Evaluation of Anti-Mullerian Hormone Levels, Antral Follicle Counts, and Mean Ovarian Volumes in Chemotherapy-Induced Amenorrhea among Breast Cancer Patients: A Prospective Clinical Study
Abstract
1. Introduction
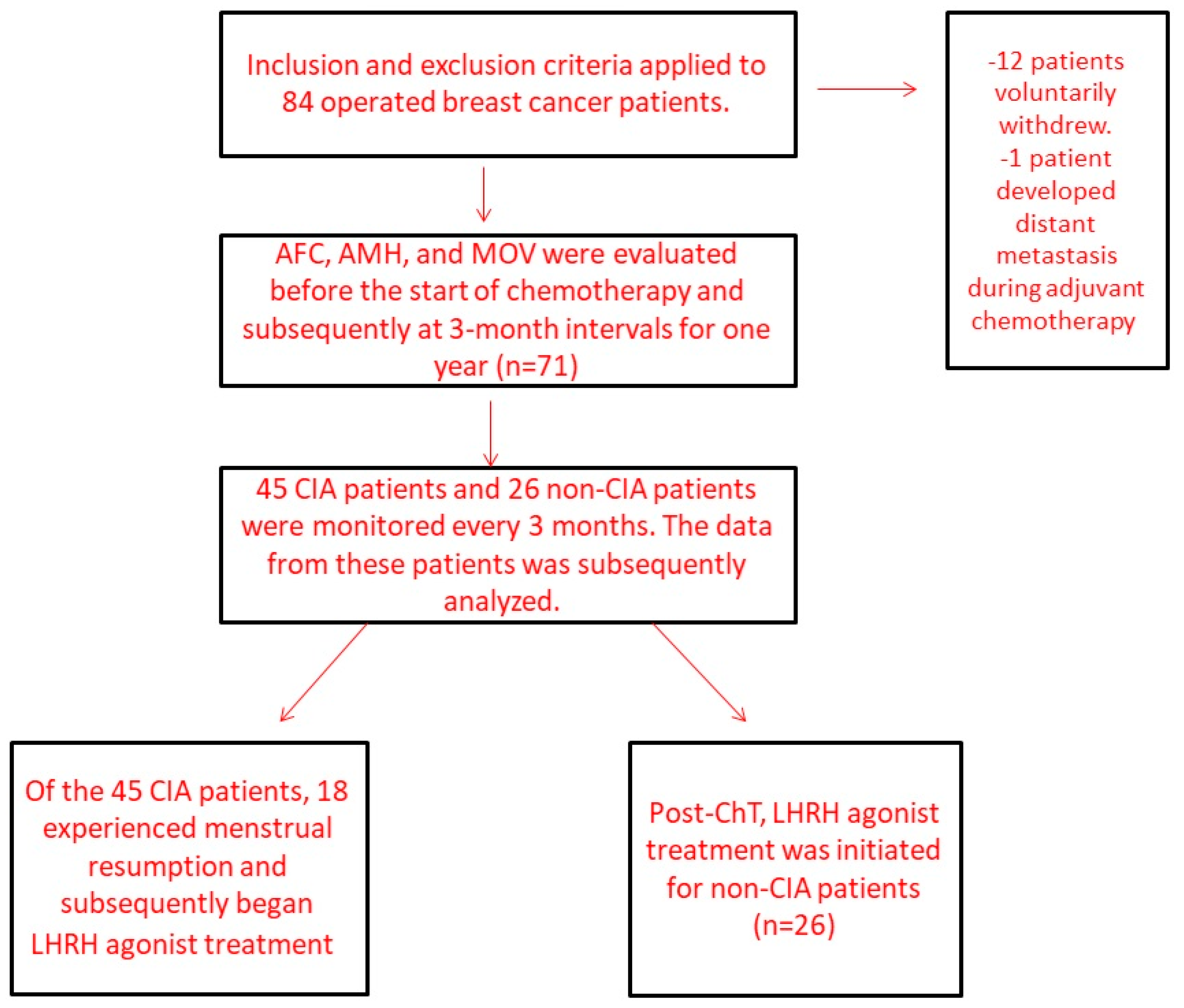
2. Patients and Methods
2.1. Study Design
2.2. Patients
- -
- Pathologically diagnosed stage I–III breast cancer with complete medical records of menses;
- -
- Perimenopausal status (regular menses at least for one year);
- -
- Undergoing adjuvant chemotherapy based on tumor board’s decision.
