Fast Track Management of Primary Thyroid Lymphoma in the Very Elderly Patient
Abstract
1. Introduction
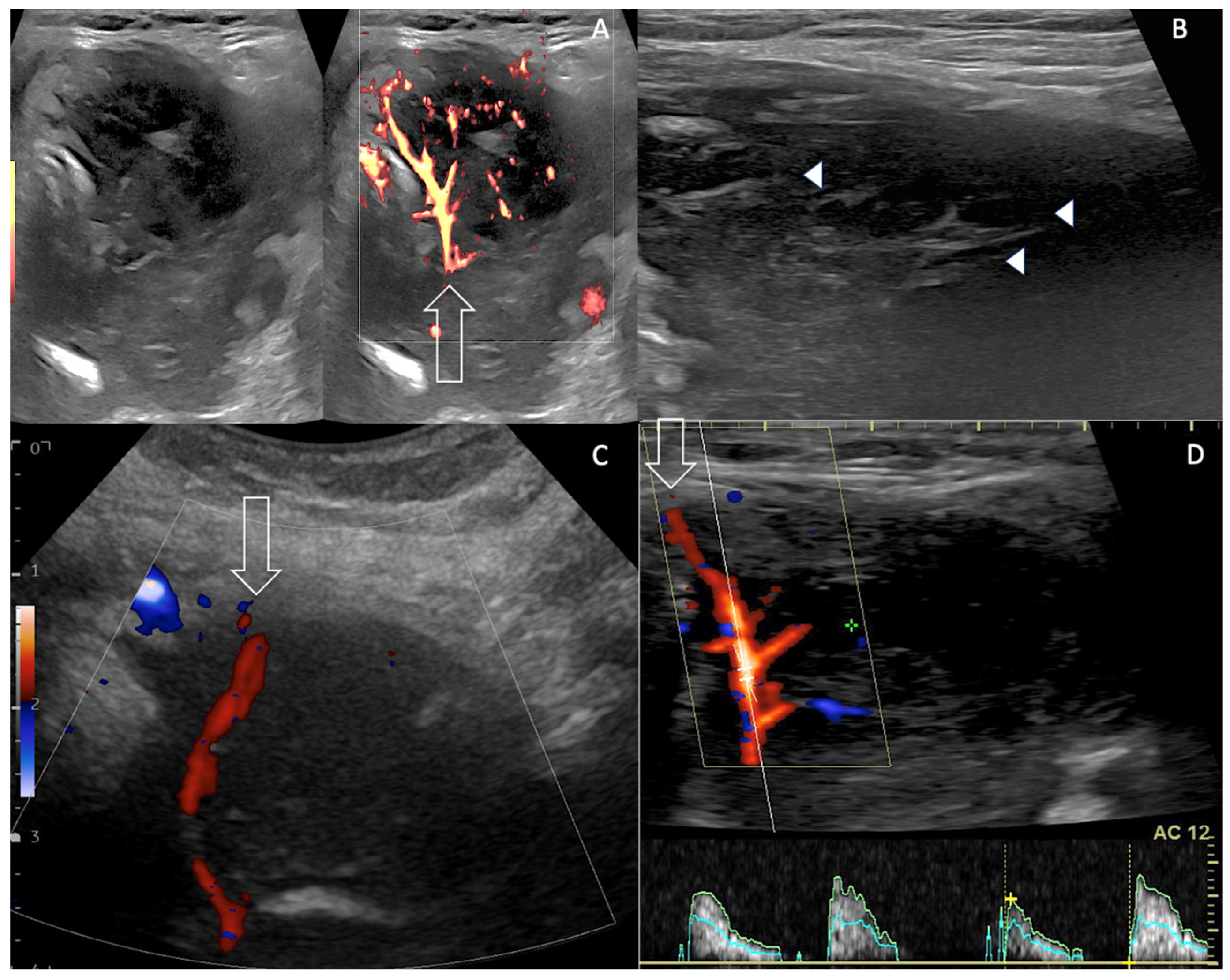
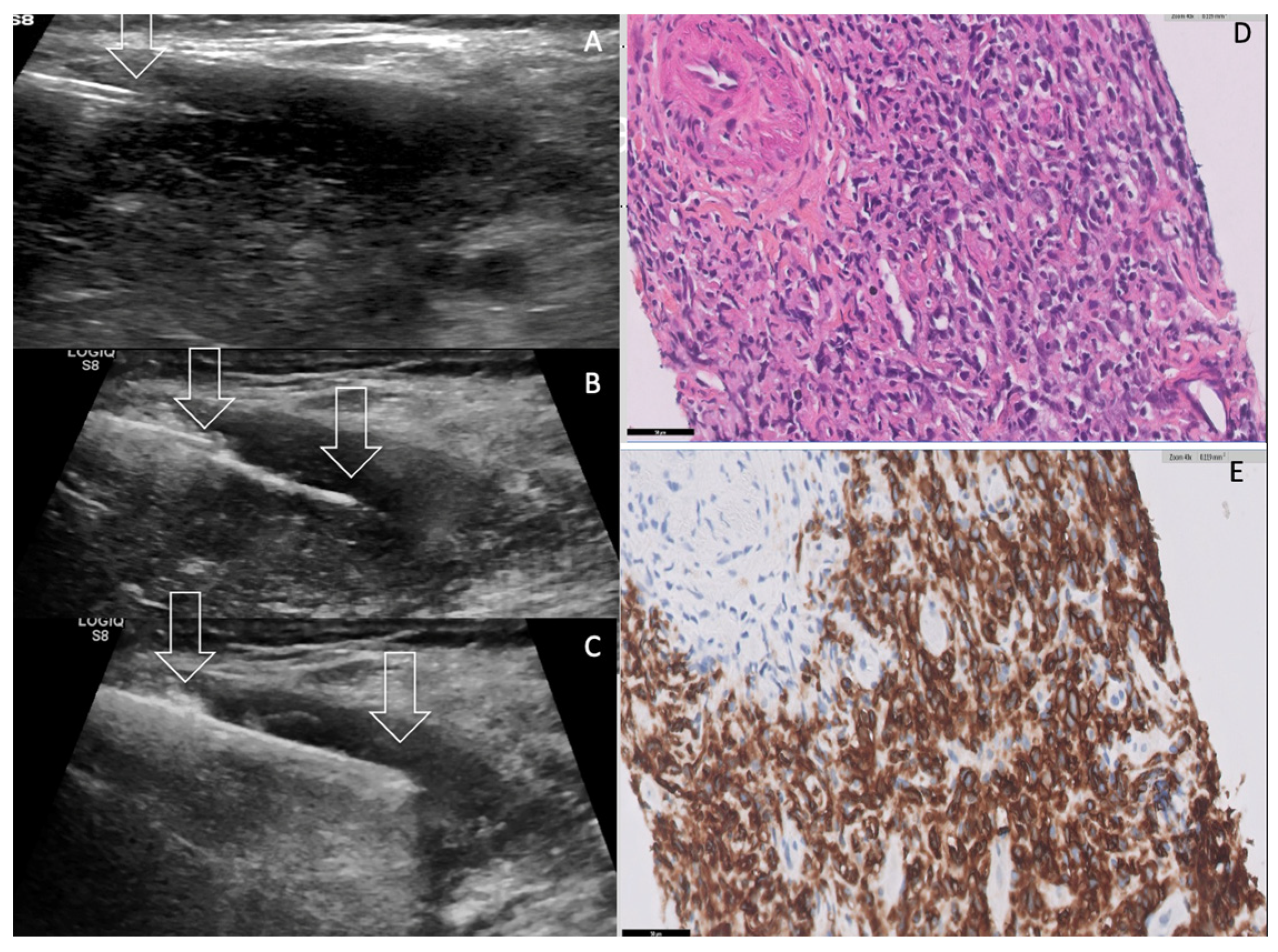
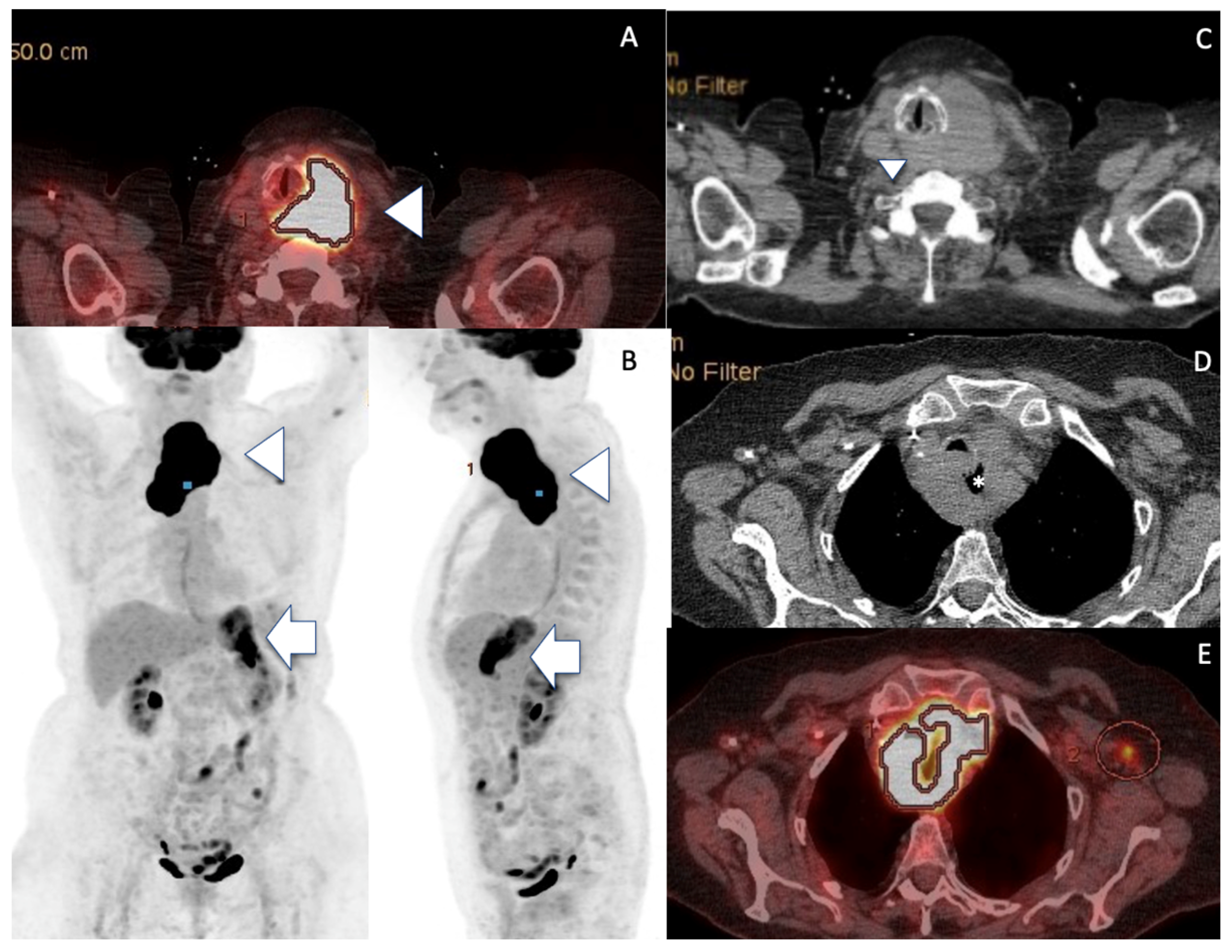
2. Case Presentation
3. Discussion
| Etiologies % of malignancies Sex Ratio F/M | PTL (1–5%) 2.5/1 | ATC (2–3%) 1.5–2/1 | MTT (0.36%, 1.9–24% autopsy) 1.1–1.4/1 |
| Hashimoto’s Thyroiditis | Common | Uncommon | Uncommon |
| Thyroid Function | Hypothyroidism (concomitant HT) | Euthyroidism Hyperthyroidism | Euthyroidism Hypo/hyperthyroidism Hypoparathyroidism * |
| Diagnosis FNA sensitivity CNB sensitivity | 44.8% 94.3% | 54% 80.1% | 73.7–87% Useful in inconclusive cases |
| Imaging Features US Doppler EuTIRADS score IJV invasion Computed Tomography | Reticular pattern More homogeneous than thyroid carcinoma No necrosis/calcification Sword sign Eu-TIRADS 5 Rare No cystic necrosis No gross calcification Homogeneous Nodes +/0 Mild vasculature Unclear boundaries + Vessel invasion not usual Tracheal compression + | No reticular pattern Heterogeneous Necrosis /calcification Sword sign Eu-TIRADS 5 Not rare Cystic necrosis Gross calcifications Heterogeneous Nodes ++/− Few vessels Unclear boundaries ++ Vessel invasion (33%) Tracheal compression ++ | No reticular pattern Inhomogeneous Depends on pre-existing thyroid No Sword sign Eu-TIRADS 5 > Eu-TIRADS 4 Exceptional No cystic necrosis No gross calcification Nodular Nodes +/− Increased Vasculature (renal) Unclear boundaries 0/+ Vessel invasion very rare (renal) Tracheal compression 0/+ |
| PET CT median SUVmax | +++ 22.7 | +++ 24.8 | ++/- variable |
One year OS 5 years OS Average survival | 84.66% (52%) 71.66% (24%) Depends on subtype, stage, age | 20% - 5months | - 58% 43.2mo (after surgery) Depends on primary histology |
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fagin, J.A.; Wells, S.A., Jr. Biologic and Clinical Perspectives on Thyroid Cancer. N. Engl. J. Med. 2016, 375, 1054–1067. [Google Scholar] [CrossRef]
