Identifying Optimal Candidates for Trimodality Therapy among Nonmetastatic Muscle-Invasive Bladder Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
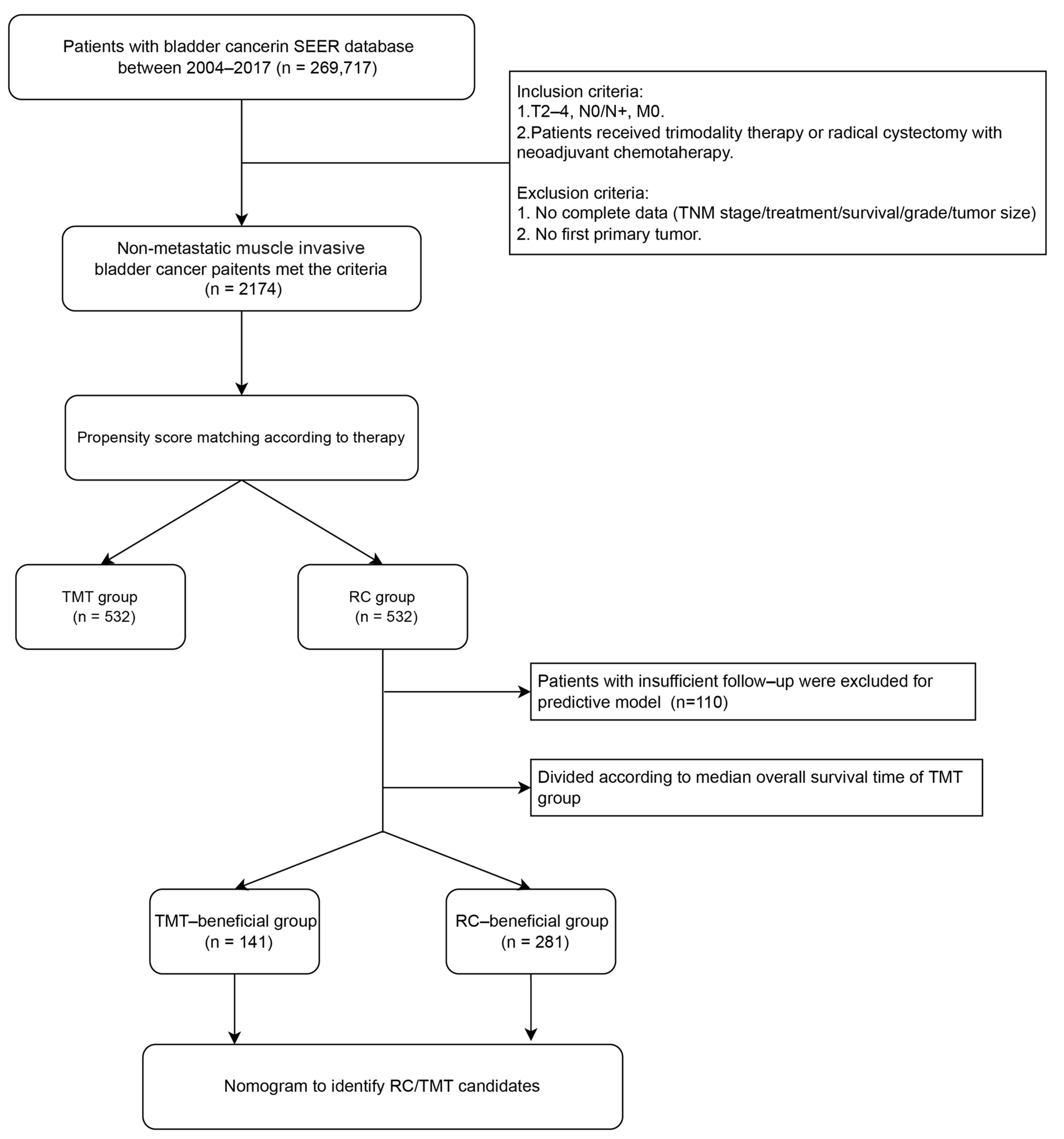
2.1. Data Source and Patient Selection
2.2. Statistical Analysis
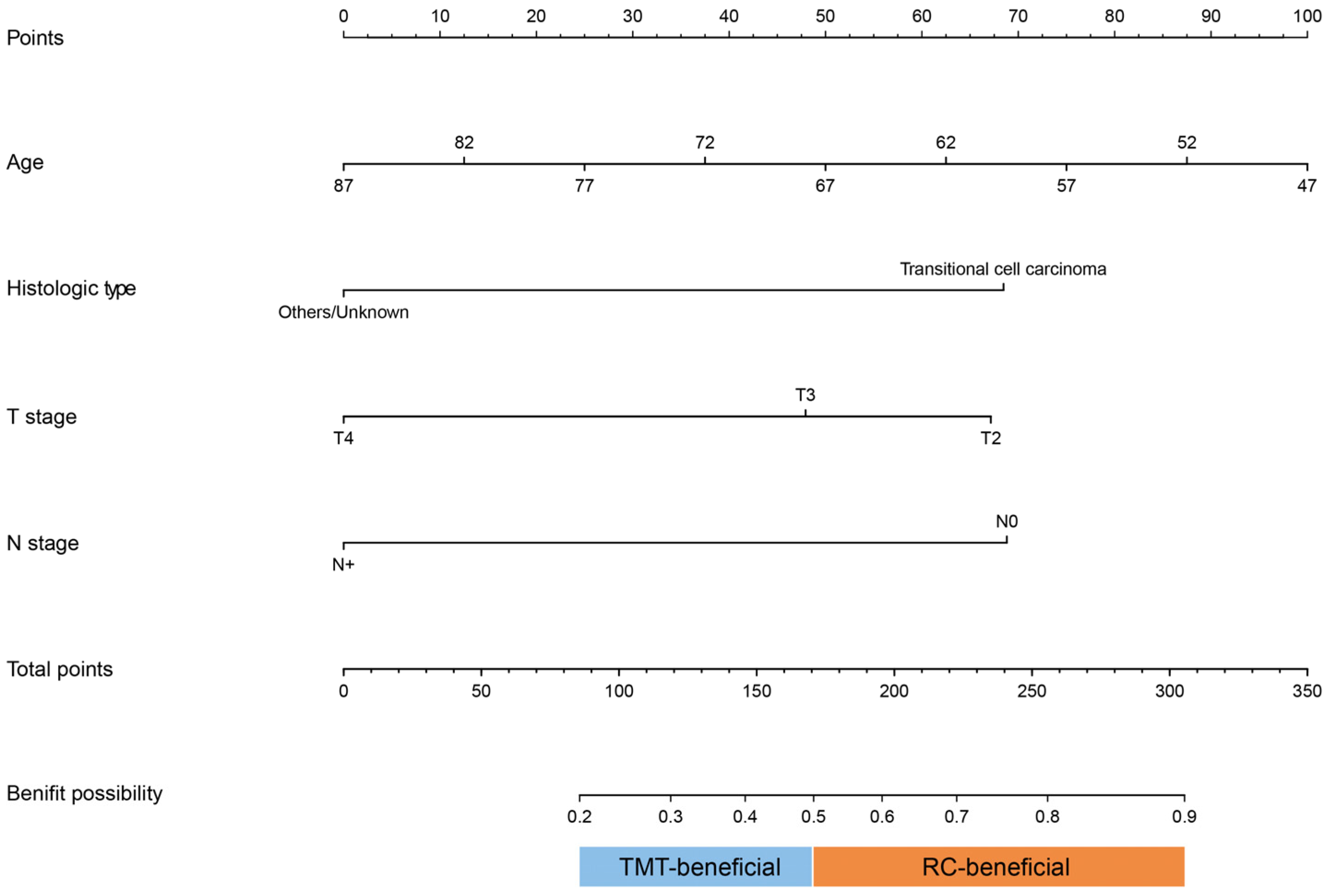
2.3. Construction, Validation, and Clinical Application of the Nomogram
3. Results
3.1. Selection of Patients and Characteristics
3.2. Survival in Nonmetastatic MIBC Patients
3.3. Nomogram to Identify Candidates for RC or TMT
3.4. Validation and Clinical Application of Prediction Nomogram
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Bellmunt, J.; Comperat, E.; De Santis, M.; Huddart, R.; Loriot, Y.; Necchi, A.; Valderrama, B.P.; Ravaud, A.; Shariat, S.F.; et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, J.; Ruan, W.; Huang, M.; Wang, C.; Wang, H.; Jiang, Z.; Wang, S.; Liu, Z.; Liu, C.; et al. Urine DNA methylation assay enables early detection and recurrence monitoring for bladder cancer. J. Clin. Investig. 2020, 130, 6278–6289. [Google Scholar] [CrossRef] [PubMed]
- Malmström, P.U.; Rintala, E.; Wahlqvist, R.; Hellström, P.; Hellsten, S.; Hannisdal, E. Five-year followup of a prospective trial of radical cystectomy and neoadjuvant chemotherapy: Nordic Cystectomy Trial I. The Nordic Cooperative Bladder Cancer Study Group. J. Urol. 1996, 155, 1903–1906. [Google Scholar] [CrossRef]
- Burger, M.; Catto, J.W.F.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.; Sfakianos, J.P.; Renström-Koskela, L.; Mortezavi, A.; Falagario, U.G.; Egevad, L.; Hosseini, A.; Mehrazin, R.; Galsky, M.D.; Steineck, G.; et al. The natural history of untreated muscle-invasive bladder cancer. BJU Int. 2020, 125, 270–275. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef]
- Chang, S.S.; Bochner, B.H.; Chou, R.; Dreicer, R.; Kamat, A.M.; Lerner, S.P.; Lotan, Y.; Meeks, J.J.; Michalski, J.M.; Morgan, T.M.; et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J. Urol. 2017, 198, 552–559. [Google Scholar] [CrossRef]
- Zlotta, A.R.; Ballas, L.K.; Niemierko, A.; Lajkosz, K.; Kuk, C.; Miranda, G.; Drumm, M.; Mari, A.; Thio, E.; Fleshner, N.E.; et al. Radical cystectomy versus trimodality therapy for muscle-invasive bladder cancer: A multi-institutional propensity score matched and weighted analysis. Lancet Oncol. 2023, 24, 669–681. [Google Scholar] [CrossRef]
