Mistletoe Extracts during the Oncological Perioperative Period: A Systematic Review and Meta-Analysis of Human Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Included Studies
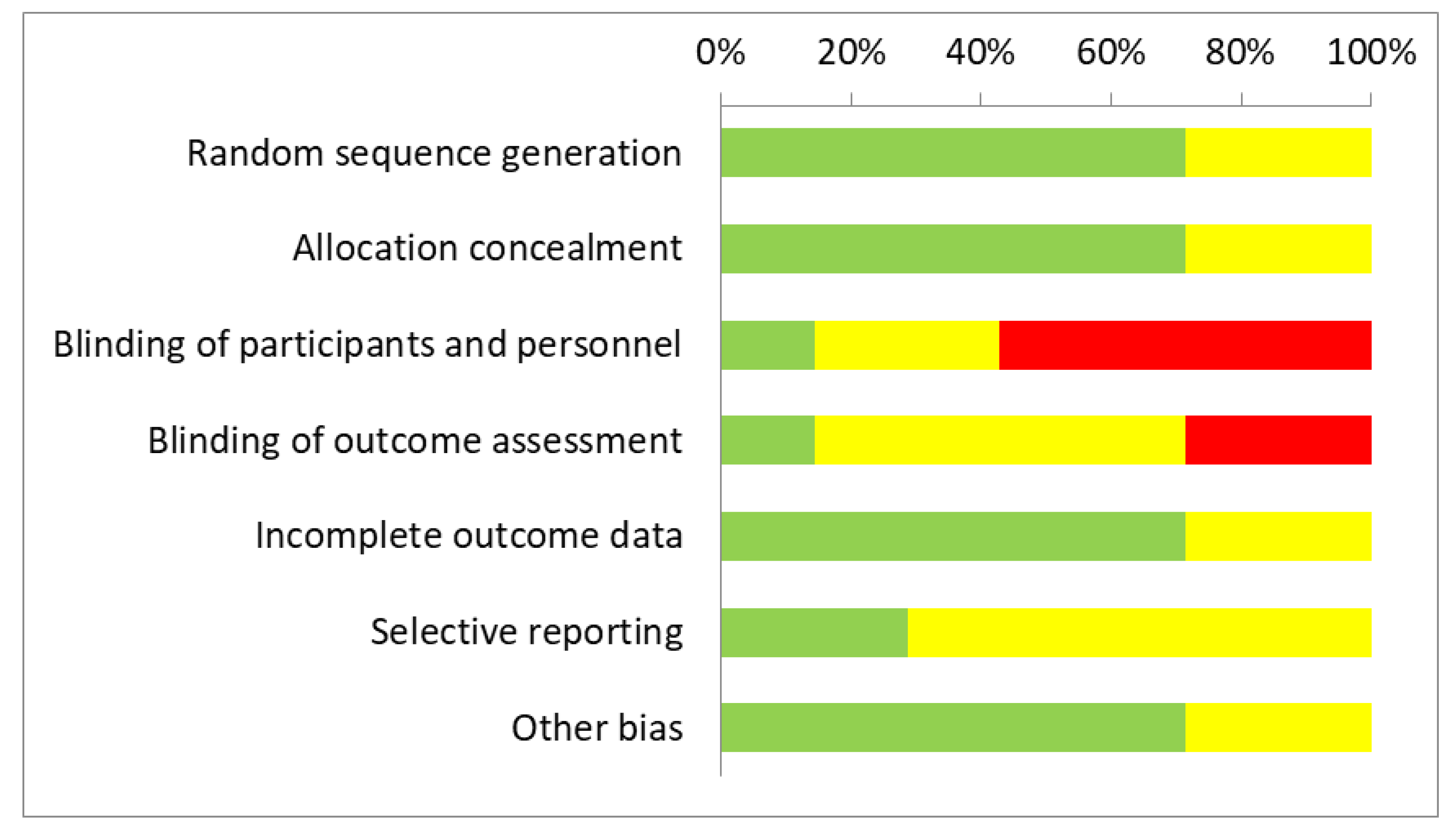
3.2. Methodological Quality Assessment and Risk of Bias
3.3. Results of the RCTs by Comparators
- Mistletoe versus no added treatments;
- Mistletoe versus etoposide;
- Mistletoe versus BCG.
