An Unconventional Regimen of Carboplatin and Paclitaxel in Metastatic Colorectal Carcinosarcoma: A Case Report and Review of Literature
Abstract
1. Introduction
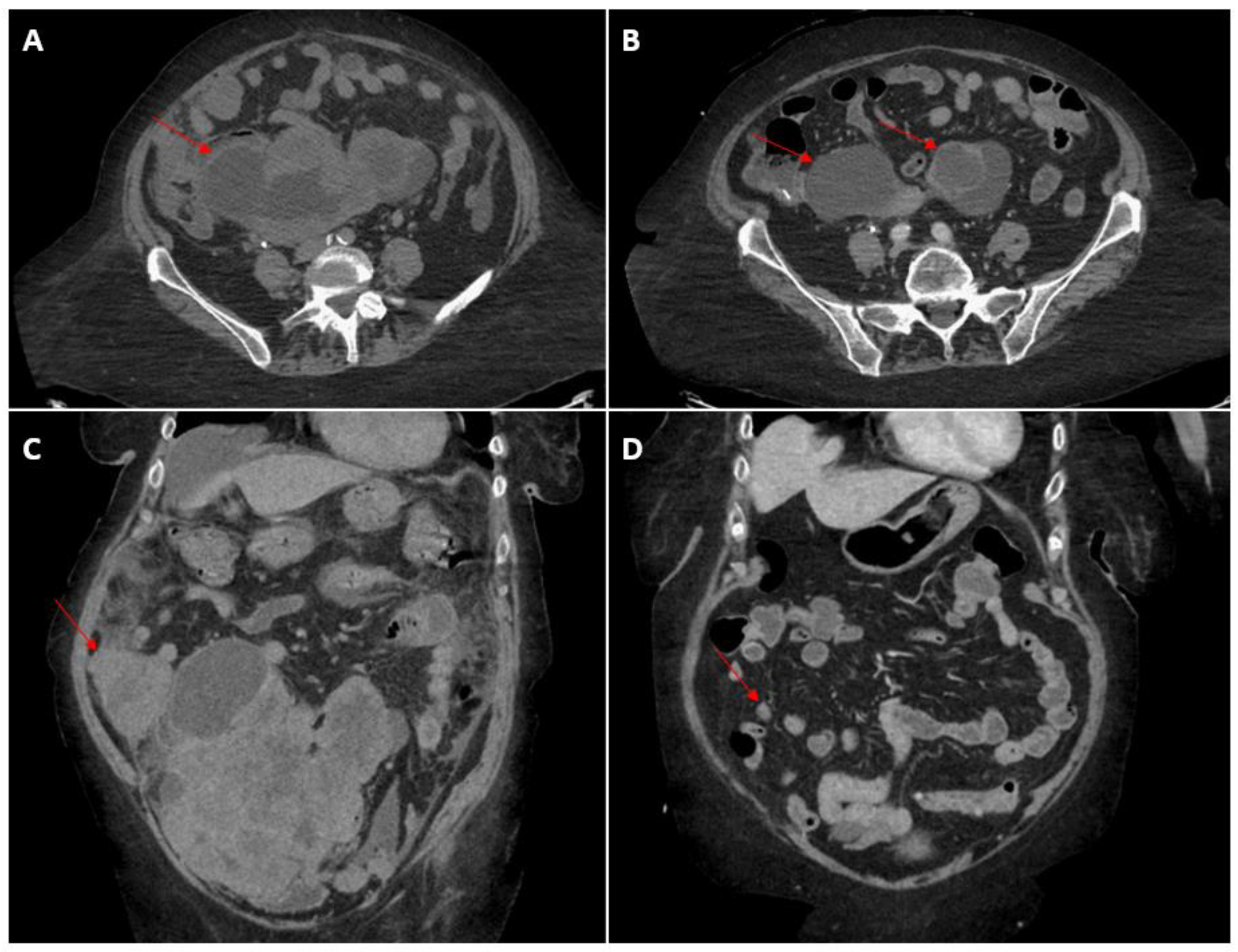
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, L.; Cai, D.; Muhetaer, A.; Yang, Y.-L.; Ren, F.; Yishake, M.; Zhang, H.; Fang, Y.; Wushou, A. Spindle cell carcinoma: The general demographics, basic clinico-pathologic characteristics, treatment, outcome and prognostic factors. Oncotarget 2017, 8, 43228–43236. [Google Scholar] [CrossRef] [PubMed]
- Zidar, N.; Gale, N. Carcinosarcoma and spindle cell carcinoma—Monoclonal neoplasms undergoing epithelial-mesenchymal transition. Virchows Arch. 2015, 466, 357–358. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Klar, M.; Matsuzaki, S.; Roman, L.D.; Sood, A.K.; Matsuo, K. Uterine carcinosarcoma: Contemporary clinical summary, molecular updates, and future research opportunity. Gynecol. Oncol. 2021, 160, 586–601. [Google Scholar] [CrossRef] [PubMed]
- Ersek, J.L.; Symanowski, J.T.; Han, Y.; Howard, A.; Dumas, K.; Ahrens, W.; Kim, E.; Kim, E.S. Pulmonary Carcinosarcoma: A Surveillance, Epidemiology, and End Results (SEER) Analysis. Clin. Lung Cancer 2020, 21, 160–170. [Google Scholar] [CrossRef] [PubMed]
- He, H.-L.; Liu, Z.-L.; Ma, C.-Y.; Li, X.-G.; He, Y. Clinicopathological features of carcinosarcoma in oromaxillofacial head and neck region. Shanghai J. Stomatol. 2017, 26, 569–572. [Google Scholar] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Przegla̜d Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.K.; Ghandour, F.; El Hajj, F.G.; El Hajj, I.; Saikaly, E. Colonic Carcinosarcoma: Report of a Rare Colorectal Malignancy and Review of Literature. Gastrointest. Tumors 2020, 8, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, A.; Liu, M.H.; Waters, G.; Velchuru, V.R. Carcinosarcoma of the Rectum: Report of a Rare Colorectal Malignancy and Review of the Literature. Case Rep. Surg. 2015, 2015, 412918. [Google Scholar] [CrossRef] [PubMed]
- Weidner, N.; Zekan, P. Carcinosarcoma of the colon. Report of a unique case with light and immunohistochemical studies. Cancer 1986, 58, 1126–1130. [Google Scholar] [CrossRef] [PubMed]
- Bertram, P.; Treutner, K.H.; Tietze, L.; Schumpelick, V. True carcinosarcoma of the colon. Case report. Langenbecks Arch. Chir. 1997, 382, 173–174. [Google Scholar] [PubMed]
- Kim, J.H.; Moon, W.S.; Kang, M.J.; Park, M.J.; Lee, D.G. Sarcomatoid carcinoma of the colon: A case report. J. Korean Med Sci. 2001, 16, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Katsumata, K.; Suzuki, S.; Matsuda, D.; Hara, T.; Hayashida, Y.; Enomoto, M.; Wada, T.; Tsuchida, A.; Aoki, T.; et al. Carcinosarcoma of the Sigmoid Colon: Report of a Case. Case Rep. Gastroenterol. 2010, 4, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini-Spaltro, A.; Vaira, V.; Braidotti, P.; Rovati, M.P.L.; Ferrero, S.; Bosari, S. Carcinosarcoma of the colon: Report of a case with morphological, ultrastructural and molecular analysis. BMC Cancer 2006, 6, 185. [Google Scholar] [CrossRef] [PubMed]