- Softness, K.; Kaul, S.; Fleishman, A.; Efstathiou, J.; Bellmunt, J.; Kim, S.P.; Korets, R.; Chang, P.; Wagner, A.; Olumi, A.F.; et al. Radical cystectomy versus trimodality therapy for muscle-invasive urothelial carcinoma of the bladder. Urol. Oncol. 2022, 40, 272.e1–272.e9. [Google Scholar] [CrossRef]
- Fahmy, O.; Khairul-Asri, M.G.; Schubert, T.; Renninger, M.; Malek, R.; Kübler, H.; Stenzl, A.; Gakis, G. A systematic review and meta-analysis on the oncological long-term outcomes after trimodality therapy and radical cystectomy with or without neoadjuvant chemotherapy for muscle-invasive bladder cancer. Urol. Oncol. 2018, 36, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, G.S.; Hermanns, T.; Wei, Y.; Bhindi, B.; Satkunasivam, R.; Athanasopoulos, P.; Bostrom, P.J.; Kuk, C.; Li, K.; Templeton, A.J.; et al. Propensity Score Analysis of Radical Cystectomy Versus Bladder-Sparing Trimodal Therapy in the Setting of a Multidisciplinary Bladder Cancer Clinic. J. Clin. Oncol. 2017, 35, 2299–2305. [Google Scholar] [CrossRef] [PubMed]
- Efstathiou, J.A.; Spiegel, D.Y.; Shipley, W.U.; Heney, N.M.; Kaufman, D.S.; Niemierko, A.; Coen, J.J.; Skowronski, R.Y.; Paly, J.J.; McGovern, F.J.; et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: The MGH experience. Eur. Urol. 2012, 61, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Mignot, F.; Fabiano, E.; Xylinas, E.; Alati, A.; Méjean, A.; Masson-Lecomte, A.; Hermieu, J.-F.; Desgrandchamps, F.; Hennequin, C.; Durdux, C.; et al. Clinical outcomes of adapted hypofractionated radiotherapy for bladder cancer in elderly patients. BJU Int. 2023, 132, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Swinton, M.; Mariam, N.B.G.; Tan, J.L.; Murphy, K.; Elumalai, T.; Soni, M.; Ferrera, A.; Richardson, C.; Walshaw, R.; Mistry, H.; et al. Bladder-Sparing Treatment With Radical Dose Radiotherapy Is an Effective Alternative to Radical Cystectomy in Patients With Clinically Node-Positive Nonmetastatic Bladder Cancer. J. Clin. Oncol. 2023, 41, 4406–4415. [Google Scholar] [CrossRef] [PubMed]
- Reignier, P.-L.; Gauthier, H.; Hennequin, C.; Aussedat, Q.; Xylinas, E.; Desgrandchamps, F.; Culine, S.; Masson-Lecomte, A.; Dumont, C. Survival after sequential neoadjuvant chemotherapy followed by trimodal treatment or radical cystectomy for muscle-invasive bladder cancer. World J. Urol. 2023, 41, 3249–3255. [Google Scholar] [CrossRef] [PubMed]