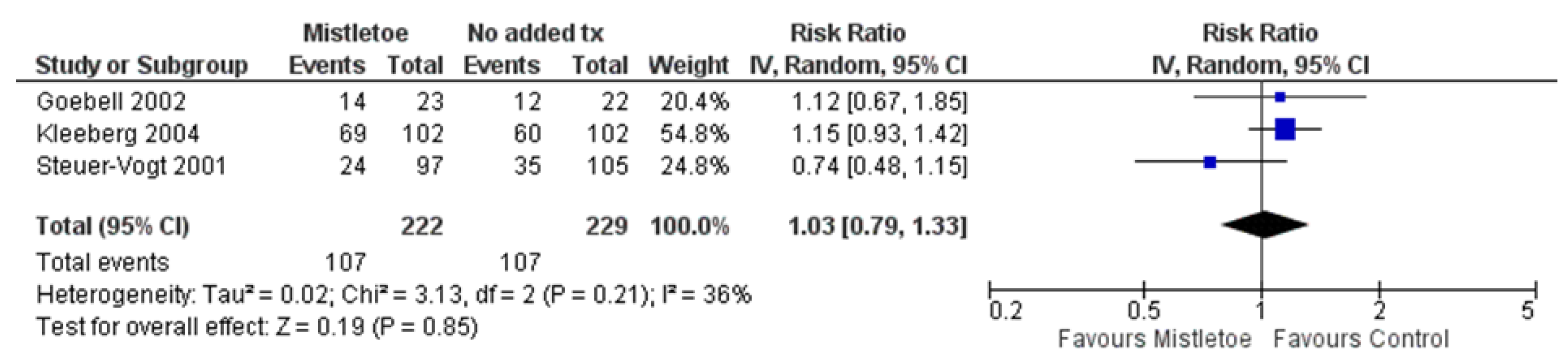
3.3.1. Mistletoe Versus No Added Treatments (5 RCTs)
Primary Outcomes (First Comparison)
- Mortality, Recurrence and Metastasis
Secondary Outcomes (First Comparison)
- Quality of Life
- Immune Cells and Inflammatory Markers
- Blood Loss
- Other Complications and Adverse Events
3.3.2. Mistletoe versus Etoposide (Second Comparison (1 RCT)
Primary Outcomes (Second Comparison)
Secondary Outcomes (Second Comparison)
- Quality of Life
- Immune Cells and Inflammatory Markers
- Other Complications and Adverse Events (RCTs)
3.3.3. Mistletoe versus BCG (1 RCT) (Third Comparison)
Primary Outcomes (Third Comparison)
- Recurrences and Metastasis
Secondary Outcomes (Third Comparison)
- Other Complications and Adverse Events (third comparison)
3.4. Results of Non-Randomized and Observational Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kienle, G.S.; Grugel, R.; Kiene, H. Safety of higher dosages of Viscum album L. in animals and humans—Systematic review of immune changes and safety parameters. BMC Complement. Altern. Med. 2011, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Kienle, G.S.; Kiene, H. Review article: Influence of Viscum album L. (European mistletoe) extracts on quality of life in cancer patients: A systematic review of controlled clinical studies. Integr. Cancer Ther. 2010, 9, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Mistletoe—Professional Monograph; Unpublished Data; The Center of Health Innovation: Ottawa, ON, Canada, 2022; Available online: https://thechi.ca/research/ (accessed on 17 July 2023).
- Szurpnicka, A.; Kowalczuk, A.; Szterk, A. Biological activity of mistletoe: In vitro and in vivo studies and mechanisms of action. Arch. Pharmacal Res. 2020, 43, 593–629. [Google Scholar] [CrossRef] [PubMed]
- Bussing, A. Mistletoe: The Genus Viscum; Hardwood Academic Publishers: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Lev, E.; Ephraim, M.; Ben-Arye, E. European and Oriental mistletoe: From mythology to contemporary integrative cancer care. Eur. J. Integr. Med. 2011, 3, e133–e137. [Google Scholar] [CrossRef]
- Steiner, R. Geisteswissenschaft und Medizin; Rudolf Steiner Verlag: Dornach, Switzerland, 1985. [Google Scholar]
- Ostermann, T.; Raak, C.; Büssing, A. Survival of cancer patients treated with mistletoe extract (Iscador): A systematic literature review. BMC Cancer 2009, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Ben-Arye, E.; Attias, S.; Tadmor, T.; Schiff, E. Herbs in hemato-oncological care: An evidence-based review of data on efficacy, safety, and drug interactions. Leuk. Lymphoma 2010, 51, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Horneber, M.A.; Bueschel, G.; Huber, R.; Linde, K.; Rostock, M. Mistletoe therapy in oncology. Cochrane Database Syst. Rev. 2008, 2008, CD003297. [Google Scholar] [CrossRef]
- Büssing, A. Induction of apoptosis by the mistletoe lectins: A review on the mechanisms of cytotoxicity mediated by Viscum album L. Apoptosis 1996, 1, 25–32. [Google Scholar] [CrossRef]
- Stein, G.M.; Schaller, G.; Pfüller, U.; Schietzel, M.; Büssing, A. Thionins from Viscum album L: Influence of the viscotoxins on the activation of granulocytes. Anticancer Res. 1999, 19, 1037–1042. [Google Scholar]
- Gabius, H.J.; Gabius, S.; Joshi, S.S.; Koch, B.; Schroeder, M.; Manzke, W.M.; Westerhausen, M. From ill-defined extracts to the immunomodulatory lectin: Will there be a reason for oncological application of mistletoe? Planta Med. 1994, 60, 2–7. [Google Scholar] [CrossRef]
- Samtleben, R.; Hajto, T.; Hostanska, K.; Wagner, H. Mistletoe lectins as immunostimulants (chemistry, pharmacology and clinic). In Immunomodulatory Agents from Plants; Wagner, H., Ed.; Birkhäuser: Basel, Switzerland, 1999; pp. 223–241. [Google Scholar]
- Enesel, M.B.; Acalovschi, I.; Grosu, V.; Sbarcea, A.; Rusu, C.; Dobre, A.; Weiss, T.; Zarkovic, N. Perioperative application of the Viscum album extract Isorel in digestive tract cancer patients. Anticancer Res. 2005, 25, 4583–4590. [Google Scholar] [PubMed]
- PDQ Integrative, Alternative, and Complementary Therapies Editorial Board. Mistletoe Extracts (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries; National Cancer Institute (US): Bethesda, MD, USA, 2002.