- Suzuki, N.; Watanabe, N.; Noh, J.Y.; Yoshimura, R.; Mikura, K.; Kinoshita, A.; Suzuki, A.; Mitsumatsu, T.; Fukushita, M.; Matsumoto, M.; et al. The Relationship Between Primary Thyroid Lymphoma and Various Types of Thyroid Autoimmunity: A Retrospective Cohort Study of 498 Cases, Including 9 Cases with Graves’ Disease. Thyroid 2022, 32, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Derringer, G.A.; Thompson, L.D.; Frommelt, R.A.; Bijwaard, K.E.; Heffess, C.S.; Abbondanzo, S.L. Malignant lymphoma of the thyroid gland: A clinicopathologic study of 108 cases. Am. J. Surg. Pathol. 2000, 24, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.K.; Baek, J.H.; Na, D.G.; Oh, Y.L.; Yi, K.H.; Kang, H.C. Practice guidelines for thyroid core needle biopsy: A report of the Clinical Practice Guidelines Development Committee of the Korean Thyroid Association. J. Pathol. Transl. Med. 2020, 54, 64–86. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.J.; Hong, J.H.; Koo do, H.; Yu, H.W.; Lee, J.H.; Kwon, H.; Kim, S.J.; Choi, J.Y.; Lee, K.E. Clinicopathological characteristics and treatment outcomes of 38 cases of primary thyroid lymphoma: A multicenter study. Ann. Surg. Treat. Res. 2015, 89, 295–299. [Google Scholar] [CrossRef]
- Sun, X.S.; Bay, J.O.; Marcy, P.Y.; Hammoud, Y.; Lacout, A.; Michels, J.J.; Guevara, N.; Thariat, J. Treatment of primary thyroid lymphomas. Bull. Cancer 2013, 10, 1031–1042. (In French) [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Shin, S.J.; Yun, H.J.; Kim, S.M.; Chang, H.; Lee, Y.S.; Chang, H.S. Primary thyroid lymphoma: A single-center experience. Front. Endocrinol. 2023, 14, 1064050. [Google Scholar] [CrossRef]
- Buffet, C.; Allard, L.; Guillerm, E.; Ghander, C.; Mathy, E.; Lussey-Lepoutre, C.; Julien, N.; Touma, E.; Quilhot, P.; Godiris-Petit, G.; et al. Detection of BRAFV600E by digital PCR on fine-needle aspirate enables rapid initiation of dabrafenib and trametinib in unresectable anaplastic thyroid carcinoma. Eur. J. Endocrinol. 2022, 187, K33–K38. [Google Scholar] [CrossRef] [PubMed]