- -
- Age > 45 years;
- -
- History of other cancer(s);
- -
- Previously underwent chemotherapy;
- -
- Underwent oophorectomy;
- -
- Have a disease causing metabolically abnormalities (renal, hepatic, or cardiac disorders or metabolic disease);
- -
- Individuals who wish or plan to conceive during the post-chemotherapy follow-up period.
2.3. Treatment Strategy
- Weekly paclitaxel (80 mg/m2) was administered for 12 weeks, potentially accompanied by trastuzumab, with the following schedule:
- Initial loading dose: 4 mg/kg; subsequent doses: 2 mg/kg, weekly for 12 weeks, concurrent with paclitaxel.
- Maintenance phase: a single dose of 6 mg/kg trastuzumab every 3 weeks to complete a full year of trastuzumab therapy.
- 2.
- Docetaxel (75 mg/m2) was administered every 3 weeks over four cycles, potentially accompanied by trastuzumab, with the following schedule:
- Initial loading dose: 8 mg/kg; subsequent doses: 6 mg/kg, every 3 weeks, concurrent with docetaxel.
- Maintenance phase: a single dose of 6 mg/kg trastuzumab every 3 weeks to complete a full year of trastuzumab therapy.
2.4. Laboratory Analysis
2.5. Statistical Analysis
3. Results
- AMH levels were significantly higher in participants aged 35 or younger at both the initial and later stages (p = 0.001, p = 0.002).
- The AFC displayed higher median values in the younger age group at both time points, with a significant difference initially (p = 0.007).
- MOV showed no significant difference between the groups at any stage.
- Age group ≤ 40 years vs. >40 years:
- The AMH levels were considerably higher in individuals aged 40 or younger at both the beginning and later periods (p < 0.001, p = 0.001).
- AFC and MOV demonstrated no significant difference between the groups at any time point.
- The median pre-treatment AMH values were 0.755 for the CIA group vs. 1.520 for the non-CIA group.
- The median pre-treatment AFC values were 4.50 for the CIA group vs. 12 for the non-CIA group.
- The median AMH value at the end of the first year was 0.010 for the CIA group and 0.073 for the non-CIA group.
- Age (≤35 vs. >35).
- ER positivity.
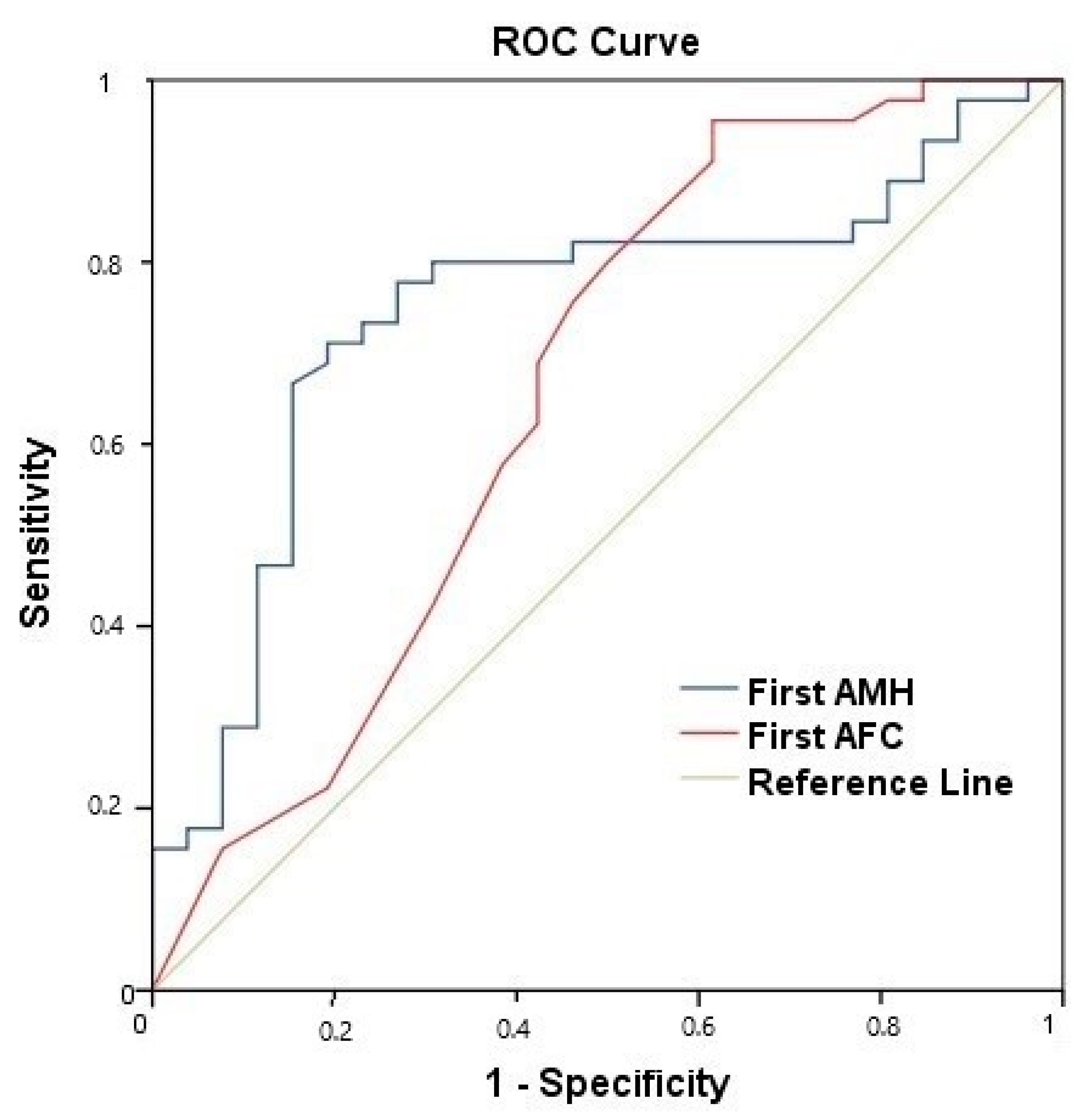
- Pre-treatment AMH level.
- Pre-treatment AFC.
4. Discussion
4.1. Clinical Implications
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ünal, I.Ö.; Ordu, C. Decoding Caregiver Burden in Cancer: Role of Emotional Health, Rumination, and Coping Mechanisms. Healthcare 2023, 11, 2700. [Google Scholar] [CrossRef] [PubMed]
