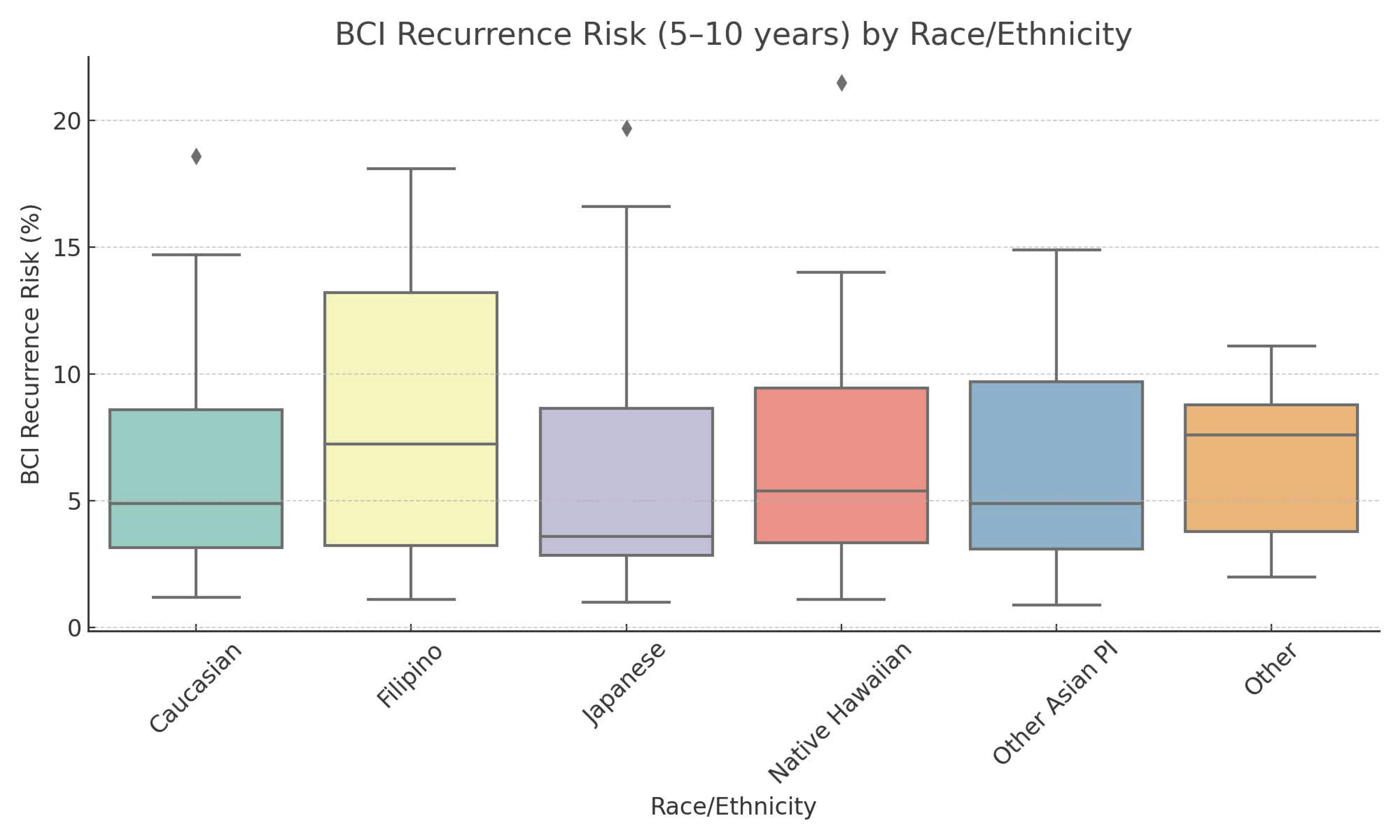
| Race | Caucasian | 20.9 | 1.00 | 9.3 | 1.00 | 69.8 | 1.00 | ||
| Filipino | 10.3 | 1.00 | 11.3 | 2.46 | 0.51 | 78.4 | 2.28 | 0.43 | |
| Japanese | 79.9 | 1.00 | 8.1 | 0.23 | 0.16 | 12.1 | 0.05 | 0.0002 | |
| Native Hawaiian | 25.6 | 1.00 | 12.6 | 1.11 | 0.93 | 61.8 | 0.73 | 0.73 | |
| Other Asian PI | 62.0 | 1.00 | 18.8 | 0.68 | 0.76 | 19.2 | 0.09 | 0.02 | |
| Other | 8.8 | 1.00 | 39.9 | 10.15 | 0.17 | 51.3 | 1.74 | 0.68 | |
| Laterality | Left | 36.4 | 1.00 | 14.3 | 1.00 | 49.4 | 1.00 | ||
| Right | 23.6 | 1.00 | 15.2 | 1.64 | 0.49 | 61.3 | 1.92 | 0.24 | |
| Prediction | No | 40.2 | 1.00 | 14.4 | 1.00 | 45.4 | 1.00 | ||
| Yes | 8.3 | 1.00 | 14.4 | 4.81 | 0.04 | 77.3 | 8.21 | 0.001 | |
| Recurrence Score | 0–13 | 35.0 | 1.00 | 10.0 | 1.00 | 55.0 | 1.00 | ||
| 14–18 | 47.4 | 1.00 | 16.1 | 1.19 | 0.85 | 36.5 | 0.49 | 0.34 | |
| 19+ | 9.8 | 1.00 | 28.8 | 10.29 | 0.06 | 61.4 | 3.99 | 0.22 | |
| N/A | 24.7 | 1.00 | 10.4 | 1.48 | 0.69 | 64.9 | 1.67 | 0.45 | |
| Tumor Size (cm) | 0.0–0.9 | 28.9 | 1.00 | 7.9 | 1.00 | 63.2 | 1.00 | ||
| 1.0–1.9 | 32.1 | 1.00 | 14.9 | 1.69 | 0.61 | 53.0 | 0.76 | 0.68 | |
| 2.0 + | 16.8 | 1.00 | 26.8 | 5.87 | 0.10 | 56.4 | 1.54 | 0.58 | |
| Tumor Grade | 1 | 56.4 | 1.00 | 9.1 | 1.00 | 34.5 | 1.00 | ||
| 2–3 | 8.2 | 1.00 | 14.3 | 10.79 | 0.003 | 77.6 | 15.45 | <0.0001 | |
| Lymph Nodes | Negative | 28.7 | 1.00 | 18.9 | 1.00 | 52.5 | 1.00 | ||
| Positive | 40.9 | 1.00 | 0.4 | 0.02 | 0.04 | 58.7 | 0.78 | 0.73 | |
| Histology | Ductal | 29.7 | 1.00 | 15.2 | 1.00 | 55.1 | 1.00 | ||