- Simcock, R.; Wright, J. Beyond Performance Status. Clin. Oncol. R. Coll. Radiol. 2020, 32, 553–561. [Google Scholar] [CrossRef]
- Hoppe, R.T.; Advani, R.H.; Ai, W.Z.; Ambinder, R.F.; Armand, P.; Bello, C.M.; Benitez, C.M.; Chen, W.; Dabaja, B.; Daly, M.E.; et al. NCCN Guidelines® Insights: Hodgkin Lymphoma, Version 2.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid. J. 2017, 6, 225–237. [Google Scholar] [CrossRef]
- Nam, M.; Shin, J.H.; Han, B.K.; Ko, E.Y.; Ko, E.S.; Hahn, S.Y.; Chung, J.H.; Oh, Y.L. Thyroid lymphoma: Correlation of radiologic and pathologic features. J. Ultrasound Med. 2012, 31, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Lacout, A.; Marcy, P.Y.; Thariat, J. RE: Role of Duplex Doppler US for thyroid nodules: Looking for the “sword” sign. Korean J. Radiol. 2011, 12, 400–401. [Google Scholar] [CrossRef]
- Robbins, K.T.; Shaha, A.R.; Medina, J.E.; Califano, J.A.; Wolf, G.T.; Ferlito, A.; Som, P.M.; Day, T.A.; Committee for Neck Dissection Classification, American Head and Neck Society. Consensus statement on the classification and terminology of neck dissection. Arch. Otolaryngol. Head. Neck Surg. 2008, 134, 536–538. [Google Scholar] [CrossRef] [PubMed]
- Matsuzuka, F.; Miyauchi, A.; Katayama, S.; Narabayashi, I.; Ikeda, H.; Kuma, K.; Sugawara, M. Clinical aspects of primary thyroid lymphoma: Diagnosis and treatment based on our experience of 119 cases. Thyroid 1993, 3, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Mirabile, A.; Biafora, M.; Giordano, L.; Arrigoni, G.; Cangi, M.G.; Dell’Oca, I.; Lira Luce, F.; Di Santo, D.; Galli, A.; Tulli, M.; et al. Uncommon Site of Metastasis and Prolonged Survival in Patients with Anaplastic Thyroid Carcinoma: A Systematic Review of the Literature. Cancers 2020, 12, 2585. [Google Scholar] [CrossRef] [PubMed]