- Kamran, S.C.; Efstathiou, J.A. The Legacy of RTOG/NRG Protocols in Shaping Current Bladder Preservation Therapy in North America. Semin. Radiat. Oncol. 2023, 33, 26–34. [Google Scholar] [CrossRef]
- Francolini, G.; Borghesi, S.; Fersino, S.; Magli, A.; Jereczek-Fossa, B.A.; Cristinelli, L.; Rizzo, M.; Corvò, R.; Pappagallo, G.L.; Arcangeli, S.; et al. Treatment of muscle-invasive bladder cancer in patients without comorbidities and fit for surgery: Trimodality therapy vs radical cystectomy. Development of GRADE (Grades of Recommendation, Assessment, Development and Evaluation) recommendation by the Italian Association of Radiotherapy and Clinical Oncology (AIRO). Crit. Rev. Oncol. /Hematol. 2021, 159, 103235. [Google Scholar] [CrossRef]
- Zhong, J.; Switchenko, J.; Jegadeesh, N.K.; Cassidy, R.J.; Gillespie, T.W.; Master, V.; Nieh, P.; Alemozaffar, M.; Kucuk, O.; Carthon, B.; et al. Comparison of Outcomes in Patients With Muscle-invasive Bladder Cancer Treated With Radical Cystectomy Versus Bladder Preservation. Am. J. Clin. Oncol. 2019, 42, 36–41. [Google Scholar] [CrossRef]
- Giacalone, N.J.; Shipley, W.U.; Clayman, R.H.; Niemierko, A.; Drumm, M.; Heney, N.M.; Michaelson, M.D.; Lee, R.J.; Saylor, P.J.; Wszolek, M.F.; et al. Long-term Outcomes After Bladder-preserving Tri-modality Therapy for Patients with Muscle-invasive Bladder Cancer: An Updated Analysis of the Massachusetts General Hospital Experience. Eur. Urol. 2017, 71, 952–960. [Google Scholar] [CrossRef]
- Büchser, D.; Zapatero, A.; Rogado, J.; Talaya, M.; Martín de Vidales, C.; Arellano, R.; Bocardo, G.; Cruz Conde, A.; Pérez, L.; Murillo, M.T. Long-term Outcomes and Patterns of Failure Following Trimodality Treatment With Bladder Preservation for Invasive Bladder Cancer. Urology 2019, 124, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Svatek, R.S.; Ji, N.; de Leon, E.; Mukherjee, N.Z.; Kabra, A.; Hurez, V.; Nicolas, M.; Michalek, J.E.; Javors, M.; Wheeler, K.; et al. Rapamycin Prevents Surgery-Induced Immune Dysfunction in Patients with Bladder Cancer. Cancer Immunol. Res. 2019, 7, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Sapre, N.; Anderson, P.; Foroudi, F. Management of local recurrences in the irradiated bladder: A systematic review. BJU Int. 2012, 110 (Suppl. 4), 51–57. [Google Scholar] [CrossRef] [PubMed]