- Hajto, T.; Hostanska, K.; Gabius, H.J. Modulatory potency of the beta-galactoside-specific lectin from mistletoe extract (Iscador) on the host defense system in vivo in rabbits and patients. Cancer Res. 1989, 49, 4803–4808. [Google Scholar] [PubMed]
- Beuth, J.; Stoffel, B.; Ko, H.L.; Buss, G.; Tunggal, L.; Pulverer, G. Immunoactive effects of various mistletoe lectin-1 dosages in mammary carcinoma patients. Arzneimittelforschung 1995, 45, 505–507. [Google Scholar] [PubMed]
- Park, Y.K.; Do, Y.R.; Jang, B.C. Apoptosis of K562 leukemia cells by Abnobaviscum F®, a European mistletoe extract. Oncol. Rep. 2012, 28, 2227–2232. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wu, M.; Wang, J.; Ren, D.; Zhao, J.; Yang, G. 1,7-Bis(4-hydroxyphenyl)-1,4-heptadien-3-one induces lung cancer cell apoptosis via the PI3K/Akt and ERK1/2 pathways. J. Cell. Physiol. 2019, 234, 6336–6349. [Google Scholar] [CrossRef] [PubMed]
- Janssen, O.; Scheffler, A.; Kabelitz, D. In vitro effects of mistletoe extracts and mistletoe lectins. Cytotoxicity towards tumor cells due to the induction of programmed cell death (apoptosis). Arzneimittelforschung 1993, 43, 1221–1227. [Google Scholar] [PubMed]
- Mishra, R.; Sharma, S.; Sharma, R.S.; Singh, S.; Sardesai, M.M.; Sharma, S.; Mishra, V. Viscum articulatum Burm. f. aqueous extract exerts antiproliferative effect and induces cell cycle arrest and apoptosis in leukemia cells. J. Ethnopharmacol. 2018, 219, 91–102. [Google Scholar] [CrossRef]
- Elluru, S.R.; Duong Van Huyen, J.P.; Delignat, S.; Prost, F.; Heudes, D.; Kazatchkine, M.D.; Friboulet, A.; Kaveri, S.V. Antiangiogenic properties of Viscum album extracts are associated with endothelial cytotoxicity. Anticancer Res. 2009, 29, 2945–2950. [Google Scholar]
- Olsnes, S.; Stirpe, F.; Sandvig, K.; Pihl, A. Isolation and characterization of viscumin, a toxic lectin from Viscum album L. (mistletoe). J. Biol. Chem. 1982, 257, 13263–13270. [Google Scholar] [CrossRef]
- Stirpe, F.; Sandvig, K.; Olsnes, S.; Pihl, A. Action of viscumin, a toxic lectin from mistletoe, on cells in culture. J. Biol. Chem. 1982, 257, 13271–13277. [Google Scholar] [CrossRef]
- Büssing, A.; Schaller, G.; Pfüller, U. Generation of reactive oxygen intermediates (ROI) by the thionins from Viscum album L. Anticancer Res. 1998, 18, 4291–4296. [Google Scholar] [PubMed]
- Elluru, S.R.; Duong van Huyen, J.P.; Delignat, S.; Kazatchkine, M.D.; Friboulet, A.; Kaveri, S.V.; Bayry, J. Induction of maturation and activation of human dendritic cells: A mechanism underlying the beneficial effect of Viscum album as complimentary therapy in cancer. BMC Cancer 2008, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Hwang, Y.H.; Kang, K.Y.; Kim, I.; Kim, J.B.; Park, J.H.; Yoo, Y.C.; Yee, S.T. Enhanced dendritic cell maturation by the B-chain of Korean mistletoe lectin (KML-B), a novel TLR4 agonist. Int. Immunopharmacol. 2014, 21, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Steinborn, C.; Klemd, A.M.; Sanchez-Campillo, A.S.; Rieger, S.; Scheffen, M.; Sauer, B.; Garcia-Käufer, M.; Urech, K.; Follo, M.; Ücker, A.; et al. Viscum album neutralizes tumor-induced immunosuppression in a human in vitro cell model. PLoS ONE 2017, 12, e0181553. [Google Scholar] [CrossRef] [PubMed]
- Podlech, O.; Harter, P.N.; Mittelbronn, M.; Pöschel, S.; Naumann, U. Fermented mistletoe extract as a multimodal antitumoral agent in gliomas. Evid.-Based Complement. Altern. Med. 2012, 2012, 501796. [Google Scholar] [CrossRef] [PubMed]
- Hajto, T. Immunomodulatory effects of iscador: A Viscum album preparation. Oncology 1986, 43 (Suppl. S1), 51–65. [Google Scholar] [CrossRef] [PubMed]
- Hajto, T.; Hostanska, K.; Frei, K.; Rordorf, C.; Gabius, H.J. Increased secretion of tumor necrosis factors alpha, interleukin 1, and interleukin 6 by human mononuclear cells exposed to beta-galactoside-specific lectin from clinically applied mistletoe extract. Cancer Res. 1990, 50, 3322–3326. [Google Scholar] [PubMed]
- Kohl, B.A.; Deutschman, C.S. The inflammatory response to surgery and trauma. Curr. Opin. Crit. Care 2006, 12, 325–332. [Google Scholar] [CrossRef]
- Aller, M.A.; Arias, J.L.; Nava, M.P.; Arias, J. Posttraumatic inflammation is a complex response based on the pathological expression of the nervous, immune, and endocrine functional systems. Exp. Biol. Med. 2004, 229, 170–181. [Google Scholar] [CrossRef]
- Arias, J.I.; Aller, M.A.; Arias, J. Surgical inflammation: A pathophysiological rainbow. J. Transl. Med. 2009, 7, 19. [Google Scholar] [CrossRef]
- Dabrowska, A.M.; Slotwinski, R. The immune response to surgery and infection. Cent. Eur. J. Immunol. 2014, 39, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef]
- Bone, R.C.; Grodzin, C.J.; Balk, R.A. Sepsis: A new hypothesis for pathogenesis of the disease process. Chest 1997, 112, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, F.; Pape, H.C.; Krettek, C. The importance of cytokines in the posttraumatic inflammatory reaction. Unfallchirurg 2005, 108, 793–803. [Google Scholar] [CrossRef]
- Bonamin, L.V.; de Carvalho, A.C.; Waisse, S. Viscum album (L.) in experimental animal tumors: A meta-analysis. Exp. Ther. Med. 2017, 13, 2723–2740. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Sawada, S.; Yoshioka, I.; Ohashi, Y.; Matsuo, M.; Harimaya, Y.; Tsukada, K.; Saiki, I. Increased surgical stress promotes tumor metastasis. Surgery 2003, 133, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Glasner, A.; Avraham, R.; Rosenne, E.; Benish, M.; Zmora, O.; Shemer, S.; Meiboom, H.; Ben-Eliyahu, S. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J. Immunol. 2010, 184, 2449–2457. [Google Scholar] [CrossRef]
