Oophorectomy in Premenopausal Patients with Estrogen Receptor-Positive Breast Cancer: New Insights into Long-Term Effects
Abstract
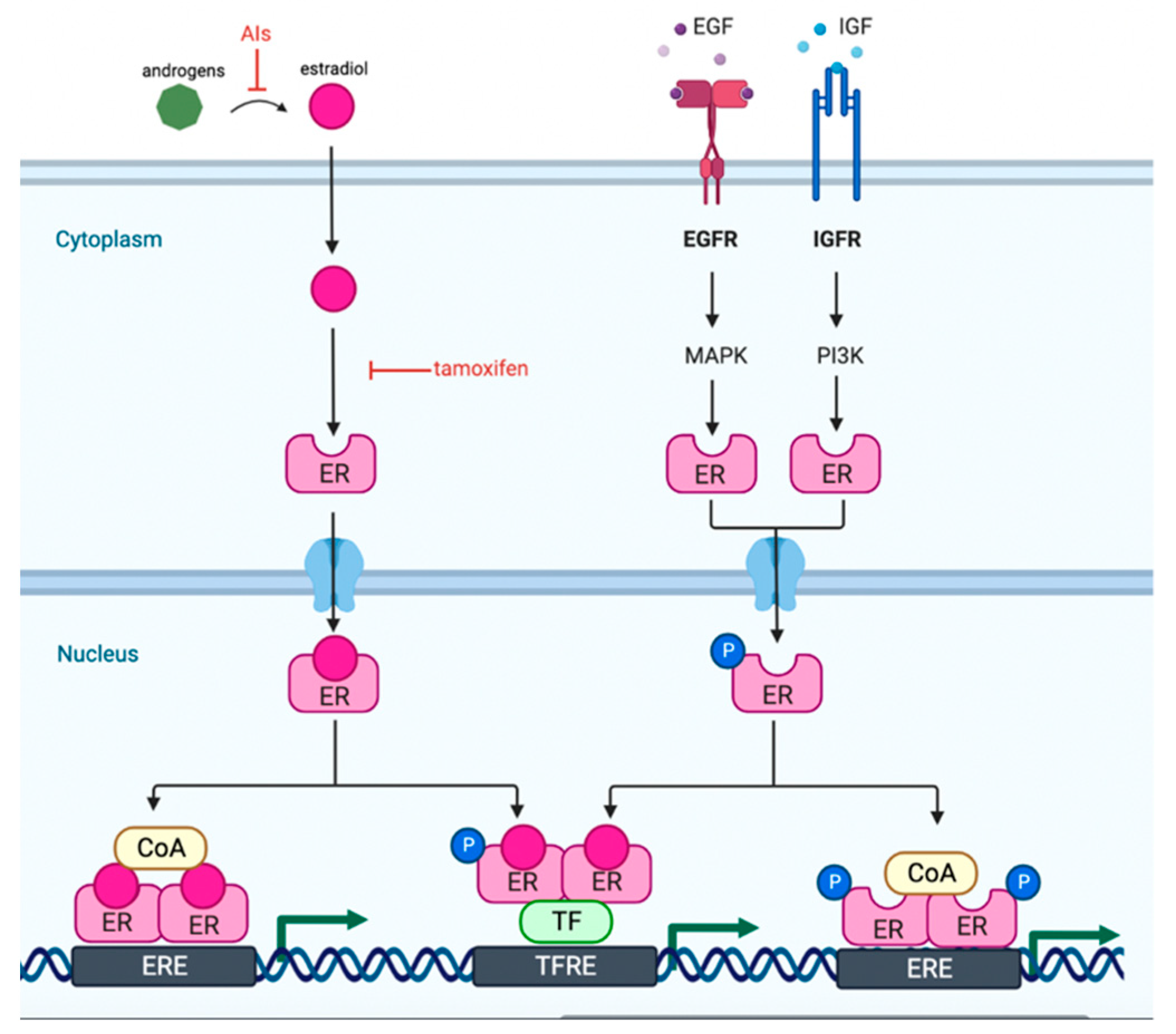
1. Introduction
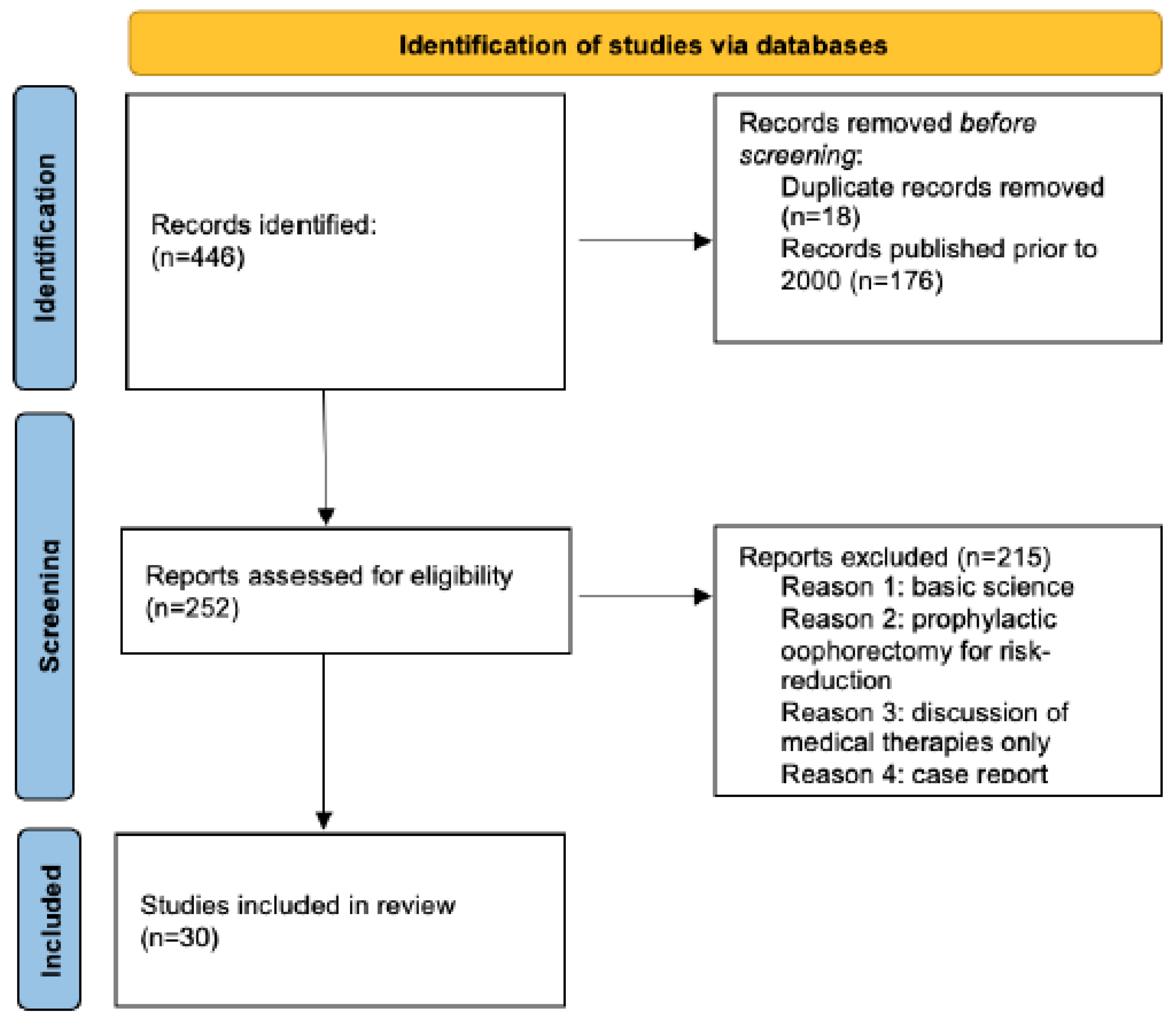
2. Methods
3. Principal Findings
4. Results
4.1. Oophorectomy vs. OFS in Premenopausal Patients Receiving SERMs
4.2. Oophorectomy vs. OFS in Premenopausal Patients Receiving AIs
4.3. Adverse Effects of Early Oophorectomy
4.3.1. Cardiovascular Effects
4.3.2. Bone Density
4.3.3. Quality of Life
4.3.4. Overall Mortality
5. Research Implications
6. Clinical Implications
7. Limitations
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Thomas, A.; Rhoads, A.; Pinkerton, E.; Schroeder, M.C.; Conway, K.M.; Hundley, W.G.; McNally, L.R.; Oleson, J.; Lynch, C.F.; A Romitti, P. Incidence and survival among young women with stage I-III breast cancer: SEER 2000–2015. JNCI Cancer Spectr. 2019, 3, 2–3. [Google Scholar] [CrossRef]
- Bui, K.T.; Willson, M.L.; Goel, S.; Beith, J.; Goodwin, A. Ovarian suppression for adjuvant treatment of hormone receptor-positive early breast cancer. Cochrane Database Syst. Rev. 2020, 3, 2. [Google Scholar] [CrossRef]
- Warner, E.T.; Colditz, G.A.; Palmer, J.R.; Partridge, A.H.; Rosner, B.A.; Tamimi, R.M. Reproductive factors and risk of premenopausal breast cancer by age at diagnosis: Are there differences before and after age 40? Breast Cancer Res. Treat. 2013, 142, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.C.; Mazzara, C.; Pagani, O. Diagnosis and Treatment of Breast Cancer in Young Women. Curr. Treat. Options Oncol. 2019, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Loizzi, V.; Dellino, M.; Cerbone, M.; Arezzo, F.; Chiariello, G.; Lepera, A.; Cazzato, G.; Cascardi, E.; Damiani, G.R.; Cicinelli, E.; et al. Hormone replacement therapy in BRCA mutation carriers: How shall we do no harm? Hormones 2023. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Loizzi, V.; Dellino, M.; Cerbone, M.; Arezzo, F.; Cazzato, G.; Damiani, G.R.; Pinto, V.; Silvestris, E.; Kardhashi, A.; Cicinelli, E.; et al. The Role of Hormonal Replacement Therapy in BRCA Mutated Patients: Lights and Shadows. Int. J. Mol. Sci. 2023, 24, 764. [Google Scholar] [CrossRef] [PubMed]
- LHRH-agonists in Early Breast Cancer Overview group. Use of luteinizing-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone- receptor-positive breast cancer: A meta-analysis of individual patient data from randomized adjuvant trials. Lancet 2007, 369, 1711–1723. [Google Scholar] [CrossRef]