- Hegerova, L.; Griebeler, M.L.; Reynolds, J.P.; Henry, M.R.; Gharib, H. Metastasis to the thyroid gland: Report of a large series from the Mayo Clinic. Am. J. Clin. Oncol. 2015, 38, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Rovira, A.; Carroll, P.; Simo, R. Thyroid lymphoma. Curr. Opin. Otolaryngol. Head. Neck Surg. 2023, 31, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Wang, W.; Xu, J.; Chen, X.; Zhong, Z.; Sun, C. Evidence from an updated meta-analysis of the prognostic impacts of postoperative radiotherapy and chemotherapy in patients with anaplastic thyroid carcinoma. Onco Targets Ther. 2018, 11, 2251–2257. [Google Scholar] [CrossRef] [PubMed]
- Shonka, D.C., Jr.; Ho, A.; Chintakuntlawar, A.V.; Geiger, J.L.; Park, J.C.; Seetharamu, N.; Jasim, S.; Abdelhamid Ahmed, A.H.; Bible, K.C.; Brose, M.S.; et al. American Head and Neck Society Endocrine Surgery Section and International Thyroid Oncology Group consensus statement on mutational testing in thyroid cancer: Defining advanced thyroid cancer and its targeted treatment. Head Neck. 2022, 44, 1277–1300. [Google Scholar] [CrossRef] [PubMed]
- Stergianos, S.; Juhlin, C.C.; Zedenius, J.; Calissendorff, J.; Falhammar, H. Metastasis to the thyroid gland: Characterization and survival of an institutional series spanning 28 years. Eur. J. Surg. Oncol. 2021, 47, 1364–1369. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Wang, Z. Metastases to the Thyroid. Gland: What Can. We Do? Cancers 2022, 14, 3017. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.Y.; Tran, T.B.; Brumund, K.T.; Weisman, R.A.; Bouvet, M. Metastases to the thyroid: A review of the literature from the last decade. Thyroid 2012, 22, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Ghossein, C.A.; Khimraj, A.; Dogan, S.; Xu, B. Metastasis to the thyroid gland: A single-institution 16-year experience. Histopathology 2021, 78, 508–519. [Google Scholar] [CrossRef]
- Matsuzuka, F.; Amino, N.; Kuma, K.; Miyauchi, A. Serial changes in thyroid ultrasonogram in a patient with Hashimoto’s thyroiditis who developed malignant lymphoma. Thyroid 2005, 15, 742–743. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Hu, X.; Ma, B. Aggressive primary thyroid lymphoma invading the internal jugular vein: A case series. Quant. Imaging Med. Surg. 2023, 13, 1213–1220. [Google Scholar] [CrossRef]