- McCrath, D.J.; Cerboni, E.; Frumento, R.J.; Hirsh, A.L.; Bennett-Guerrero, E. Thromboelastography maximum amplitude predicts postoperative thrombotic complications including myocardial infarction. Anesth. Analg. 2005, 100, 1576–1583. [Google Scholar] [CrossRef]
- Seth, R.; Tai, L.H.; Falls, T.; de Souza, C.T.; Bell, J.C.; Carrier, M.; Atkins, H.; Boushey, R.; Auer, R.A. Surgical stress promotes the development of cancer metastases by a coagulation-dependent mechanism involving natural killer cells in a murine model. Ann. Surg. 2013, 258, 158–168. [Google Scholar] [CrossRef]
- Tartter, P.I.; Steinberg, B.; Barron, D.M.; Martinelli, G. The prognostic significance of natural killer cytotoxicity in patients with colorectal cancer. Arch. Surg. 1987, 122, 1264–1268. [Google Scholar] [CrossRef]
- Tai, L.H.; de Souza, C.T.; Belanger, S.; Ly, L.; Alkayyal, A.A.; Zhang, J.; Rintoul, J.L.; Ananth, A.A.; Lam, T.; Breitbach, C.J.; et al. Preventing postoperative metastatic disease by inhibiting surgery-induced dysfunction in natural killer cells. Cancer Res. 2013, 73, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Shakhar, G.; Ben-Eliyahu, S. Potential prophylactic measures against postoperative immunosuppression: Could they reduce recurrence rates in oncological patients? Ann. Surg. Oncol. 2003, 10, 972–992. [Google Scholar] [CrossRef] [PubMed]
- Li, T.S.; Kaneda, Y.; Ueda, K.; Hamano, K.; Zempo, N.; Esato, K. The influence of tumour resection on angiostatin levels and tumour growth—An experimental study in tumour-bearing mice. Eur. J. Cancer 2001, 37, 2283–2288. [Google Scholar] [CrossRef] [PubMed]
- Kumara, H.M.; Feingold, D.; Kalady, M.; Dujovny, N.; Senagore, A.; Hyman, N.; Cekic, V.; Whelan, R.L. Colorectal resection is associated with persistent proangiogenic plasma protein changes: Postoperative plasma stimulates in vitro endothelial cell growth, migration, and invasion. Ann. Surg. 2009, 249, 973–977. [Google Scholar] [CrossRef] [PubMed]
- van der Bilt, J.D.; Borel Rinkes, I.H. Surgery and angiogenesis. Biochim. Biophys. Acta 2004, 1654, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.E.; Parikh, A.A. Assessment of the risk of antiangiogenic agents before and after surgery. Cancer Treat. Rev. 2018, 68, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Stöcker, A.; Mehnert-Theuerkauf, A.; Hinz, A.; Ernst, J. Utilization of complementary and alternative medicine (CAM) by women with breast cancer or gynecological cancer. PLoS ONE 2023, 18, e0285718. [Google Scholar] [CrossRef]
- Huebner, J.; Muenstedt, K.; Prott, F.J.; Stoll, C.; Micke, O.; Buentzel, J.; Muecke, R.; Senf, B. Online survey of patients with breast cancer on complementary and alternative medicine. Breast Care 2014, 9, 60–63. [Google Scholar] [CrossRef]
- Grimm, D.A.-O.; Voiss, P.; Paepke, D.; Dietmaier, J.; Cramer, H.; Kümmel, S.; Beckmann, M.W.; Woelber, L.; Schmalfeldt, B.; Freitag, U.; et al. Gynecologists’ attitudes toward and use of complementary and integrative medicine approaches: Results of a national survey in Germany. Arch. Gynecol. Obstet. 2021, 303, 967–980. [Google Scholar] [CrossRef]
- Bussing, A.; Raak, C.; Ostermann, T. Quality of life and related dimensions in cancer patients treated with mistletoe extract (iscador): A meta-analysis. Evid.-Based Complement. Altern. Med. 2012, 2012, 219402. [Google Scholar] [CrossRef]
- Beuth, J. Evidence-based complementary oncology: Innovative approaches to optimise standard therapy strategies. Anticancer Res. 2010, 30, 1767–1771. [Google Scholar] [PubMed]
- Cogo, E.; Papadogianis, P. Characteristics of 218 Recent Reviews on Natural Health Products in Integrative Cancer Care: A bibliometric analysis of trends in the human research literature. J. Orthomol. Med. 2018, 33, 1–13. [Google Scholar]
- Cogo, E.; Elsayed, M.; Liang, V.; Cooley, K.; Guerin, C.; Psihogios, A.; Papadogianis, P. Are Supplemental Branched-Chain Amino Acids Beneficial during the Oncological Peri-Operative Period: A Systematic Review and Meta-Analysis. Integr. Cancer Ther. 2021, 20, 1534735421997551. [Google Scholar] [CrossRef] [PubMed]
- Cogo, E.; Elsayed, M.; Liang, V.; Cooley, K.; Guerin, C.; Psihogios, A.; Papadogianis, P. Probiotics Evaluation in Oncological Surgery: A Systematic Review of 36 Randomized Controlled Trials Assessing 21 Diverse Formulations. Curr. Oncol. 2021, 28, 5192–5214. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A.E. Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2 (Updated February 2021); Cochrane: London, UK, 2021; Available online: www.training.cochrane.org/handbook (accessed on 1 September 2021).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Page, M.A.-O.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiol. 2016, 75, 40–46. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 September 2021).
- STATA, version 12; Computer Program; StataCorp LLC: College Station, TX, USA, 2011.
- CIOMS Working Group X. Evidence Synthesis and Meta-Analysis for Drug Safety: Report of CIOMS Working Group X; Council for International Organizations of Medical Sciences (CIOMS): Geneva, Switzerland, 2016; Available online: https://cioms.ch/publications/product/evidence-synthesis-and-meta-analysis-report-of-cioms-working-group-x/ (accessed on 1 September 2021).