- Ozonder Unal, I.; Ordu, C. Alexithymia, Self-Compassion, Emotional Resilience, and Cognitive Emotion Regulation: Charting the Emotional Journey of Cancer Patients. Curr. Oncol. 2023, 30, 8872–8887. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ünal, Ç.; Özmen, T.; Ordu, Ç.; Pilanci, K.N.; İ lgün, A.S.; Gökmen, E.; Almuradova, E.; Özdoğan, M.; Güler, N.; Uras, C.; et al. Survival results according to Oncotype Dx recurrence score in patients with hormone receptor positive HER-2 negative early-stage breast cancer: First multicenter Oncotype Dx recurrence score survival data of Turkey. Front. Oncol. 2023, 13, 1151733. [Google Scholar] [CrossRef]
- Rosenberg, S.M.; Newman, L.A.; Partridge, A.H. Breast Cancer in Young Women: Rare Disease or Public Health Problem? JAMA Oncol. 2015, 1, 877–878. [Google Scholar] [CrossRef]
- Ozmen, V.; Ozmen, T.; Dogru, V. Breast Cancer in Turkey; An Analysis of 20.000 Patients with Breast Cancer. Eur. J. Breast Health 2019, 15, 141–146. [Google Scholar] [CrossRef]
- Kim, S.E.; Kim, W.-J.; Choi, D.; Lee, D.-Y. Comparison of goserelin and leuprorelin for ovarian protection during chemotherapy in young patients with breast cancer. Breast Cancer Res. Treat. 2023, 198, 231–237. [Google Scholar] [CrossRef]
- Fabiani, C.; Guarino, A.; Meneghini, C.; Licata, E.; Paciotti, G.; Miriello, D.; Schiavi, M.C.; Spina, V.; Corno, R.; Gallo, M.; et al. Oocyte Quality Assessment in Breast Cancer: Implications for Fertility Preservation. Cancers 2022, 14, 5718. [Google Scholar] [CrossRef]
- Bedoschi, G.; Navarro, P.A.; Oktay, K. Chemotherapy-induced damage to ovary: Mechanisms and clinical impact. Futur. Oncol. 2016, 12, 2333–2344. [Google Scholar] [CrossRef]
- Wu, M.; Zhu, Q.; Huang, Y.; Tang, W.; Dai, J.; Guo, Y.; Xiong, J.; Zhang, J.; Zhou, S.; Fu, F.; et al. Ovarian reserve in reproductive-aged patients with cancer before gonadotoxic treatment: A systematic review and meta-analysis. Hum. Reprod. Open 2023, 2023, hoad024. [Google Scholar] [CrossRef]
- Ünal, Ç.; Tunçer, G.; Çopur, B.; Pilanci, K.N.; Okutur, K.S.; Yararbaş, K.; Alan, Ö.; Sakin, A.; Simsek, M.; Ünal, İ.Ö.; et al. Clinical and inflammation marker features of cancer patients with COVID-19: Data of Istanbul, Turkey multicenter cancer patients (2020–2022). Curr. Med. Res. Opin. 2023, 39, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Kalelioglu, T.; Karamustafalioglu, N.; Emul, M.; Celikel, G.; Ozonder, I.; Kara, A.; Kilic, C.; Yalcin, S.; Celik, E.; Kilic, U.; et al. Detecting biomarkers associated with antipsychotic-induced extrapyramidal syndromes by using machine learning techniques. J. Psychiatr. Res. 2023, 158, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Bedenk, J.; Vrtačnik-Bokal, E.; Virant-Klun, I. The role of anti-Müllerian hormone (AMH) in ovarian disease and infertility. J. Assist. Reprod. Genet. 