A Population Description of Young Women with Breast Cancer in Newfoundland and Labrador
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canada, Public Health Agency of Canadian Cancer Statistics. 2021. Available online: https://www.canada.ca/en/public-health/services/reports-publications/health-promotion-chronic-disease-prevention-canada-research-policy-practice/vol-41-no-11-2021/canadian-cancer-statistics-2021.html (accessed on 31 March 2023).
- Street, W. Breast Cancer Facts & Figures 2019–2020; American Cancer Society: Atlanta, GA, USA, 2019. [Google Scholar]
- Thomas, A.; Rhoads, A.; Pinkerton, E.; Schroeder, M.C.; Conway, K.M.; Hundley, W.G.; McNally, L.R.; Oleson, J.; Lynch, C.F.; Romitti, P.A. Incidence and Survival among Young Women with Stage I–III Breast Cancer: SEER 2000–2015. JNCI Cancer Spectr. 2019, 3, pkz040. [Google Scholar] [CrossRef] [PubMed]
- Azim, H.A.; Partridge, A.H. Biology of Breast Cancer in Young Women. Breast Cancer Res. BCR 2014, 16, 427. [Google Scholar] [CrossRef] [PubMed]
- Rudra, S.; Yu, D.S.; Yu, E.S.; Switchenko, J.M.; Mister, D.; Torres, M.A. Locoregional and Distant Recurrence Patterns in Young versus Elderly Women Treated for Breast Cancer. Int. J. Breast Cancer 2015, 2015, 213123. [Google Scholar] [CrossRef] [PubMed]
- Gnerlich, J.L.; Deshpande, A.D.; Jeffe, D.B.; Sweet, A.; White, N.; Margenthaler, J.A. Elevated Breast Cancer Mortality in Women Younger than Age 40 Years Compared with Older Women Is Attributed to Poorer Survival in Early-Stage Disease. J. Am. Coll. Surg. 2009, 208, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Consensus Recommendations—Breast Cancer in Young Women (BCY5)|ESMO. Available online: https://www.esmo.org/guidelines/guidelines-by-topic/breast-cancer/consensus-recommendations-breast-cancer-in-young-women-bcy5 (accessed on 1 April 2023).
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene Expression Patterns of Breast Carcinomas Distinguish Tumor Subclasses with Clinical Implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, S.; Freedman, R.A.; Partridge, A.H. The Impact of Young Age at Diagnosis (Age <40 Years) on Prognosis Varies by Breast Cancer Subtype: A U. S. SEER Database Analysis. Breast 2022, 61, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Sabiani, L.; Houvenaeghel, G.; Heinemann, M.; Reyal, F.; Classe, J.M.; Cohen, M.; Garbay, J.R.; Giard, S.; Charitansky, H.; Chopin, N.; et al. Breast Cancer in Young Women: Pathologic Features and Molecular Phenotype. Breast Edinb. Scotl. 2016, 29, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.; Mullins, M.; Cheang, M.C.U.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised Risk Predictor of Breast Cancer Based on Intrinsic Subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Kim, D.; Jain, E.; Snow, C.; Kirkner, G.J.; Rosenberg, S.M.; Oh, C.; Poorvu, P.D.; Ruddy, K.J.; Tamimi, R.M.; et al. Somatic and Germline Genomic Alterations in Very Young Women with Breast Cancer. Clin. Cancer Res. 2022, 28, 2339–2348. [Google Scholar] [CrossRef] [PubMed]
- Lalloo, F.; Varley, J.; Moran, A.; Ellis, D.; O’dair, L.; Pharoah, P.; Antoniou, A.; Hartley, R.; Shenton, A.; Seal, S.; et al. BRCA1, BRCA2 and TP53 Mutations in Very Early-Onset Breast Cancer with Associated Risks to Relatives. Eur. J. Cancer Oxf. Engl. 2006, 42, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Cathcart-Rake, E.J.; Ruddy, K.J.; Bleyer, A.; Johnson, R.H. Breast Cancer in Adolescent and Young Adult Women Under the Age of 40 Years. JCO Oncol. Pract. 2021, 17, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Klarenbach, S.; Sims-Jones, N.; Lewin, G.; Singh, H.; Thériault, G.; Tonelli, M.; Doull, M.; Courage, S.; Garcia, A.J.; Thombs, B.D. Recommendations on Screening for Breast Cancer in Women Aged 40–74 Years Who Are Not at Increased Risk for Breast Cancer. CMAJ 2018, 190, E1441–E1451. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, K.J.; Gelber, S.; Tamimi, R.M.; Schapira, L.; Come, S.E.; Meyer, M.E.; Winer, E.P.; Partridge, A.H. Breast Cancer Presentation and Diagnostic Delays in Young Women. Cancer 2014, 120, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Howard-Anderson, J.; Ganz, P.A.; Bower, J.E.; Stanton, A.L. Quality of Life, Fertility Concerns, and Behavioral Health Outcomes in Younger Breast Cancer Survivors: A Systematic Review. J. Natl. Cancer Inst. 2012, 104, 386–405. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.L.; Day, C.N.; Hoskin, T.L.; Habermann, E.B.; Boughey, J.C. Adolescents and Young Adults with Breast Cancer Have More Aggressive Disease and Treatment Than Patients in Their Forties. Ann. Surg. Oncol. 2019, 26, 3920–3930. [Google Scholar] [CrossRef] [PubMed]
- Rahman, P.; Jones, A.; Curtis, J.; Bartlett, S.; Peddle, L.; Fernandez, B.A.; Freimer, N.B. The Newfoundland Population: A Unique Resource for Genetic Investigation of Complex Diseases. Hum. Mol. Genet. 2003, 12, R167–R172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adult Obesity by Province Canada. 2021. Available online: https://www.statista.com/statistics/936787/obesity-among-canadians-by-province/ (accessed on 30 April 2023).