- Goebell, P.J.; Otto, T.; Suhr, J.; Rübben, H. Evaluation of an unconventional treatment modality with mistletoe lectin to prevent recurrence of superficial bladder cancer: A randomized phase II trial. J. Urol. 2002, 168, 72–75. [Google Scholar] [CrossRef]
- Ibrahiem, E.; Hekal, I. UP-3.040: Mistletoe Extract as an Adjuvant Therapy after Resection of Superficial Bladder Cancer: Prospective Clinical Randomized Study. Urology 2009, 74, S306. [Google Scholar] [CrossRef]
- Kleeberg, U.R.; Suciu, S.; Bröcker, E.B.; Ruiter, D.J.; Chartier, C.; Liénard, D.; Marsden, J.; Schadendorf, D.; Eggermont, A.M. Final results of the EORTC 18871/DKG 80-1 randomised phase III trial. rIFN-alpha2b versus rIFN-gamma versus ISCADOR M versus observation after surgery in melanoma patients with either high-risk primary (thickness > 3 mm) or regional lymph node metastasis. Eur. J. Cancer 2004, 40, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Longhi, A.; Mariani, E.; Kuehn, J.J. A randomized study with adjuvant mistletoe versus oral Etoposide on post relapse disease-free survival in osteosarcoma patients. Eur. J. Integr. Med. 2009, 1, 27–33. [Google Scholar] [CrossRef]
- Longhi, A.; Reif, M.; Mariani, E.; Ferrari, S. A Randomized Study on Postrelapse Disease-Free Survival with Adjuvant Mistletoe versus Oral Etoposide in Osteosarcoma Patients. Evid.-Based Complement. Altern. Med. 2014, 2014, 210198. [Google Scholar] [CrossRef]
- Longhi, A.; Cesari, M.; Serra, M.; Mariani, E. Long-Term Follow-up of a Randomized Study of Oral Etoposide versus Viscum album Fermentatum Pini as Maintenance Therapy in Osteosarcoma Patients in Complete Surgical Remission after Second Relapse. Sarcoma 2020, 2020, 8260730. [Google Scholar] [CrossRef] [PubMed]
- Schink, M.; Tröger, W.; Dabidian, A.; Goyert, A.; Scheuerecker, H.; Meyer, J.; Fischer, I.U.; Glaser, F. Mistletoe extract reduces the surgical suppression of natural killer cell activity in cancer patients. a randomized phase III trial. Forsch. Komplementärmedizin 2007, 14, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Steuer-Vogt, M.K.; Bonkowsky, V.; Ambrosch, P.; Scholz, M.; Neiss, A.; Strutz, J.; Hennig, M.; Lenarz, T.; Arnold, W. The effect of an adjuvant mistletoe treatment programme in resected head and neck cancer patients: A randomised controlled clinical trial. Eur. J. Cancer 2001, 37, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Elsässer-Beile, U.; Leiber, C.; Wetterauer, U.; Bühler, P.; Wolf, P.; Lucht, M.; Mengs, U. Adjuvant intravesical treatment with a standardized mistletoe extract to prevent recurrence of superficial urinary bladder cancer. Anticancer Res. 2005, 25, 4733–4736. [Google Scholar]
- Augustin, M.; Bock, P.R.; Hanisch, J.; Karasmann, M.; Schneider, B. Safety and efficacy of the long-term adjuvant treatment of primary intermediate- to high-risk malignant melanoma (UICC/AJCC stage II and III) with a standardized fermented European mistletoe (Viscum album L.) extract. Results from a multicenter, comparative, epidemiological cohort study in Germany and Switzerland. Arzneimittelforschung 2005, 55, 38–49. [Google Scholar] [CrossRef]
- Büssing, A.; Bischof, M.; Hatzmann, W.; Bartsch, F.; Soto-Vera, D.; Fronk, E.M.; Gmeindl, M.; Stein, G.M. Prevention of surgery-induced suppression of granulocyte function by intravenous application of a fermented extract from Viscum album L. in breast cancer patients. Anticancer Res. 2005, 25, 4753–4757. [Google Scholar]
- Tröger, W.; Zdrale, Z.; Tišma, N.; Matijašević, M. Additional Therapy with a Mistletoe Product during Adjuvant Chemotherapy of Breast Cancer Patients Improves Quality of Life: An Open Randomized Clinical Pilot Trial. Evid.-Based Complement. Altern. Med. 2014, 2014, 430518. [Google Scholar] [CrossRef]
- Pelzer, F.; Tröger, W.; Nat, D.R. Complementary Treatment with Mistletoe Extracts During Chemotherapy: Safety, Neutropenia, Fever, and Quality of Life Assessed in a Randomized Study. J. Altern. Complement. Med. 2018, 24, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Yook, J.H.; Eisenbraun, J.; Kim, B.S.; Huber, R. Quality of life, immunomodulation and safety of adjuvant mistletoe treatment in patients with gastric carcinoma—A randomized, controlled pilot study. BMC Complement. Altern. Med. 2012, 12, 172. [Google Scholar] [CrossRef] [PubMed]
- Tröger, W.; Jezdić, S.; Zdrale, Z.; Tišma, N.; Hamre, H.J.; Matijašević, M. Quality of life and neutropenia in patients with early stage breast cancer: A randomized pilot study comparing additional treatment with mistletoe extract to chemotherapy alone. Breast Cancer 2009, 3, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Semiglazov, V.F.; Stepula, V.V.; Dudov, A.; Schnitker, J.; Mengs, U. Quality of life is improved in breast cancer patients by Standardised Mistletoe Extract PS76A2 during chemotherapy and follow-up: A randomised, placebo-controlled, double-blind, multicentre clinical trial. Anticancer Res. 2006, 26, 1519–1529. [Google Scholar] [PubMed]
