Risk Stratified Follow-Up for Endometrial Cancer: The Clinicians’ Perspective
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Online Questionnaire
3.2. Audience Survey
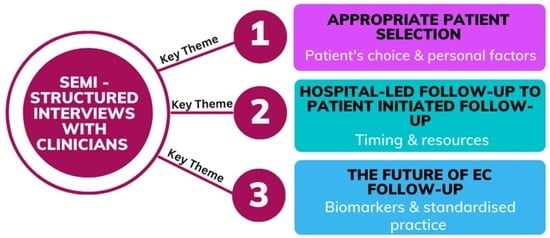
3.3. Qualitative Interviews
3.3.1. Theme 1: Appropriate Patient Selection
Subtheme: Managing Patient Expectations
Subtheme: Patient Characteristics and Good Communication
“If they don’t speak English fluently, then they can’t be on patient-initiated follow-up, because they need to understand obviously if something goes wrong, or [if] they have a worry they need to be able to contact us to discuss their concerns, and if they can’t speak English then there is no way that we can contact them.” (CNS 9)
“Elderly patients who can’t really assess themselves and too anxious patients, patients who have got psychiatric illness—these are the two types of patients we can’t really analyse—because anxiety is something where they are anxious for each and everything you know and if we don’t give them follow-up, they just climb up the wall.” (GO2)
“We do have some ladies who don’t want to be a bother and if you don’t ask, they won’t tell you until they are really struggling.” (GO8)
“You need to look after the psychological aspect—if you just leave them on PIFU—if they suffer in silence, you wouldn’t know.” (GO2)
Subtheme: Survivor’s Choice: Follow-Up and Survivorship Programmes
“They certainly should have ‘survivorship programme’ for every type of cancer. As cancer treatment gets better, you are creating a huge cohort of patients who are surviving cancer, living with the aftermath of the cancer … complications and side effects … they cannot revert back to where they were.” (GO7)
“… when the treatment is done and the cancer is treated, then they are living with the aftermath of what has been done and quite a few times I have seen people who have actually struggled to cope with surgeries, complications, side effects from surgery.” (GO3)
3.3.2. Theme 2: Change from HFU to PIFU
Subtheme: Acceptance to Both Patients and Clinicians
“Most of these patients would be doing quite well but when they get the letter about their appointment or when they have to come, that’s when a lot of patients say that ‘I don’t remember I had this disease’ and—when they come for the interview or assessment—’that’s when I get stressed’.” (GO7)
Subtheme: Appropriate Time for Discharge/Transfer to PIFU
“I advise patients to contact our CNS team rather than go through GP, as I feel it would be better managed than waiting for the referral process.” (ULG6)
Subtheme: COVID 19 Impact on the NHS Resource Management
3.3.3. Theme 3: The Future of EC Follow-Up Schemes
Subtheme: Standardisation
“The disadvantage is if it is applied as a blanket policy, we should have the discussion with the patients and take into account their concerns, other risk factors, which might prevent them from presenting to you; this is how it should be for every patient.” (GO7)
“The main advantage is that it should give the patients confidence and the clinicians confidence that this is what we should do; there would be recurrences regardless of how early-stage or low-grade they are and it’s easier if we have a national protocol.” (GO7)
Subtheme: A Biomarker to Detect EC Recurrence
“If you are detecting a recurrence early, then you need to demonstrate that your intervention is acceptable—it’s going to improve quality and quantity of life.” (GO10)
“We have to be realistic, so for some of those patients there is not much value to detect an early recurrence because the therapeutic options are not there, so you have to be selective—so which ones do we want to monitor?” (GO1)
“Is there a way to detect it early i.e., at a stage where it is salvageable—is the marker capable of doing that? We want good sensitivity and good specificity. Sensitivity would give more confidence.” (ONC1)
“If you have a test that has high specificity rather than sensitivity—because you will end up screening half the people, if you have high sensitivity—I think that would be useful.” (GO9)
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coleman, L.; Newton, C. Patient initiated follow up after gynaecological malignancy: National survey of current UK practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 248, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Polymeros, K.; Wickham-Joseph, R.; Luqman, I.; Charadva, C.; Morris, T.; Collins, A.; Barber, S.; Khunti, K.; Moss, E.L. Comparing Characteristics of Endometrial Cancer in Women of South Asian and White Ethnicity in England. Cancers 2021, 13, 6123. [Google Scholar] [CrossRef] [PubMed]
- Ignatov, T.; Eggemann, H.; Costa, S.D.; Ortmann, O.; Ignatov, A. Endometrial cancer subtypes are associated with different patterns of recurrence. J. Cancer Res. Clin. Oncol. 2018, 144, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Taylor, A.; Guttery, D.S.; Moss, E.L. Innovative Follow-up Strategies for Endometrial Cancer. Clin. Oncol. 2021, 33, e383–e392. [Google Scholar] [CrossRef]
- Department of Health, Macmillan Cancer Support & NHS Improvement. National Cancer Survivorship Initiative. Living with and Beyond Cancer: Taking Action to Improve Outcomes. 2013. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/181054/9333-TSO-2900664-NCSI_Report_FINAL.pdf (accessed on 18 November 2022).