- Onozawa, M.; Miyanaga, N.; Hinotsu, S.; Miyazaki, J.; Oikawa, T.; Kimura, T.; Takaoka, E.; Kawai, K.; Shimazui, T.; Sakurai, H.; et al. Analysis of Intravesical Recurrence After Bladder-preserving Therapy for Muscle-invasive Bladder Cancer. Jpn. J. Clin. Oncol. 2012, 42, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, K.; Ko, H.; Huang, R.; Tsai, P.; Chen, M.; Lin, Y.; Hwang, T.; Juang, G.; Chi, K.J.R.o. Trimodality bladder-sparing approach without neoadjuvant chemotherapy for node-negative localized muscle-invasive urinary bladder cancer resulted in comparable cystectomy-free survival. Radiat. Oncol. 2014, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Krasnow, R.E.; Drumm, M.; Roberts, H.J.; Niemierko, A.; Wu, C.L.; Wu, S.; Zhang, J.; Heney, N.M.; Wszolek, M.F.; Blute, M.L.; et al. Clinical Outcomes of Patients with Histologic Variants of Urothelial Cancer Treated with Trimodality Bladder-sparing Therapy. Eur. Urol. 2017, 72, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Gao, S.; Lu, C.; Wu, X.; Zuo, L.; Zhang, L. Comparing Prognosis Associated with Partial Cystectomy and Trimodal Therapy for Muscle-Invasive Bladder Cancer Patients. Urol. Int. 2023, 107, 46–57. [Google Scholar] [CrossRef]
- Nagumo, Y.; Kawai, K.; Kojima, T.; Shiga, M.; Kojo, K.; Tanaka, K.; Kandori, S.; Kimura, T.; Kawahara, T.; Okuyama, A.; et al. Prognostic significance of non-urothelial carcinoma of bladder: Analysis of nationwide hospital-based cancer registry data in Japan. Jpn. J. Clin. Oncol. 2020, 50, 1068–1075. [Google Scholar] [CrossRef]
- Deuker, M.; Martin, T.; Stolzenbach, F.; Rosiello, G.; Collà Ruvolo, C.; Nocera, L.; Tian, Z.; Becker, A.; Kluth, L.; Roos, F.C.; et al. Bladder Cancer: A Comparison Between Non-urothelial Variant Histology and Urothelial Carcinoma Across All Stages and Treatment Modalities. Clin. Genitourin. Cancer 2021, 19, 60–68.e1. [Google Scholar] [CrossRef]
- Jiang, D.M.; Chung, P.; Kulkarni, G.S.; Sridhar, S.S. Trimodality Therapy for Muscle-Invasive Bladder Cancer: Recent Advances and Unanswered Questions. Curr. Oncol. Rep. 2020, 22, 14. [Google Scholar] [CrossRef]