- Francis, P.A.; Regan, M.M.; Fleming, G.F.; Láng, I.; Ciruelos, E.; Bellet, M.; Bonnefoi, H.R.; Climent, M.A.; Da Prada, G.A.; Burstein, H.J.; et al. Adjuvant ovarian suppression in premenopausal breast cancer. N. Engl. J. Med. 2015, 372, 436–446. [Google Scholar] [CrossRef]
- Francis, P.A.; Pagani, O.; Fleming, G.F.; Walley, B.A.; Colleoni, M.; Láng, I.; Gómez, H.L.; Tondini, C.; Ciruelos, E.; Burstein, H.J.; et al. Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer. N. Engl. J. Med. 2018, 379, 122–137. [Google Scholar] [CrossRef]
- Oliveira, J.C.; Sousa, F.C.; Gante, I.; Dias, M.F. Awareness of the causes leading to surgical ablation of ovarian function in premenopausal breast cancer-a single-center analysis. Medicina 2021, 57, 385. [Google Scholar] [CrossRef]
- Armstrong, K.; Schwartz, J.S.; Randall, T.; Rubin, S.C.; Weber, B. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: A decision analysis. J. Clin. Oncol. 2004, 22, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Makita, K.; Konukai, S.; Nozawa, S. Bone resorption versus estrogen loss following oophorectomy and menopause. Maturitas 2002, 43, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Rocca, W.A.; Bower, J.H.; Maraganore, D.M.; Ahlskog, J.E.; Grossardt, B.R.; De Andrade, M.; Melton, L. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 2007, 69, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Rocca, W.A.; Bower, J.H.; Maraganore, D.M.; Ahlskog, J.E.; Grossardt, B.R.; de Andrade, M.; Melton, L.J. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology 2008, 70, 200–209. [Google Scholar] [CrossRef]
- Parker, W.H. Bilateral oophorectomy versus ovarian conservation: Effects on long-term women’s health. J. Minim. Invasive Gynecol. 2010, 17, 161–166. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Blair, S.L.; Burstein, H.J.; Dang, C.; Elias, A.D.; et al. NCCN guidelines insights: Breast cancer, version 4.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 484–493. [Google Scholar] [CrossRef]
- Kirkham, A.A.; Beaudry, R.I.; Paterson, D.I.; Mackey, J.R.; Haykowsky, M.J. Curing breast cancer and killing the heart: A novel model to explain elevated cardiovascular disease and mortality risk among women with early-stage breast cancer. Prog. Cardiovasc. Dis. 2019, 62, 116–126. [Google Scholar] [CrossRef]