- Bar-Sela, G.; Haim, N. Abnoba-viscum (mistletoe extract) in metastatic colorectal carcinoma resistant to 5-fluorouracil and leucovorin-based chemotherapy. Med. Oncol. 2004, 21, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Piao, B.K.; Wang, Y.X.; Xie, G.R.; Mansmann, U.; Matthes, H.; Beuth, J.; Lin, H.S. Impact of complementary mistletoe extract treatment on quality of life in breast, ovarian and non-small cell lung cancer patients. A prospective randomized controlled clinical trial. Anticancer Res. 2004, 24, 303–309. [Google Scholar] [PubMed]
- Tröger, W.; Galun, D.; Reif, M.; Schumann, A.; Stanković, N.; Milićević, M. Quality of life of patients with advanced pancreatic cancer during treatment with mistletoe: A randomized controlled trial. Dtsch. Ärztebl. Int. 2014, 111, 493–502. [Google Scholar] [CrossRef]
- Beuth, J.; Schneider, B.; Schierholz, J.M. Impact of complementary treatment of breast cancer patients with standardized mistletoe extract during aftercare: A controlled multicenter comparative epidemiological cohort study. Anticancer Res. 2008, 28, 523–527. [Google Scholar]
- Loef, M.; Walach, H. Quality of life in cancer patients treated with mistletoe: A systematic review and meta-analysis. BMC Complement. Med. Ther. 2020, 20, 227. [Google Scholar] [CrossRef]
- Mansky, P.J.; Wallerstedt, D.B.; Sannes, T.S.; Stagl, J.; Johnson, L.L.; Blackman, M.R.; Grem, J.L.; Swain, S.M.; Monahan, B.P. NCCAM/NCI Phase 1 Study of Mistletoe Extract and Gemcitabine in Patients with Advanced Solid Tumors. Evid.-Based Complement. Altern. Med. 2013, 2013, 964592. [Google Scholar] [CrossRef]
- Kienle, G.S.; Berrino, F.; Büssing, A.; Portalupi, E.; Rosenzweig, S.; Kiene, H. Mistletoe in cancer—A systematic review on controlled clinical trials. Eur. J. Med. Res. 2003, 8, 109–119. [Google Scholar] [PubMed]
- Kienle, G.S.; Kiene, H. Complementary cancer therapy: A systematic review of prospective clinical trials on anthroposophic mistletoe extracts. Eur. J. Med. Res. 2007, 12, 103–119. [Google Scholar] [PubMed]
- Freuding, M.; Keinki, C.; Kutschan, S.; Micke, O.; Buentzel, J.; Huebner, J. Mistletoe in oncological treatment: A systematic review: Part 2: Quality of life and toxicity of cancer treatment. J. Cancer Res. Clin. Oncol. 2019, 145, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Freuding, M.; Keinki, C.; Micke, O.; Buentzel, J.; Huebner, J. Mistletoe in oncological treatment: A systematic review: Part 1: Survival and safety. J. Cancer Res. Clin. Oncol. 2019, 145, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, T.; Appelbaum, S.; Poier, D.; Boehm, K.; Raak, C.; Büssing, A. A Systematic Review and Meta-Analysis on the Survival of Cancer Patients Treated with a Fermented Viscum album L. Extract (Iscador): An Update of Findings. Complement. Med. Res. 2020, 27, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Matthes, H.; Hofheinz, R.D.; Bar-Sela, G.; Galun, D.; Martin, D.; Huber, R.; Langhorts, J.; Matthiessen, P.F.; Schad, F. Letter to the editors of the Journal of Cancer Research and Clinical Oncology. J. Cancer Res. Clin. Oncol. 2019, 145, 2405–2407. [Google Scholar] [CrossRef] [PubMed]
- Matthes, H.; Thronicke, A.; Hofheinz, R.D.; Baars, E.; Martin, D.; Huber, R.; Breitkreuz, T.; Bar-Sela, G.; Galun, D.; Schad, F. Statement to an Insufficient Systematic Review on Viscum album L. Therapy. Evid.-Based Complement. Altern. Med. 2020, 2020, 7091039. [Google Scholar] [CrossRef]
- Huebner, J.; Freuding, M.; Keinki, C.; Micke, O.; Buentzel, J. Answer to the letter to the editors by Matthes and colleagues regarding our systematic reviews on mistletoe. J. Cancer Res. Clin. Oncol. 2019, 145, 2409–2410. [Google Scholar] [CrossRef]
- Ackerman, R.S.; Muncey, A.R.; Aldawoodi, N.N.; Kotha, R.; Getting, R.E.G. Cancer Immunotherapies: What the Perioperative Physician Needs to Know. Curr. Oncol. Rep. 2022, 24, 399–414. [Google Scholar] [CrossRef]
- Qiu, B.; Cai, K.; Chen, C.; Chen, J.; Chen, K.N.; Chen, Q.X.; Cheng, C.; Dai, T.Y.; Fan, J.; Fan, Z.; et al. Expert consensus on perioperative immunotherapy for local advanced non-small cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 3713–3736. [Google Scholar] [CrossRef]
- Bakos, O.; Lawson, C.; Rouleau, S.; Tai, L.H. Combining surgery and immunotherapy: Turning an immunosuppressive effect into a therapeutic opportunity. J. Immunother. Cancer 2018, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Matzner, P.; Sandbank, E.; Neeman, E.; Zmora, O.; Gottumukkala, V.; Ben-Eliyahu, S. Harnessing cancer immunotherapy during the unexploited immediate perioperative period. Nat. Rev. Clin. Oncol. 2020, 17, 313–326. [Google Scholar] [CrossRef] [PubMed]