- Mak, K.S.; Smith, A.B.; Eidelman, A.; Clayman, R.; Niemierko, A.; Cheng, J.S.; Matthews, J.; Drumm, M.R.; Nielsen, M.E.; Feldman, A.S.; et al. Quality of Life in Long-term Survivors of Muscle-Invasive Bladder Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Zietman, A.L.; Sacco, D.; Skowronski, U.; Gomery, P.; Kaufman, D.S.; Clark, J.A.; Talcott, J.A.; Shipley, W.U. Organ conservation in invasive bladder cancer by transurethral resection, chemotherapy and radiation: Results of a urodynamic and quality of life study on long-term survivors. J. Urol. 2003, 170, 1772–1776. [Google Scholar] [CrossRef] [PubMed]
- Kachnic, L.A.; Shipley, W.U.; Griffin, P.P.; Zietman, A.L.; Kaufman, D.S.; Althausen, A.F.; Heney, N.M. Combined modality treatment with selective bladder conservation for invasive bladder cancer: Long-term tolerance in the female patient. Cancer J. Sci. Am. 1996, 2, 79–84. [Google Scholar] [PubMed]
- Royce, T.J.; Feldman, A.S.; Mossanen, M.; Yang, J.C.; Shipley, W.U.; Pandharipande, P.V.; Efstathiou, J.A. Comparative Effectiveness of Bladder-preserving Tri-modality Therapy Versus Radical Cystectomy for Muscle-invasive Bladder Cancer. Clin. Genitourin. Cancer 2019, 17, 23–31.e3. [Google Scholar] [CrossRef]
- Bruins, H.M.; Veskimäe, E.; Hernández, V.; Neuzillet, Y.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Espinós, E.L.; Lorch, A.; et al. The Importance of Hospital and Surgeon Volume as Major Determinants of Morbidity and Mortality After Radical Cystectomy for Bladder Cancer: A Systematic Review and Recommendations by the European Association of Urology Muscle-invasive and Metastatic Bladder Cancer Guideline Panel. Eur. Urol. Oncol. 2020, 3, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.B.; Shan, Y.; Jazzar, U.; Mehta, H.B.; Baillargeon, J.G.; Huo, J.; Senagore, A.J.; Orihuela, E.; Tyler, D.S.; Swanson, T.A.; et al. Comparing Survival Outcomes and Costs Associated With Radical Cystectomy and Trimodal Therapy for Older Adults With Muscle-Invasive Bladder Cancer. JAMA Surg. 2018, 153, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.P.; Lieskovsky, G.; Cote, R.; Groshen, S.; Feng, A.C.; Boyd, S.; Skinner, E.; Bochner, B.; Thangathurai, D.; Mikhail, M.; et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J. Clin. Oncol. 2001, 19, 666–675. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Pansadoro, V.; Calabrò, F.; Schnetzer, S.; Giannarelli, D.; Emiliozzi, P.; De Paula, F.; Scarpone, P.; De Carli, P.; Pizzo, M.; et al. Can patient selection for bladder preservation be based on response to chemotherapy? Cancer 2003, 97, 1644–1652. [Google Scholar] [CrossRef]