- Johnston, S.R.; Harbeck, N.; Hegg, R.; Toi, M.; Martin, M.; Shao, Z.M.; Zhang, Q.Y.; Rodriguez, J.L.; Campone, M.; Hamilton, E.; et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node- positive, high-risk, early breast cancer (monarchE). J. Clin. Oncol. 2020, 38, 3987–3998. [Google Scholar] [CrossRef] [PubMed]
- Jaiyesimi, I.A.; Buzdar, A.U.; Decker, D.A.; Hortobagyi, G.N. Use of tamoxifen for breast cancer: Twenty-eight years later. J. Clin. Oncol. 1995, 13, 513–529. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Tamoxifen and uterine cancer. Committee Opinion No. 601. Obs. Gynecol. 2014, 123, 1394–1397. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Anderson, B.O.; Balassanian, R.; Blair, S.L.; Burstein, H.J.; Cyr, A.; Elias, A.D.; Farrar, W.B.; Forero, A.; Giordano, S.H.; et al. NCCN Guidelines Insights: Breast Cancer, Version 1.2017. J. Natl. Compr. Cancer Netw. 2017, 15, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Adjuvant Breast Cancer Trials Collaborative Group. Ovarian ablation or suppression in premenopausal early breast cancer: Results from the international adjuvant breast cancer ovarian ablation or suppression randomized trial. J. Natl. Cancer Inst. 2007, 99, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Fleming, G.F.; Francis, P. Survival after adjuvant oophorectomy and tamoxifen in operable breast cancer in premenopausal women. J. Clin. Oncol. 2008, 26, 253–257. [Google Scholar] [CrossRef]
- Tevaarwerk, A.J.; Wang, M.; Zhao, F.; Fetting, J.H.; Cella, D.; Wagner, L.I.; Martino, S.; Ingle, J.N.; Sparano, J.A.; Solin, L.J.; et al. Phase III comparison of tamoxifen versus tamoxifen plus ovarian function suppression in premenopausal women with node-negative, hormone receptor-positive breast cancer (E- 3193, INT-0142): A trial of the Eastern Cooperative Oncology Group. J. Clin. Oncol. 2014, 32, 3948–3958. [Google Scholar] [CrossRef]
- Kim, H.A.; Lee, J.W.; Nam, S.J.; Park, B.W.; Im, S.A.; Lee, E.S. Adding Ovarian Suppression to Tamoxifen for Premenopausal Breast Cancer: A Randomized Phase III Trial. J. Clin. Oncol. 2020, 38, 434–443. [Google Scholar] [CrossRef]
- Baek, S.Y.; Noh, W.C.; Ahn, S.H.; Kim, H.A.; Ryu, J.M.; Kim, S.I.; Lee, E.G.; Im, S.A.; Jung, Y.; Park, M.H.; et al. Adding ovarian suppression to tamoxifen in young women with hormone-sensitive breast cancer who remain premenopausal or resume menstruation after chemotherapy: 8-year follow-up of the randomized ASTRRA trial. J. Clin. Oncol. 2022, 40 (Suppl. S16), 506. [Google Scholar] [CrossRef]