| Lobular | 70.0 | 1.00 | 4.8 | 0.13 | 0.07 | 25.2 | 0.19 | 0.05 | |
| Mixed | 2.4 | 1.00 | 91.1 | 75.33 | 0.06 | 6.5 | 1.49 | 0.82 | |
| ER | 0–74 | 14.3 | 1.00 | 14.3 | 1.00 | 71.4 | 1.00 | ||
| 75–100 | 20.7 | 1.00 | 24.1 | 1.16 | 0.91 | 55.2 | 0.53 | 0.57 | |
| PR | 0–49 | 25.5 | 1.00 | 19.1 | 1.00 | 55.3 | 1.00 | ||
| 50–100 | 13.3 | 1.00 | 14.0 | 1.39 | 0.69 | 72.7 | 2.51 | 0.19 | |
| HER2 | Negative | 25.0 | 1.00 | 7.3 | 1.00 | 67.7 | 1.00 | ||
| Positive | 42.5 | 1.00 | 31.6 | 2.55 | 0.20 | 25.8 | 0.22 | 0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.H.; Siu-Li, N.; Pagano, I.; Fukui, J.A. Examining a Genomic Test in Predicting Extended Endocrine Benefit and Recurrence Risk in a Diverse Breast Cancer Population. Curr. Oncol. 2025, 32, 537. https://doi.org/10.3390/curroncol32100537
Lee HH, Siu-Li N, Pagano I, Fukui JA. Examining a Genomic Test in Predicting Extended Endocrine Benefit and Recurrence Risk in a Diverse Breast Cancer Population. Current Oncology. 2025; 32(10):537. https://doi.org/10.3390/curroncol32100537
Chicago/Turabian StyleLee, Ho Hyun, Nicholas Siu-Li, Ian Pagano, and Jami Aya Fukui. 2025. "Examining a Genomic Test in Predicting Extended Endocrine Benefit and Recurrence Risk in a Diverse Breast Cancer Population" Current Oncology 32, no. 10: 537. https://doi.org/10.3390/curroncol32100537
APA StyleLee, H. H., Siu-Li, N., Pagano, I., & Fukui, J. A. (2025). Examining a Genomic Test in Predicting Extended Endocrine Benefit and Recurrence Risk in a Diverse Breast Cancer Population. Current Oncology, 32(10), 537. https://doi.org/10.3390/curroncol32100537