- Hu, J.; Chen, J.; Ou, Z.; Chen, H.; Liu, Z.; Chen, M.; Zhang, R.; Yu, A.; Cao, R.; Zhang, E.; et al. Neoadjuvant immunotherapy, chemotherapy, and combination therapy in muscle-invasive bladder cancer: A multi-center real-world retrospective study. Cell Rep. Med. 2022, 3, 100785. [Google Scholar] [CrossRef]
- Pedrosa, J.A.; Koch, M.O.; Cheng, L. Lymph node-positive bladder cancer: Surgical, pathologic, molecular and prognostic aspects. Expert Rev. Anticancer. Ther. 2013, 13, 1281–1295. [Google Scholar] [CrossRef]




| Variable | Overall Cohort | p | Matched Cohort | p | ||
|---|---|---|---|---|---|---|
| RC | TMT | RC | TMT | |||
| n = 1170(%) | n = 1004(%) | n = 532(%) | n = 532(%) | |||
| Age (median (IQR)) | 62.00 [57.00, 72.00] | 77.00 [67.00, 82.00] | <0.001 | 67.00 [62.00, 72.00] | 67.00 [62.00, 72.00] | 0.999 |
| Gender | 0.001 | 1.000 | ||||
| Male | 782 (66.8) | 738 (73.5) | 386 (72.6) | 385 (72.4) | ||
| Female | 388 (33.2) | 266 (26.5) | 146 (27.4) | 147 (27.6) | ||
| Race | 0.752 | 0.272 | ||||
| White | 1017 (86.9) | 868 (86.5) | 470 (88.3) | 457 (85.9) | ||
| Others/unknown | 153 (13.1) | 136 (13.5) | 62 (11.7) | 75 (14.1) | ||
| Primary site | 0.020 | 0.999 | ||||
| Trigone of bladder | 83 (7.1) | 74 (7.4) | 40 (7.5) | 39 (7.3) | ||
| Bladder neck | 24 (2.1) | 28 (2.8) | 11 (2.1) | 11 (2.1) | ||
| Lateral wall of bladder | 255 (21.8) | 253 (25.2) | 122 (22.9) | 119 (22.4) | ||
| Posterior wall of bladder | 91 (7.8) | 102 (10.2) | 49 (9.2) | 51 (9.6) | ||
| Others/Unknown | 717 (61.3) | 547 (54.5) | 310 (58.3) | 312 (58.6) | ||
| Histologic type | 1.000 | 0.845 | ||||
| Urothelium carcinoma | 1058 (90.4) | 908 (90.4) | 475 (89.3) | 472 (88.7) | ||
| Others/unknown | 112 (9.6) | 96 (9.6) | 57 (10.7) | 60 (11.3) | ||
| Grade | 0.090 | 0.862 | ||||
| G 1/2 | 31 (2.6) | 40 (4.0) | 18 (3.4) | 16 (3.0) | ||
| G 3/4 | 1139 (97.4) | 964 (96.0) | 514 (96.6) | 516 (97.0) | ||
| T stage | <0.001 | 0.772 | ||||
| T 2 | 590 (50.4) | 837 (83.4) | 379 (71.2) | 381 (71.6) | ||
| T 3 | 391 (33.4) | 91 (9.1) | 81 (15.2) | 86 (16.2) | ||
| T 4 | 189 (16.2) | 76 (7.6) | 72 (13.5) | 65 (12.2) | ||
| N stage | <0.001 | 0.428 | ||||
| N 0 | 325 (27.8) | 76 (7.6) | 80 (15.0) | 70 (13.2) | ||
| N + | 845 (72.2) | 928 (92.4) | 452 (85.0) | 462 (86.8) | ||