- Marcy, P.Y.; Thariat, J.; Bozec, A.; Poissonnet, G.; Benisvy, D.; Dassonville, O. Venous obstruction of thyroid malignancy origin: The Antoine Lacassagne Institute experience. World J. Surg. Oncol. 2009, 7, 40. [Google Scholar] [CrossRef]
- Takashima, S.; Morimoto, S.; Ikezoe, J.; Takai, S.; Kobayashi, T.; Koyama, H.; Nishiyama, K.; Kozuka, T. CT evaluation of anaplastic thyroid carcinoma. AJR Am. J. Roentgenol. 1990, 154, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Tamaki, Y.; Takahashi, M.; Higuchi, K.; Sakaino, K.; Nonaka, T.; Shioya, M.; Mitsuhashi, N.; Niibe, H. Comparison of primary thyroid lymphoma with anaplastic thyroid carcinoma on computed tomographic imaging. Radiat. Med. 2002, 20, 9–15. [Google Scholar] [PubMed]
- Gupta, N.; Nijhawan, R.; Srinivasan, R.; Rajwanshi, A.; Dutta, P.; Bhansaliy, A.; Sharma, S.C. Fine needle aspiration cytology of primary thyroid lymphoma: A report of ten cases. Cytojournal 2005, 2, 21. [Google Scholar] [CrossRef]
- Ha, E.J.; Suh, C.H.; Baek, J.H. Complications following ultrasound-guided core needle biopsy of thyroid nodules: A sysmatic review and meta-analysis. Eur. Radiol. 2018, 28, 3848–3860. [Google Scholar] [CrossRef] [PubMed]
- Vander Poorten, V.; Goedseels, N.; Triantafyllou, A.; Sanabria, A.; Clement, P.M.; Cohen, O.; Golusinski, P.; Guntinas-Lichius, O.; Piazza, C.; Randolph, G.W.; et al. Effectiveness of core needle biopsy in the diagnosis of thyroid lymphoma and anaplastic thyroid carcinoma: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 971249. [Google Scholar] [CrossRef]
- Ha, E.J.; Baek, J.H.; Lee, J.H.; Kim, J.K.; Choi, Y.J.; Sung, T.Y.; Kim, T.Y. Complications following US-guided core-needle biopsy for thyroid lesions: A retrospective study of 6,169 consecutive patients with 6,687 thyroid nodules. Eur. Radiol. 