- Macaigne, G.; Aouad, K.; Boivin, J.F.; Bellaïche, A.; Auriault, M.L.; Picard, D.; Deplus, R. Sarcomatoid carcinoma of the colon: Report of a case and review of the literature. Gastroenterol. Clin. Biol. 2004, 28 Pt 1, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.H.; Dang, S.; Bentley, F.R.; Julka, R.N.; Olden, K.W.; Aduli, F. Carcinosarcoma of the colon: A rare cause of colovesical fistula. Am. Surg. 2009, 75, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Cho, M.; Fakih, M. RAS and BRAF in metastatic colorectal cancer management. J. Gastrointest. Oncol. 2016, 7, 687–704. [Google Scholar] [CrossRef] [PubMed]


| Ref. | Metastatic Sites | Surgery? | Systemic Treatment | Best Response |
|---|---|---|---|---|
| [13] | Liver | Yes | Capecitabine | Stable disease sustained for 2 years |
| [14] | Liver, peritoneum | Yes | Doxorubicin | Disease progression |
| [15] | Liver | Yes | Gemcitabine + Docetaxel | Unknown |
| [9] | Peritoneum | Yes | 1: Mitomycin C + 5-FU 2: Cyclophosphamide + doxorubicin + carboplatin | Disease progression |
| [10] | Liver | Yes | 5-FU + Leucovorin | Disease progression |
| [11] | Liver, Lung, Brain | Yes | 5-FU + Leucovorin | Disease progression |
| [12] | Lung | Yes | FOLFIRI + Bevacizumab | Disease progression |
| * | Liver, peritoneum | No | Carboplatin + Paclitaxel | Partial response |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.L.; Rajadurai, C.V.; Ton Nu, T.N.; Mandilaras, V. An Unconventional Regimen of Carboplatin and Paclitaxel in Metastatic Colorectal Carcinosarcoma: A Case Report and Review of Literature. Curr. Oncol. 2023, 30, 4897-4903. https://doi.org/10.3390/curroncol30050369
Park CL, Rajadurai CV, Ton Nu TN, Mandilaras V. An Unconventional Regimen of Carboplatin and Paclitaxel in Metastatic Colorectal Carcinosarcoma: A Case Report and Review of Literature. Current Oncology. 2023; 30(5):4897-4903. https://doi.org/10.3390/curroncol30050369
Chicago/Turabian StylePark, Changsu Lawrence, Charles Vincent Rajadurai, Tuyet Nhung Ton Nu, and Victoria Mandilaras. 2023. "An Unconventional Regimen of Carboplatin and Paclitaxel in Metastatic Colorectal Carcinosarcoma: A Case Report and Review of Literature" Current Oncology 30, no. 5: 4897-4903. https://doi.org/10.3390/curroncol30050369
APA StylePark, C. L., Rajadurai, C. V., Ton Nu, T. N., & Mandilaras, V. (2023). An Unconventional Regimen of Carboplatin and Paclitaxel in Metastatic Colorectal Carcinosarcoma: A Case Report and Review of Literature. Current Oncology, 30(5), 4897-4903. https://doi.org/10.3390/curroncol30050369