2020, 37, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Amir, E.; Freedman, O.; Allen, L.; Colgan, T.; Clemons, M. Defining ovarian failure in amenorrheic young breast cancer patients. Breast 2010, 19, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Arecco, L.; Blondeaux, E.; Bruzzone, M.; Ceppi, M.; Latocca, M.M.; Marrocco, C.; Boutros, A.; Spagnolo, F.; Razeti, M.G.; Favero, D.; et al. Safety of fertility preservation techniques before and after anticancer treatments in young women with breast cancer: A systematic review and meta-analysis. Hum. Reprod. 2022, 37, 954–968. [Google Scholar] [CrossRef]
- Song, Y.; Liu, H. A review on the relationship between anti-mullerian hormone and fertility in treating young breast cancer patients. BMC Women’s Health 2021, 21, 1–10. [Google Scholar] [CrossRef]
- Furlanetto, J.; Marmé, F.; Seiler, S.; Thode, C.; Untch, M.; Schmatloch, S.; Schneeweiss, A.; Bassy, M.; Fasching, P.A.; Strik, D.; et al. Chemotherapy-induced ovarian failure in young women with early breast cancer: Prospective analysis of four randomised neoadjuvant/adjuvant breast cancer trials. Eur. J. Cancer 2021, 152, 193–203. [Google Scholar] [CrossRef]
- Zavos, A.; Valachis, A. Risk of chemotherapy-induced amenorrhea in patients with breast cancer: A systematic review and meta-analysis. Acta Oncol. 2016, 55, 664–670. [Google Scholar] [CrossRef]
- Mir, M.A.; Mir, A.Y. Current Treatment Approaches to Breast Cancer. In Therapeutic Potential of Cell Cycle Kinases in Breast Cancer; Springer Nature Singapore: Singapore, 2023; pp. 23–51. [Google Scholar]
- Gracia, C.R.; Jeruss, J.S. Lives in the Balance: Women With Cancer and the Right to Fertility Care. J. Clin. Oncol. 2013, 31, 668–669. [Google Scholar] [CrossRef]
- Giovannopoulou, E.; Karakasi, M.-V.; Kouroupi, M.; Giatromanolaki, A.; Tsikouras, P.; Pavlidis, P. Safety and efficacy of ovarian tissue autotransplantation: A systematic literature review. Folia Medica 2023, 65, 362–370. [Google Scholar] [CrossRef]
- Çelebi, F.; Ordu, Ç.; Ilgün, S.; Oztürk, A.; Erdoğan Iyigün, Z.; Alço, G.; Duymaz, T.; Aktepe, F.; Soybir, G.; Baysal, B.; et al. 2020, The Effect of Systemic Chemotherapy on Ovarian Function: A Prospective Clinical Trial. Eur. J. Breast Health 2020, 16, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Park, I.H.; Han, H.S.; Lee, H.; Lee, K.S.; Kang, H.S.; Lee, S.; Kim, S.W.; Jung, S.; Ro, J. Resumption or persistence of menstruation after cytotoxic chemotherapy is a prognostic factor for poor disease-free survival in premenopausal patients with early breast cancer. Ann. Oncol. 2012, 23, 2283–2289. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Karwoski, A.; Abdulrahman, L.; Chaparala, S.; Chaudhary, M.; Nagarsheth, K. Neutrophil-to-Lymphocyte Ratio as a Predictor of Mortality for COVID-19-Related Acute Respiratory Distress Syndrome (ARDS) Patients Requiring Extracorporeal Membrane Oxygenation Therapy. Cureus 2023, 15, e46238. [Google Scholar] [CrossRef]