- Kumarakulasingam, P.; McDermott, H.; Patel, N.; Boutler, L.; Tincello, D.G.; Peel, D.; Moss, E.L. Acceptability and utilisation of patient-initiated follow-up for endometrial cancer amongst women from diverse ethnic and social backgrounds: A mixed methods study. Eur. J. Cancer Care 2019, 28, e12997. [Google Scholar] [CrossRef]
- Beaver, K.; Williamson, S.; Sutton, C.; Hollingworth, W.; Gardner, A.; Allton, B.; Abdel-Aty, M.; Blackwood, K.; Burns, S.; Curwen, D.; et al. Comparing hospital and telephone follow-up for patients treated for stage-I endometrial cancer (ENDCAT trial): A randomised, multicentre, non-inferiority trial. BJOG 2017, 124, 150–160. [Google Scholar] [CrossRef]
- Luqman, I.; Wickham-Joseph, R.; Cooper, N.; Boulter, L.; Patel, N.; Kumarakulasingam, P.; Moss, E. Patient-initiated follow-up for low risk endometrial cancer: A cost-analysis evaluation. Int. J. Gynecol. Cancer 2020, 30, 1000–1004. [Google Scholar] [CrossRef]
- Coleridge, S.; Morrison, J. Patient-initiated follow-up after treatment for low risk endometrial cancer: A prospective audit of outcomes and cost benefits. Int. J. Gynecol. Cancer 2020, 30, 1177–1182. [Google Scholar] [CrossRef]
- Jeppesen, M.M.; Jensen, P.T.; Hansen, D.G.; Christensen, R.D.; Mogensen, O. Patient-initiated follow up affects fear of recurrence and healthcare use: A randomised trial in early-stage endometrial cancer. BJOG 2018, 125, 1705–1714. [Google Scholar] [CrossRef]
- Newton, C.; Nordin, A.; Rolland, P.; Ind, T.; Larsen-Disney, P.; Martin-Hirsch, P.; Beaver, K.; Bolton, H.; Peevor, R.; Fernandes, A. British Gynaecological Cancer Society recommendations and guidance on patient-initiated follow-up (PIFU). Int. J. Gynecol. Cancer 2020, 30, 695–700. [Google Scholar] [CrossRef]
- NHS England and NHS Improvement. Living with and Beyond Cancer, Implementing Personalised Stratified Follow Up Pathways. 2020. Available online: https://www.england.nhs.uk/wp-content/uploads/2020/04/cancer-stratified-follow-up-handbook-v1-march-2020.pdf (accessed on 18 November 2022).
- Leeson, S.; Stuart, N.; Sylvestre, Y.; Hall, L.; Whitaker, R. Gynaecological cancer follow-up: National survey of current practice in the UK. BMJ Open 2013, 3, e002859. [Google Scholar] [CrossRef]
- Zola, P.; Ciccone, G.; Piovano, E.; Fuso, L.; Di Cuonzo, D.; Castiglione, A.; Pagano, E.; Peirano, E.; Landoni, F.; Sartori, E.; et al. Effectiveness of Intensive Versus Minimalist Follow-Up Regimen on Survival in Patients With Endometrial Cancer (TOTEM Study): A Randomized, Pragmatic, Parallel Group, Multicenter Trial. J. Clin. Oncol. 2022, 40, 3817–3827. [Google Scholar] [CrossRef]
- Manchanda, R.; Oxley, S.; Ghaem-Maghami, S.; Sundar, S. COVID-19 and the impact on gynecologic cancer care. Int. J. Gynaecol. Obs. 2021, 155 (Suppl. 1), 94–101. [Google Scholar] [CrossRef]
- Nikolopoulos, M.; Godfrey, M.A.L.; Sohrabi, F.; Wong, M.; Bhatte, D.; Wuntakal, R. Stage one endometrioid endometrial adenocarcinoma: Is there a role of traditional hospital follow-up in the detection of cancer recurrence in women after treatment? Obs. Gynecol. Sci. 2021, 64, 506–516. [Google Scholar] [CrossRef]
- Aung, L.; Howells, R.E.; Lim, K.C.; Hudson, E.; Jones, P.W. Why routine clinical follow-up for patients with early stage endometrial cancer is not always necessary: A study on women in South Wales. Int. J. Gynecol. Cancer 2014, 24, 556–563. [Google Scholar] [CrossRef]