| Study Author, Year (Study Period) | Country | Sample Size a | Funding | Surgical Period of Mistletoe Exposure b | Post-op Mistletoe Initiation Timing b | Cancer Types | Cancer Severity | Age (y, var.) | % Female | Adjunct tx (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Schink, 2007 (2002–2004) [74] | Germany | 32 | public | Intra-operative | NA | Colorectal cancer | primary and locally relapsed, stages II–IV | 71 (NR) | 45 | Antibiotic (NR) |
| Enesel, 2005 (NR) [15] | Romania | 70 | NR | Mixed periods | NA | Digestive tract cancers (51% colorectal) | NR | NR (NR) | 43 | NR |
| Kleeberg, 2004 (1988–2003) [70] c | 13 countries: Germany; France; Switzerland; Austria; Belgium; Great Britain; Yugoslavia; Israel; Czechia; Estonia; Spain; Greece; Poland | 204 | mixed public and private | Post-operative | within 6 weeks | melanoma | High-risk stage IIb (thickness > 3 mm) and stage III (positive lymph nodes) without distant metastasis (49% were Stage Iib) | 52 (range 14–84) | 41 | NR |
| Goebell, 2002 (NR) [68] | Germany | 45 | public | Post-operative | 2 weeks postop | bladder cancer | pTa G1–2 | 65 d (NR) | 27 | NR |
| Ibrahiem, 2009 (2006–2008) [69] e | Egypt | 60 | none | Post-operative | 1–2 weeks postop | Superficial bladder cancer | Ta or T1; 82% were Grade 2 | 56.1 d (NR) | NR | NR |
| Steuer-Vogt, 2001 (1993–2000) [75] f | Germany | 202 | public | Mixed periods f | NA | Head and neck cancers | All stages | 58 (range 30–70) | 9 | Antibiotic (88%) |
| Longhi, 2020 g (2007–2019) [73] g | Italy | 20 | private | Post-operative | mean 1.9 (range 0.7–5.9) months | Osteosarcoma | 2nd relapse in the lung (stages I–III) | 33.9 (range 11–65) | 45 | NR |
| Study Author, Year | Group/ Brand Name | Characteristics of the Intervention and Control | Dosage Frequency | Route of Admin | Tx Duration Pre-op | Tx Duration Post-op | Tx Duration TOTAL |
|---|---|---|---|---|---|---|---|
| Schink 2007 [74] | Iscador® M special (Weleda, Schwäbisch Gmünd, Germany) | Mistletoe extract, 50 mg, IO | once | IV | NA | NA | Over 1 h |
| Control | no added tx | ||||||
| Enesel 2005 [15] | Isorel® A a (V. album grown on abietis extract) | Mistletoe extract, 2 vials (60 mg/mL, but the volume per vial was not reported). Protocol: in the 1st pre-operative week, 1 vial, then 2 vials, then 3 vials every second day and in the 2nd pre-operative week 3, then 2, then 1 vial; and postoperative treatments were repeated in the same manner. | 3 × per week | SC | 2 | 2 | 4 weeks |
| Control | no added tx | ||||||
| Kleeberg 2004 [70] | Iscador® M (Viscum, mali extract) | Mistletoe extract, the dose and the unit were not mentioned. Protocol was started at dose 0 and then was escalated every other day over 2 weeks starting from 0.01 to reach to 1.0 mg/mL, followed by a 3 day break. Treatment then resumed with 14 doses of 20 mg/mL over 28 days, followed by 7 days of no treatment. Volume per dose NR, but presumption is that 1 mL was given per dose. | 3 × per week | Injection | NA | 12 | 12 months or until tumour progression |
| Control | no added tx | ||||||
| Goebell 2002 [68] | V. album lectin extract (brand name NR) | Standardized to mistletoe lectin I (galactoside-specific lectin), 1 mL | 2 × week | SC | NA | 9 months, 2 cycles of 3 months of treatment separated by a 3-month break | 9 months |
| Control | no added tx | ||||||
| Steuer-Vogt 2001 [75] | Eurixor® (biosyn Arzneimittel GmbH, Fellbach, Germany) (mistletoe extract) | Mistletoe lectin-1 (ML-1), 1 ng of ML-1 per kg body weight | 2 × week | SC | 1–4 days | 60 weeks | 60 weeks b |
| Control | no added tx | ||||||
| Ibrahiem 2009 [69] | Mistletoe extract (brand name was NR) | Lectin concentration, 10.000 ng c | NR | Intravesical | NA | NR | NR |
| Control | Bacillus Calmette–Guérin (BCG), standard therapy c | NR | Intravesical d | NA | NR | NR | |
| Longhi 2020 [73], Longhi 2014 [72], Longhi 2009 [71] f | Iscador® P (V. album fermentatum Pini extract) | Mistletoe, 20 mg e | 3 × per week | SC | NA | 12 months | 12 months |
| Control | Etoposide, 50 mg/m2 | QD | oral | NA | 6 months; 3 weeks per cycle, 6 cycles, separated by 1-week break | 6 months |
| Author Year | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Bias |
|---|---|---|---|---|---|---|---|
| Enesel 2005 [15] | ? | ? | - | - | ? | ? | + |
| Goebell 2012 [68] | + | + | + | + | + | ? | + |
| Ibrahiem 2009 [69] | ? | ? | ? | ? | + | ? | ? |
| Kleeberg 2004 [70] | + | + | ? | ? | ? | + | + |
| Longhi 2020 [73] | + | + | - | - | + | + | ? |
| Schink 2007 [74] | + | + | - | ? | + | ? | + |
| Steuer-Vogt 2001 [75] | + | + | - | ? | + | ? | + |
| Study Author, Year | Selection | Comparability | Outcome |
|---|---|---|---|
| Augustin, 2005 [77] | ★★★★ | ★★ | ★★★ |
| Bussing, 2005 [78] | ★★★ | ★ | ★★ |
| Elsasser-Beile, 2005 [76] | ★★★ | ★ | ★ |
| Study Author, Year | Tx Name | Sample Size | Follow -Up Time and Unit | All Adverse Effects (AEs)/Most Common AEs (N) (Definition) | Serious AEs (N) (Definition | Severe AEs (N) (Definition) | Surgical Complications (N) (Definition) | Drug Reduction /Treatment Discontinuation Number (Definition) | OC (N) (Definition) |