- Kalinsky, K.; Barlow, W.E.; Gralow, J.R.; Meric-Bernstam, F.; Albain, K.S.; Hayes, D.F.; Lin, N.U.; Perez, E.A.; Goldstein, L.J.; Chia, S.K.; et al. 21-Gene assay to inform chemotherapy benefit in node-positive breast cancer. N. Engl. J. Med. 2021, 385, 2336–2347. [Google Scholar] [CrossRef]
- Jain, S.; Santa-Maria, C.A.; Gradishar, W.J. The Role of Ovarian Suppression in Premenopausal Women With Hormone Receptor-Positive Early-Stage Breast Cancer. Oncology 2015, 29, 473–478. [Google Scholar]
- Suh, K.J.; Kim, S.H.; Lee, K.H.; Kim, T.Y.; Kim, Y.J.; Han, S.W.; Kang, E.; Kim, E.K.; Kim, K.; No, J.H.; et al. Bilateral Salpingo- oophorectomy Compared to Gonadotropin-Releasing Hormone Agonists in Premenopausal Hormone Receptor-Positive Metastatic Breast Cancer Patients Treated with Aromatase Inhibitors. Cancer Res. Treat. 2017, 49, 1153–1163. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.-P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics--2015 update: A report from the American Heart Association. Circulation 2015, 131, 29–322. [Google Scholar] [CrossRef]
- Mytton, J.; Evison, F.; Chilton, P.J.; Lilford, R.J. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: Study using routine data and data linkage. BMJ 2017, 356, 372. [Google Scholar] [CrossRef]
- Dørum, A.; Tonstad, S.; Liavaag, A.H.; Michelsen, T.M.; Hildrum, B.; Dahl, A.A. Bilateral oophorectomy before 50 years of age is significantly associated with the metabolic syndrome and Framingham risk score: A controlled, population-based study (HUNT-2). Gynecol. Oncol. 2008, 109, 377–383. [Google Scholar] [CrossRef]
- Honigberg, M.C.; Patel, A.P.; Lahm, T.; Wood, M.J.; Ho, J.E.; Kohli, P.; Natarajan, P. Association of premature menopause with incident pulmonary hypertension: A cohort study. PLoS ONE 2021, 16, e0247398. [Google Scholar] [CrossRef] [PubMed]
- Honigberg, M.; Zekavat, S.; Aragam, K.; Finneran, P.; Klarin, D.; Bhatt, D.L.; Januzzi, J.L.; Scott, N.S.; Natarajan, P. Association of Premature Natural and Surgical Menopause With Incident Cardiovascular Disease. JAMA 2019, 322, 2411–2421. [Google Scholar] [CrossRef]
- Parker, W.H. Ovarian conservation versus bilateral oophorectomy at the time of hysterectomy for benign disease. Menopause 2014, 21, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.H.; Feskanich, D.; Broder, M.S.; Chang, E.; Shoupe, D.; Farquhar, C.M. Long- term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet. Gynecol. 