- Sarwar, A.; Van Griethuysen, J.; Waterhouse, J.; Dehbi, H.M.; Eminowicz, G.; McCormack, M. Stratified follow-up for endometrial cancer: A move to more personalized cancer care. Int. J. Gynecol. Cancer 2021, 31, 1564–1571. [Google Scholar] [CrossRef]
- Lindemann, K.; Smogeli, E.; Småstuen, M.C.; Bruheim, K.; Trovik, J.; Nordberg, T.; Kristensen, G.B.; Werner, H.M.; Nakken, E. Salvage radiation for pelvic relapse after surgically treated endometrial cancer. Cancers 2021, 13, 1367. [Google Scholar] [CrossRef]
- Mehanna, H.; McConkey, C.C.; Rahman, J.K.; Wong, W.L.; Smith, A.F.; Nutting, C.; Hartley, A.G.; Hall, P.; Hulme, C.; Patel, D.K.; et al. PET-NECK: A multicentre randomised Phase III non-inferiority trial comparing a positron emission tomography-computerised tomography-guided watch-and-wait policy with planned neck dissection in the management of locally advanced (N2/N3) nodal metastases in patients with squamous cell head and neck cancer. Health Technol. Assess 2017, 21, 1–122. [Google Scholar] [CrossRef]
- Lorenc, A.; Wells, M.; Fulton-Lieuw, T.; Nankivell, P.; Mehanna, H.; Jepson, M. Clinicians’ Views of Patient-initiated Follow-up in Head and Neck Cancer: A Qualitative Study to Inform the PETNECK2 Trial. Clin. Oncol. 2022, 34, 230–240. [Google Scholar] [CrossRef]
- Silvera-Tawil, D.; Pocock, C.; Bradford, D.; Donnell, A.; Freyne, J.; Harrap, K.; Brinkmann, S. Enabling Nurse-Patient Communication With a Mobile App: Controlled Pretest-Posttest Study With Nurses and Non-English-Speaking Patients. JMIR Nurs. 2021, 4, e19709. [Google Scholar] [CrossRef]
- Delon, C.; Brown, K.F.; Payne, N.W.S.; Kotrotsios, Y.; Vernon, S.; Shelton, J. Differences in cancer incidence by broad ethnic group in England, 2013–2017. Br. J. Cancer 2022, 126, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.; Beaver, K.; Langton, S. Exploring health care professionals views on alternative approaches to cancer follow-up and barriers and facilitators to implementation of a recovery package. Eur. J. Oncol. Nurs. 2020, 46, 101759. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.C.; Allen, P.K.; Jhingran, A.; Westin, S.N.; Lu, K.H.; Eifel, P.J.; Klopp, A.H. Management of nodal recurrences of endometrial cancer with IMRT. Gynecol. Oncol. 2015, 139, 40–46. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.M.; Bariani, G.M.; Cassier, P.A.; Marabelle, A.; Hansen, A.R.; De Jesus Acosta, A.; Miller, W.H., Jr.; Safra, T.; Italiano, A.; Mileshkin, L.; et al. Pembrolizumab in Patients with Microsatellite Instability-High Advanced Endometrial Cancer: Results From the KEYNOTE-158 Study. J. Clin. Oncol. 2022, 40, 752–761. [Google Scholar] [CrossRef]
- Germanova, A.; Raspagliesi, F.; Chiva, L.; Dusek, L.; Arvas, M.; Leblanc, E.; Lengeyl, T.; Di Donato, V.; Zaal, A.; Dursun, P.; et al. Oncological outcome of surgical management in patients with recurrent uterine cancer-a multicenter retrospective cohort study-CEEGOG EX01 Trial. Int. J. Gynecol. Cancer 2019, 29, 711–720. [Google Scholar] [CrossRef]
- Moss, E.L.; Gorsia, D.N.; Collins, A.; Sandhu, P.; Foreman, N.; Gore, A.; Wood, J.; Kent, C.; Silcock, L.; Guttery, D.S. Utility of Circulating Tumor DNA for Detection and Monitoring of Endometrial Cancer Recurrence and Progression. Cancers 2020, 12, 2231. [Google Scholar] [CrossRef]
- Niroomand, S.; Youseflu, S.; Gilani, M.M.; Kazemnejad, A.; Samani, L.N. Does educational-supportive program affect anxiety in women with endometrial cancer? Result from a randomized clinical trials. Indian J. Cancer 2021, 58, 336–341. [Google Scholar] [CrossRef]