|---|---|---|---|---|---|---|---|---|---|
| Longhi, 2014 [72] | Mistletoe | 9 | 12 mon. | (16) (definition NR) | (2) (post -operative hospitalization) | (5) (definition NR) | NR (NA) | (0) Dose reduced (definition NR) (2) Medication discontinued (definition NR) (1) Medication continued after interruption (definition NR) | (2) Adverse drug reactions (Local erythema and hypotension (1)) |
| Etoposide | 10 | 12 mon. | (69) (definition NR) | (3) (hospitalizations due to surgery (1) and pneumonia a (2)). 3 | (26) (definition NR) | NR (NA) | (5) Dose reduced (definition NR) (4) Medication discontinued (definition NR) (18) Medication continued after interruption (definition NR) | (47) Adverse drug reactions (Including G2, G3 hematologic toxicity, G-CSF was necessary in 3 patients, 1 needed blood transfusion for G4 anemia. Most frequent ADRs were neutropenia, anemia, leukopenia, nausea, alopecia) | |
| Schink, 2007 [74] | Iscador® M special (mistletoe extract) | 11 | 7 days | NR | (0) (definition NR ) | NR | (4) (AEs during surgery (hypotension (2), hypertension, circulatory instability) (5) (post-op AEs UTI (2), erythocytouria, body temp >38 °C and C-reactive protein >10 mg/dL, Tension bulla beneath bandage of peridural catheter) | (0) Allergic predisposition to mistletoe extract (hypersensitivity reaction to test injection dose pre-op of mistletoe (0.1 mg) | |
| No added tx | 11 | 7 days | NR | (1) (anastomosis leakage requiring 2nd surgery) | NR | (4) (AEs during surgery hypotension) (4) (post-op. AEs (hypertension (2), erythocytouria, suppuration of surgical wound) | NR | NR | |
| Enesel, 2005 [15] | No added tx | 30 | NR | NR | NR | NR | NR | NR | NR |
| Isorel® A (V. album grown on abietis extract) | 40 | NR | NR | NR | NR | NR | NR | NR | |
| Kleeberg, 2004 [70] | Iscador® M (Viscum, mali extract) | 102 | 8 years | NR | NR | NR | NR | NR/ (5) (Tx discontinuation d/t Grade 3–4 toxicity (WHO classification)) | (0) organ toxicity (definition NR) |
| No added tx | 102 | 8 years | NR | NR | NR | NR | NR/ (0) (Tx Discontinuation d/t Grade 3–4 toxicity (WHO classification)) | (0) organ toxicity (definition NR) | |
| Goebell, 2002 [68] | V. album Lectin extract (brand NR) | 23 | 18 mon. | (0) (Adverse events after application of mistletoe lectin) (no specific Definition) | NR | NR | NR | NR | NR |
| No added tx | 22 | 18 mon. | NR | NR | NR | NR | NR | NR | |
| Ibrahiem, 2009 [69] | Mistletoe extract (brand NR) | 30 | NR | (30) Self-limited local skin reaction after first SC dose b (definition NR) (0) Biochemical or hematological changes (definition NR) | NR | NR | NR | NR | NR |
| BCG standard therapy | 30 | NR | (23) Painful bladder sensation (definition NR) | NR | NR | NR | NR | NR | |
| Steuer- Vogt, 2001 [75] | Eurixor® (mistletoe extract) | 97 | 60 weeks | (47) {At start of tx: pxs with AEs upon injections (local and/or systemic side-effects upon mistletoe injections into abdominal wall. Most common local AEs were rubor and prurigo)} (4) {At 32 weeks or later: pxs with AEs upon injections (local and/or Systemic side-effects upon mistletoe injections into abdominal wall} | NR | NR | NR | (16) Pxs who refused further injections because of mistletoe -induced AEs (definition NR) | (4) Generalized drug reactions (melalgia, fever ≤ 39 °C, sleeplessness, tiredness, coldness or heat sensation and sneezing) |
| No added tx | 105 | 60 weeks | NR | NR | NR | NR | NR | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cogo, E.; Elsayed, M.; Bhardwaj, S.; Cooley, K.; Aycho, C.; Liang, V.; Papadogianis, P.; Psihogios, A.; Seely, D. Mistletoe Extracts during the Oncological Perioperative Period: A Systematic Review and Meta-Analysis of Human Randomized Controlled Trials. Curr. Oncol. 2023, 30, 8196-8219. https://doi.org/10.3390/curroncol30090595
Cogo E, Elsayed M, Bhardwaj S, Cooley K, Aycho C, Liang V, Papadogianis P, Psihogios A, Seely D. Mistletoe Extracts during the Oncological Perioperative Period: A Systematic Review and Meta-Analysis of Human Randomized Controlled Trials. Current Oncology. 2023; 30(9):8196-8219. https://doi.org/10.3390/curroncol30090595
Chicago/Turabian StyleCogo, Elise, Mohamed Elsayed, Sukriti Bhardwaj, Kieran Cooley, Christilynn Aycho, Vivian Liang, Peter Papadogianis, Athanasios Psihogios, and Dugald Seely. 2023. "Mistletoe Extracts during the Oncological Perioperative Period: A Systematic Review and Meta-Analysis of Human Randomized Controlled Trials" Current Oncology 30, no. 9: 8196-8219. https://doi.org/10.3390/curroncol30090595
APA StyleCogo, E., Elsayed, M., Bhardwaj, S., Cooley, K., Aycho, C., Liang, V., Papadogianis, P., Psihogios, A., & Seely, D. (2023). Mistletoe Extracts during the Oncological Perioperative Period: A Systematic Review and Meta-Analysis of Human Randomized Controlled Trials. Current Oncology, 30(9), 8196-8219. https://doi.org/10.3390/curroncol30090595