2017, 27, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Jasim, S.; Reading, C.C.; Ristow, K.M.; Villasboas Bisneto, J.C.; Habermann, T.M.; Fatourechi, V.; Stan, M. Clinical Presentation and Diagnostic Challenges of Thyroid Lymphoma: A Cohort Study. Thyroid 2016, 26, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Travaglino, A.; Pace, M.; Varricchio, S.; Insabato, L.; Giordano, C.; Picardi, M.; Pane, F.; Staibano, S.; Mascolo, M. Hashimoto Thyroiditis in Primary Thyroid Non-Hodgkin Lymphoma. Am. J. Clin. Pathol. 2020, 153, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Takedani, K.; Notsu, M.; Adachi, N.; Tanaka, S.; Yamamoto, M.; Yamauchi, M.; Yamauchi, N.; Maruyama, R.; Kanasaki, K. Thyroid crisis caused by metastatic thyroid cancer: An autopsy case report. BMC Endocr. Disord. 2021, 21, 213. [Google Scholar] [CrossRef]
- Patrizio, A.; Ferrari, S.M.; Stoppini, G.; Palmisano, E.; Elia, G.; Ragusa, F.; Paparo, S.R.; Balestri, E.; Mazzi, V.; Botrini, C.; et al. Thyroid Metastasis from Primary Breast Cancer. J. Clin. Med. 2023, 12, 2709. [Google Scholar] [CrossRef]
- De Leo, S.; Trevisan, M.; Moneta, C.; Colombo, C. Endocrine-related adverse conditions induced by tyrosine kinase inhibitors. Ann. Endocrinol. 2023, 84, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Matrone, A.; De Napoli, L.; Torregrossa, L.; Aghababyan, A.; Papini, P.; Ambrosini, C.E.; Cervelli, R.; Ugolini, C.; Basolo, F.; Molinaro, E.; et al. Core Needle Biopsy Can Early and Precisely Identify Large Thyroid Masses. Front. Oncol. 2022, 12, 854755. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Ding, C.; Shen, Y.; Zhao, L.; Li, H. Clinicopathological analysis of primary thyroid non-Hodgkin lymphoma: A single-center study. Transl. Cancer Res. 2023, 12, 515–524. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Pickard, R.H. Sonography of delayed thyroid metastasis from renal cell carcinoma with jugular vein extension. AJR Am. J. Roentgenol. 2003, 181, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Marcy, P.Y.; Thariat, J.; Chevenet, C.; Lacout, A. Jugular Vein Invasion Diagnosis and Prognosis in Thyroid Carcinomas. Pol. J. Radiol. 