- Beaver, K.; Martin-Hirsch, P.; Williamson, S.; Kyrgiou, M. Exploring the acceptability and feasibility of patient-initiated follow-up for women treated for stage I endometrial cancer. Eur. J. Oncol. Nurs. 2020, 44, 101704. [Google Scholar] [CrossRef]
- de Rooij, B.H.; Ezendam, N.P.M.; Nicolaije, K.A.H.; Lodder, P.; Vos, M.C.; Pijnenborg, J.M.A.; Boll, D.; Kruitwagen, R.; van de Poll-Franse, L.V. Survivorship care plans have a negative impact on long-term quality of life and anxiety through more threatening illness perceptions in gynecological cancer patients: The ROGY care trial. Qual. Life Res. 2018, 27, 1533–1544. [Google Scholar] [CrossRef]
- Nout, R.A.; van de Poll-Franse, L.V.; Lybeert, M.L.; Warlam-Rodenhuis, C.C.; Jobsen, J.J.; Mens, J.W.; Lutgens, L.C.; Pras, B.; van Putten, W.L.; Creutzberg, C.L. Long-term outcome and quality of life of patients with endometrial carcinoma treated with or without pelvic radiotherapy in the post operative radiation therapy in endometrial carcinoma 1 (PORTEC-1) trial. J. Clin. Oncol. 2011, 29, 1692–1700. [Google Scholar] [CrossRef]
- Tauber, N.M.; O’Toole, M.S.; Dinkel, A.; Galica, J.; Humphris, G.; Lebel, S.; Maheu, C.; Ozakinci, G.; Prins, J.; Sharpe, L.; et al. Effect of Psychological Intervention on Fear of Cancer Recurrence: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2019, 37, 2899–2915. [Google Scholar] [CrossRef]
- Mulhall, J.; Donohoe, F.; Moran, S.; Corry, E.; Glennon, K.; Broderick, S.; Nixon, E.; Tara, S.; Lennon, O.; McVey, R.; et al. From physical to virtual: How the COVID-19 pandemic changed a tertiary gynaecologic oncology surveillance program in Ireland. Gynecol. Oncol. Rep. 2021, 37, 100804. [Google Scholar] [CrossRef]
- Relton, A.; Collins, A.; Guttery, D.S.; Gorsia, D.N.; McDermott, H.J.; Moss, E.L. Patient acceptability of circulating tumour DNA testing in endometrial cancer follow-up. Eur. J. Cancer Care 2021, 30, e13429. [Google Scholar] [CrossRef]
- Farrell, C.; Molassiotis, A.; Beaver, K.; Heaven, C. Exploring the scope of oncology specialist nurses’ practice in the UK. Eur. J. Oncol. Nurs. 2011, 15, 160–166. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amirthanayagam, A.; Boulter, L.; Millet, N.; McDermott, H.J.; Morrison, J.; Taylor, A.; Miles, T.; Coton, L.; Moss, E.L. Risk Stratified Follow-Up for Endometrial Cancer: The Clinicians’ Perspective. Curr. Oncol. 2023, 30, 2237-2248. https://doi.org/10.3390/curroncol30020173
Amirthanayagam A, Boulter L, Millet N, McDermott HJ, Morrison J, Taylor A, Miles T, Coton L, Moss EL. Risk Stratified Follow-Up for Endometrial Cancer: The Clinicians’ Perspective. Current Oncology. 2023; 30(2):2237-2248. https://doi.org/10.3390/curroncol30020173
Chicago/Turabian StyleAmirthanayagam, Anumithra, Louise Boulter, Nessa Millet, Hilary J. McDermott, Jo Morrison, Alexandra Taylor, Tracie Miles, Lorna Coton, and Esther L. Moss. 2023. "Risk Stratified Follow-Up for Endometrial Cancer: The Clinicians’ Perspective" Current Oncology 30, no. 2: 2237-2248. https://doi.org/10.3390/curroncol30020173
APA StyleAmirthanayagam, A., Boulter, L., Millet, N., McDermott, H. J., Morrison, J., Taylor, A., Miles, T., Coton, L., & Moss, E. L. (2023). Risk Stratified Follow-Up for Endometrial Cancer: The Clinicians’ Perspective. Current Oncology, 30(2), 2237-2248. https://doi.org/10.3390/curroncol30020173