2013, 121, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Appiah, D.; Nwabuo, C.C.; Owoade, D.R.; Samad, J.; Ebong, I.; Winters, S.J. Family history of premature myocardial infarction modifies the associations between bilateral oophorectomy and cardiovascular disease mortality in a US national cohort of postmenopausal women. Menopause 2020, 27, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Hibler, E.A.; Kauderer, J.; Greene, M.H.; Rodriguez, G.C.; Alberts, D.S. Bone loss after oophorectomy among high-risk women: An NRG oncology/gynecologic oncology group study. Menopause 2016, 23, 1228–1232. [Google Scholar] [CrossRef]
- Love, R.R.; Young, G.S.; Laudico, A.V.; Dinh, N.V.; Uy, G.B.; Quang, L.H. Bone mineral densityfollowing surgical oophorectomy and tamoxifen adjuvant therapy for breast cancer. Cancer 2013, 119, 3746–3752. [Google Scholar] [CrossRef]
- Reeves, K.W.; Pennell, M.; Foraker, R.E.; Crandall, C.J.; Stefanick, M.; Paskett, E.D. Predictors of vasomotor symptoms among breast cancer survivors. J. Cancer Surviv. 2018, 12, 379–387. [Google Scholar] [CrossRef]
- von Hippel, C.; Rosenberg, S.M.; Austin, S.B.; Sprunck-Harrild, K.; Ruddy, K.J.; Schapira, L.; Come, S.; Borges, V.F.; Partridge, A.H. Identifying distinct trajectories of change in young breast cancer survivors’ sexual functioning. Psychooncology 2019, 28, 1033–1040. [Google Scholar] [CrossRef]
- Tuesley, K.M.; Protani, M.M.; Webb, P.M.; Dixon-Suen, S.C.; Wilson, L.F.; Stewart, L.M.; Jordan, S.J. Hysterectomy with and without oophorectomy and all-cause and cause-specific mortality. Am. J. Obstet. Gynecol. 2020, 223, e1–e723. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.M.; Grossardt, B.R.; Rhodes, D.J.; Brown, R.D., Jr.; Roger, V.L.; Melton, L.J., III; Rocca, W.A. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause 2009, 16, 15–23. [Google Scholar] [CrossRef]
- Gierach, G.L.; Pfeiffer, R.M.; Patel, D.A.; Black, A.; Schairer, C.; Gill, A.; Brinton, L.A.; Sherman, M.E. Long-term overall and disease-specific mortality associated with benign gynecologic surgery performed at different ages. Menopause 2014, 21, 592–601. [Google Scholar] [CrossRef] [PubMed]
- QuickStats: Age-Adjusted Death Rates* from Female Breast Cancer, (dagger) by State—National Vital Statistics System, United States, (section sign) 2017. Morb. Mortal. Wkly. Rep. (MMWR) 2019, 68, 617. [CrossRef] [PubMed]
- Deaths, Percent of Total Deaths, and Death Rates for the 15 Leading Causes of Death in 5- Year Age Groups, by Race and sex: United States, 1999–2015. LWK1. 2015. Available online: https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm (accessed on 27 October 2022).