2016, 81, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, S.; He, X. Prognostic evaluation models for primary thyroid lymphoma, based on the SEER database and an external validation cohort. J. Endocrinol. Investig. 2022, 45, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.S.; Shadle, K.M.; Medeiros, L.J.; Wilder, R.B.; Hess, M.A.; Cabanillas, F.; Cox, J.D. Localized non-Hodgkin lymphoma involving the thyroid gland. Cancer 2001, 91, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Zucca, E.; Bertoni, F. The spectrum of MALT lymphoma at different sites: Biological and therapeutic relevance. Blood 2016, 127, 2082–2092. [Google Scholar] [CrossRef]
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Müeller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of imaging in the staging and response assessment of lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J. Clin. Oncol. 2014, 32, 3048–3058. [Google Scholar] [CrossRef]
- Schöder, H.; Noy, A.; Gönen, M.; Weng, L.; Green, D.; Erdi, Y.E.; Larson, S.M.; Yeung, H.W. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin’s lymphoma. J. Clin. Oncol. 2005, 23, 4643–4651. [Google Scholar] [CrossRef]
- Kushwaha, P.; Singh, M.; Mandal, S.; Dhingra, S.; Jain, S. DLBCL of bilateral submandibular glands and MALToma of thyroid-A rare coexistence. Cytopathology 2021, 32, 523–526. [Google Scholar] [CrossRef]
- Bauduer, F.; Robin, H.; Labadie, J.C.; Darrasse, D. MALT lymphoma of initial gastric site relapsing as simultaneous bilateral palpebral and salivary glands localizations. Presse Med. 2014, 43, 1387–1388. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Sun, Y.; Hu, C.; Zhi, Y.; Xiao, H.; Li, Y. Primary nasal diffuse large B-cell lymphoma with synchronous pulmonary involvement: A case report. Medicine 2019, 98, e15439. [Google Scholar] [CrossRef]