- Chen, G.-C.; Chen, S.-J.; Zhang, R.; Hidayat, K.; Qin, J.-B.; Zhang, Y.-S.; Qin, L.-Q. Central Obesity and Risks of Pre- and Postmenopausal Breast Cancer: A Dose-Response Meta-Analysis of Prospective Studies. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2016, 17, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Cathcart-Rake, E.J.; Ruddy, K.J.; Johnson, R.H. Modifiable Risk Factors for the Development of Breast Cancer in Young Women. Cancer J. Sudbury Mass 2018, 24, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Fagherazzi, G.; Vilier, A.; Boutron-Ruault, M.-C.; Mesrine, S.; Clavel-Chapelon, F. Alcohol Consumption and Breast Cancer Risk Subtypes in the E3N-EPIC Cohort. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP 2015, 24, 209–214. [Google Scholar] [CrossRef] [PubMed]
- SEER*Explorer Application. Available online: https://seer.cancer.gov/statistics-network/explorer/application.html?site=55&data_type=4&graph_type=6&compareBy=age_range&chk_age_range_62=62&sex=3&race=1&stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_show_apc=on&advopt_display=2 (accessed on 23 April 2023).

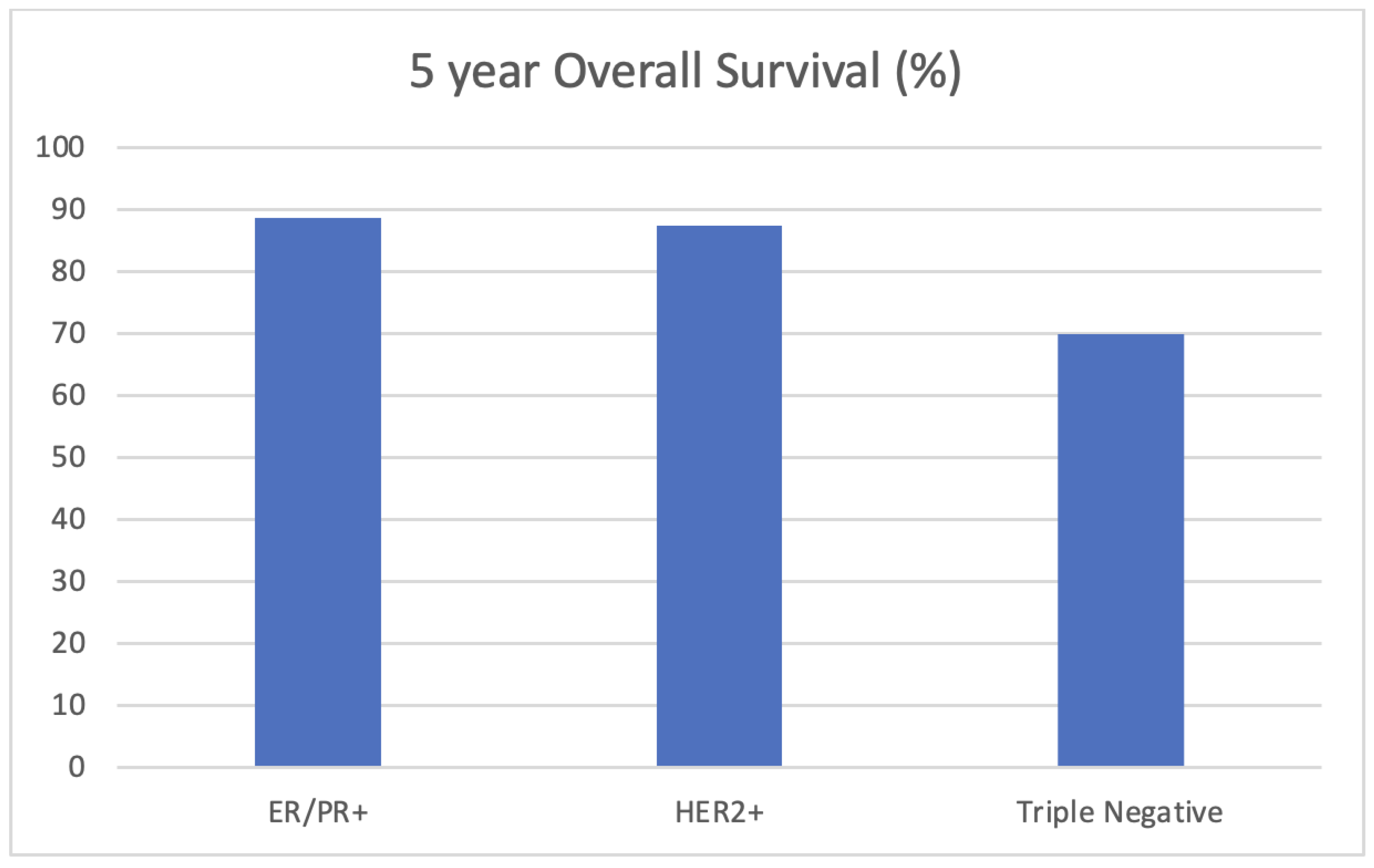

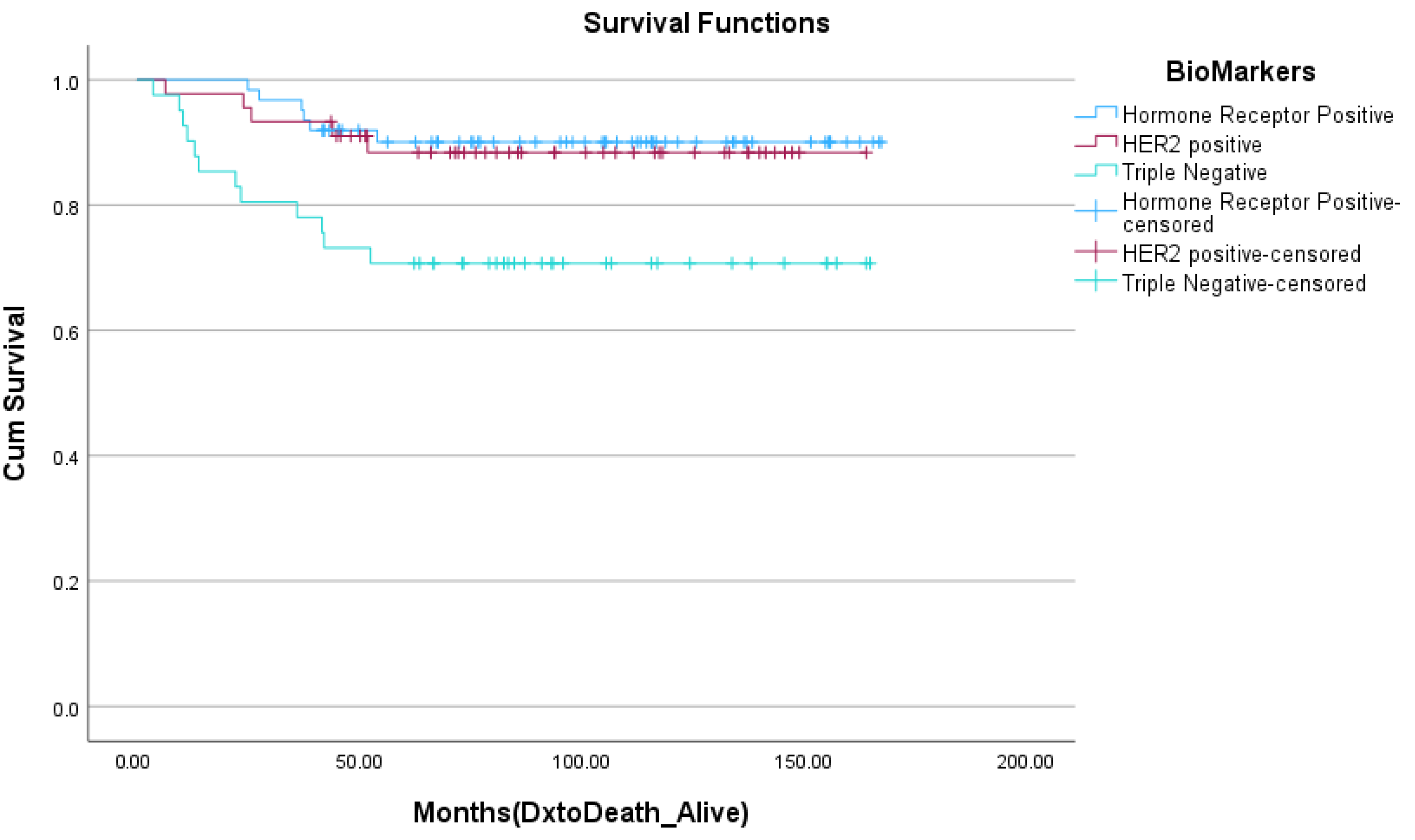