- Regan, M.M.; Pagani, O.; Fleming, G.F.; Walley, B.A.; Price, K.N.; Rabaglio, M.; Maibach, R.; Ruepp, B.; Coates, A.S.; Goldhirsch, A.; et al. Adjuvant treatment of premenopausal women with endocrine-responsive early breast cancer: Design of the TEXT and SOFT trials. Breast 2013, 22, 1094–1100. [Google Scholar] [CrossRef]
- Kwon, J.S.; Pansegrau, G.; Nourmoussavi, M.; Hammond, G.L.; Carey, M.S. Costs and Benefits of Extended Endocrine Strategies for Premenopausal Breast Cancer. J. Natl. Compr. Cancer Netw. 2017, 15, 1015–1021. [Google Scholar] [CrossRef]
- Kwon, J.S.; Pansegrau, G.; Nourmoussavi, M.; Hammond, G.L.; Carey, M.S. Long-term consequences of ovarian ablation for premenopausal breast cancer. Breast Cancer Res. Treat. 2016, 157, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Ferrandina, G.; Amadio, G.; Marcellusi, A.; Azzolini, E.; Puggina, A.; Pastorino, R.; Ricciardi, W.; Scambia, G. Bilateral Salpingo-Oophorectomy Versus GnRH Analogue in the Adjuvant Treatment of Premenopausal Breast Cancer Patients: Cost-Effectiveness Evaluation of Breast Cancer Outcome, Ovarian Cancer Prevention and Treatment. Clin. Drug Investig. 2017, 37, 1093–1102. [Google Scholar] [CrossRef]
- Love, R.R. Adjuvant surgical oophorectomy plus tamoxifen in premenopausal women with operable hormone receptor-positive breast cancer: A global treatment option. Clin. Breast Cancer 2016, 16, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Bodai, B.I.; Tuso, P. Breast cancer survivorship: A comprehensive review of long-term medical issues and lifestyle recommendations. Perm. J. 2015, 19, 48–79. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, F.; Rojas, K.; Schlumbrecht, M.; Jeudin, P. Oophorectomy in Premenopausal Patients with Estrogen Receptor-Positive Breast Cancer: New Insights into Long-Term Effects. Curr. Oncol. 2023, 30, 1794-1804. https://doi.org/10.3390/curroncol30020139
Khan F, Rojas K, Schlumbrecht M, Jeudin P. Oophorectomy in Premenopausal Patients with Estrogen Receptor-Positive Breast Cancer: New Insights into Long-Term Effects. Current Oncology. 2023; 30(2):1794-1804. https://doi.org/10.3390/curroncol30020139
Chicago/Turabian StyleKhan, Fatima, Kristin Rojas, Matthew Schlumbrecht, and Patricia Jeudin. 2023. "Oophorectomy in Premenopausal Patients with Estrogen Receptor-Positive Breast Cancer: New Insights into Long-Term Effects" Current Oncology 30, no. 2: 1794-1804. https://doi.org/10.3390/curroncol30020139
APA StyleKhan, F., Rojas, K., Schlumbrecht, M., & Jeudin, P. (2023). Oophorectomy in Premenopausal Patients with Estrogen Receptor-Positive Breast Cancer: New Insights into Long-Term Effects. Current Oncology, 30(2), 1794-1804. https://doi.org/10.3390/curroncol30020139