- Onal, C.; Li, Y.X.; Miller, R.C.; Poortmans, P.; Constantinou, N.; Weber, D.C.; Atasoy, B.M.; Igdem, S.; Ozsahin, M.; Ozyar, E. Treatment results and prognostic factors in primary thyroid lymphoma patients: A rare cancer network study. Ann. Oncol. 2011, 22, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Peyrade, F.; Jardin, F.; Thieblemont, C.; Thyss, A.; Emile, J.F.; Castaigne, S.; Coiffier, B.; Haioun, C.; Bologna, S.; Fitoussi, O.; et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2011, 12, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Pyke, C.M.; Grant, C.S.; Habermann, T.M.; Kurtin, P.J.; Van Heerden, J.A.; Bergstralh, E.J.; Kunselman, A.; Hay, I.D. Non-Hodgkin’s lymphoma of the thyroid: Is more than biopsy necessary? World J. Surg. 1992, 16, 604–609. [Google Scholar] [CrossRef]
- Thieblemont, C.; Bernard, S.; Molina, T. Management of aggressive lymphoma in very elderly patients. Hematol. Oncol. 2017, 35 (Suppl. S1), 49–53. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcy, P.Y.; Bauduer, F.; Thariat, J.; Gisserot, O.; Ghanassia, E.; Chetaille, B.; Boudin, L.; Morvan, J.B. Fast Track Management of Primary Thyroid Lymphoma in the Very Elderly Patient. Curr. Oncol. 2023, 30, 5816-5827. https://doi.org/10.3390/curroncol30060435
Marcy PY, Bauduer F, Thariat J, Gisserot O, Ghanassia E, Chetaille B, Boudin L, Morvan JB. Fast Track Management of Primary Thyroid Lymphoma in the Very Elderly Patient. Current Oncology. 2023; 30(6):5816-5827. https://doi.org/10.3390/curroncol30060435
Chicago/Turabian StyleMarcy, Pierre Yves, Frederic Bauduer, Juliette Thariat, Olivier Gisserot, Edouard Ghanassia, Bruno Chetaille, Laurys Boudin, and Jean Baptiste Morvan. 2023. "Fast Track Management of Primary Thyroid Lymphoma in the Very Elderly Patient" Current Oncology 30, no. 6: 5816-5827. https://doi.org/10.3390/curroncol30060435
APA StyleMarcy, P. Y., Bauduer, F., Thariat, J., Gisserot, O., Ghanassia, E., Chetaille, B., Boudin, L., & Morvan, J. B. (2023). Fast Track Management of Primary Thyroid Lymphoma in the Very Elderly Patient. Current Oncology, 30(6), 5816-5827. https://doi.org/10.3390/curroncol30060435