| Variable | Percent Deceased at 5 Years (n = 23) | Percent Alive at 5 Years (n = 110) | p-Value (Chi-Square/Fisher’s Exact *) |
|---|---|---|---|
| Age | |||
| 25–30 | 0.0% (0) | 1.8% (2) | 0.751 |
| 30–35 | 8.7% (2) | 6.4% (7) | |
| 35–50 | 21% (21) | 91.8% (101) | |
| BMI at diagnosis | |||
| <18.5 (Underweight) | 0.0% (0) | 0.9% (1) | 0.967 |
| 18.5–<25 (Normal) | 26.1% (6) | 27.3% (30) | |
| 25–<30 (Overweight) | 21.7% (5) | 21.8% (24) | |
| 30 or greater (Obese) Unknown | 26.1% (6) 26.1% (6) | 30.9% (34) 19.1% (21) | |
| Stage at diagnosis | |||
| Stage I | 0.0% (0) | 24.5% (27) | ≤0.05 |
| Stage II | 21.7% (5) | 51.8% (57) | |
| Stage III | 30.4% (7) | 20.0% (22) | |
| Stage IV | 43.5 (10) | 2.7% (3) | |
| Unknown | 4.3% (1) | 0.9% (1) | |
| Tumour Grade | |||
| I | 0.0% (0) | 7.3% (8) | 0.218 |
| II | 21.7% (5) | 34.5% (38) | |
| III | 65.2% (15) | 54.5% (60) | |
| Unknown | 13.0% (3) | 3.6% (4) | |
| Tumour subtype | |||
| Triple-negative | 52.2% (12) | 25.5% (28) | 0.039 |
| HER2+ | 21.7 (5) | 31.8 (35) | |
| HR+/HER2- | 26.1% (6) | 42.7% (47) | |
| Initial surgical management | |||
| Mastectomy | 77.8% (14) | 51.9% (56) | 0.121 |
| Bilateral Mastectomy | 5.6% (1) | 14.8% (16) | |
| Lupectomy | 16.7% (3) | 33.3% (36) | |