| Tumor size, mm (mean (SD)) | 45.16 (34.83) | 45.99 (20.76) | 0.511 | 46.29 (44.95) | 46.45 (20.83) | 0.940 |
| Covariate | Regression Coefficient (SE) | Odds Ratio (95% CI) | p |
|---|---|---|---|
| Age | −0.035 (0.015) | 0.966 (0.938–0.994) | 0.017 |
| Gender | |||
| Male | References | ||
| Female | −0.058 (0.262) | 0.944 (0.565–1.578) | 0.826 |
| Race | |||
| White | Reference | ||
| Others/unknown | 0.004 (0.365) | 1.004 (0.491–2.054) | 0.992 |
| Primary site | |||
| Trigone of bladder | Reference | ||
| Bladder neck | 0.428 (1.04) | 1.534 (0.2–11.779) | 0.68 |
| Lateral wall of bladder | 0.015 (0.478) | 1.015 (0.397–2.591) | 0.975 |
| Posterior wall of bladder | 0.401 (0.605) | 1.494 (0.457–4.889) | 0.507 |
| Others/unknown | −0.263 (0.435) | 0.769 (0.328–1.804) | 0.546 |
| Histologic type | |||
| Urothelium carcinoma | Reference | ||
| Others/unknown | −1.254 (0.394) | 0.285 (0.132–0.618) | 0.001 |
| Grade | |||
| G 1/2 | Reference | ||
| G 3/4 | −0.9 (0.664) | 0.407 (0.111–1.493) | 0.175 |
| T stage | |||
| T 2 | Reference | ||
| T 3 | −0.778 (0.3) | 0.459 (0.255–0.826) | 0.009 |
| T 4 | −1.277 (0.336) | 0.279 (0.144–0.539) | <0.001 |
| N stage | |||
| N 0 | Reference | ||
| N + | −1.127 (0.325) | 0.324 (0.171–0.613) | 0.001 |
| Tumor size | 0.004 (0.004) | 1.004 (0.997–1.012) | 0.272 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, S.; Yang, J.; Hu, J.; Fang, L.; He, W. Identifying Optimal Candidates for Trimodality Therapy among Nonmetastatic Muscle-Invasive Bladder Cancer Patients. Curr. Oncol. 2023, 30, 10166-10178. https://doi.org/10.3390/curroncol30120740
Ran S, Yang J, Hu J, Fang L, He W. Identifying Optimal Candidates for Trimodality Therapy among Nonmetastatic Muscle-Invasive Bladder Cancer Patients. Current Oncology. 2023; 30(12):10166-10178. https://doi.org/10.3390/curroncol30120740
Chicago/Turabian StyleRan, Shengming, Jingtian Yang, Jintao Hu, Liekui Fang, and Wang He. 2023. "Identifying Optimal Candidates for Trimodality Therapy among Nonmetastatic Muscle-Invasive Bladder Cancer Patients" Current Oncology 30, no. 12: 10166-10178. https://doi.org/10.3390/curroncol30120740
APA StyleRan, S., Yang, J., Hu, J., Fang, L., & He, W. (2023). Identifying Optimal Candidates for Trimodality Therapy among Nonmetastatic Muscle-Invasive Bladder Cancer Patients. Current Oncology, 30(12), 10166-10178. https://doi.org/10.3390/curroncol30120740