| Neoadjuvant systemic therapy | |||
| Yes | 39.1 (9) | 16.4% (18) | 0.021 |
| No | 60.9 (14) | 83.6% (92) | |
| Ovarian function suppression | |||
| Yes | 23.5% (4) | 41.7% (45) | 0.189 |
| No | 76.5% (13) | 58.3% (63) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahoney, M.; Sriranganathan, S.; Dowden, J.; Seal, M. A Population Description of Young Women with Breast Cancer in Newfoundland and Labrador. Curr. Oncol. 2023, 30, 9602-9610. https://doi.org/10.3390/curroncol30110695
Mahoney M, Sriranganathan S, Dowden J, Seal M. A Population Description of Young Women with Breast Cancer in Newfoundland and Labrador. Current Oncology. 2023; 30(11):9602-9610. https://doi.org/10.3390/curroncol30110695
Chicago/Turabian StyleMahoney, Meghan, Saranga Sriranganathan, Jeff Dowden, and Melanie Seal. 2023. "A Population Description of Young Women with Breast Cancer in Newfoundland and Labrador" Current Oncology 30, no. 11: 9602-9610. https://doi.org/10.3390/curroncol30110695
APA StyleMahoney, M., Sriranganathan, S., Dowden, J., & Seal, M. (2023). A Population Description of Young Women with Breast Cancer in Newfoundland and Labrador. Current Oncology, 30(11), 9602-9610. https://doi.org/10.3390/curroncol30110695