- Peluso, C.; de Oliveira, R.; Laporta, G.Z.; Christofolini, D.M.; Fonseca, F.L.A.; Laganà, A.S.; Barbosa, C.P.; Bianco, B. Are ovarian reserve tests reliable in predicting ovarian response? Results from a prospective, cross-sectional, single-center analysis. Gynecol. Endocrinol. 2021, 37, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Wenners, A.; Grambach, J.; Koss, J.; Maass, N.; Jonat, W.; Schmutzler, A.; Mundhenke, C. Reduced ovarian reserve in young early breast cancer patients: Preliminary data from a prospective cohort trial. BMC Cancer 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- D’Avila, A.M.; Capp, E.; von Eye Corleta, H. Antral Follicles Count and Anti-Müllerian Hormone Levels after Gonadotoxic Chemotherapy in Patients with Breast Cancer: Cohort Study. Rev. Bras. Ginecol. Obstet. 2017, 39, 162–168. [Google Scholar]
- Turnbull, A.K.; Patel, S.; Martinez-Perez, C.; Rigg, A.; Oikonomidou, O. Risk of chemotherapy-related amenorrhoea (CRA) in premenopausal women undergoing chemotherapy for early stage breast cancer. Breast Cancer Res. Treat. 2021, 186, 237–245. [Google Scholar] [CrossRef]
- Anderson, R.A.; Themmen AP, N.; Qahtani, A.A.; Groome, N.P.; Cameron, D.A. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum. Reprod. 2006, 21, 2583–2592. [Google Scholar] [CrossRef]
- Xue, C.; Wei, W.; Sun, P.; Zheng, W.; Diao, X.; Xu, F.; Huang, J.; An, X.; Xia, W.; Hong, R.; et al. Pretreatment anti-Mullerian hormone-based nomogram predicts menstruation status after chemotherapy for premenopausal women with hormone receptor-positive early breast cancer. Breast Cancer Res. Treat. 2019, 173, 619. [Google Scholar] [CrossRef]
- D’Avila, Â.M.; Biolchi, V.; Capp, E.; von Eye Corleta, H. Age, anti-müllerian hormone, antral follicles count to predict amenorrhea or oligomenorrhea after chemotherapy with cyclophosphamide. J. Ovarian Res. 2015, 8, 82. [Google Scholar] [CrossRef]
- Moolhuijsen, L.M.E.; Visser, J.A. Anti-Müllerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function. J. Clin. Endocrinol. Metab. 2020, 105, 3361–3373. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Velasco, J.A.; Domingo, J.; Cobo, A.; Martı´nez, M.; Carmona, L.; Pellicer, A. Five years’ experience using oocyte vitrification to preserve fertility for medical and nonmedical indications. Fertil. Steril. 2013, 99, 1994–1999. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Moore, H.C.; Leonard, R.C.; Loibl, S.; Munster, P.; Bruzzone, M.; Boni, L.; Unger, J.M.; Anderson, R.A.; Mehta, K.; et al. Gonadotropin-Releasing Hormone Agonists During Chemotherapy for Preservation of Ovarian Function and Fertility in Premenopausal Patients With Early Breast Cancer: A Systematic Review and Meta-Analysis of Individual Patient–Level Data. J. Clin. Oncol. 2018, 36, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.C.; Unger, J.M.; Phillips, K.-A.; Boyle, F.; Hitre, E.; Porter, D.; Francis, P.A.; Goldstein, L.J.; Gomez, H.L.; Vallejos, C.S.; et al. Goserelin for Ovarian Protection during Breast-Cancer Adjuvant Chemotherapy, POEMS/S0230. N. Engl. J. Med. 2015, 372, 923–932. [Google Scholar] [CrossRef]
- Petrek, J.A.; Naughton, M.J.; Case, L.D.; Paskett, E.D.; Naftalis, E.Z.; Singletary, S.E.; Sukumvanich, P. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: A prospective study. J. Clin. Oncol. 2006, 24, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Sukumvanich, P.; Case, L.D.; Van Zee, K.; Singletary, S.E.; Paskett, E.D.; Petrek, J.A.; Naftalis, E.; Naughton, M.J. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: A prospective study. Cancer 2010, 116, 3102–3111. [Google Scholar] [CrossRef] [PubMed]
- Ordu, Ç.; Pilancı, K.N.; Alço, G.; Elbüken, F.; Köksal, Ü.İ.; İlgun, S.; Sarsenov, D.; Aydın, A.E.; Öztürk, A.; İyigün, Z.; et al. Prognostic Significance of Adjuvant Chemotherapy Induced Amenorrhea in Luminal A and B Subtypes. Eur. J. Breast Health 2018, 14, 173–179. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Liang, J.; Zhang, N.; Yang, Q. Chemotherapy-Induced Amenorrhea and Its Prognostic Significance in Premenopausal Women With Breast Cancer: An Updated Meta-Analysis. Front. Oncol. 2022, 12, 859974. [Google Scholar] [CrossRef]
| CIA | Non-CIA | p-Value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Age groups | |||
| Age ≤ 35 years | 9 (20%) | 14 (55%) | 0.003 |
| Age > 35 years | 36 (80%) | 12 (45%) | |
| Age (median–min–max) | 41 (24–45) | 35 (23–40) | |
| Age groups | |||
| Age ≤ 40 years | 18 (40%) | 26 (55%) | <0.001 |
| Age > 40 years | 27 (60%) | 0 (0%) | |
| Body mass index | |||
| BMI≤30 | 38 (84%) | 25 (96%) | 0.13 |
| BMI>30 | 7 (16%) | 1 (4%) | |
| ER receptor | |||
| Negative | 14 (31%) | 2 (8%) | 0.02 |
| Positive | 31 (69%) | 24 (92%) | |
| PR receptor | |||
| Negative | 16 (36%) | 4 (15%) | 0.06 |
| Positive | 29 (64%) | 22 (84%) | |
| Histologic grade | |||
| Grade 1 | - | 2 (7.7%) | 0.16 |
| Grade 2 | 17 (37.8%) | 9 (34.6%) | |
| Grade 3 | 28 (63.4%) | 15 (57.7%) | |
| Molecular subtype | |||
| Luminal A | 8 (18%) | 4 (16%) | 0.15 |
| Luminal B | 22 (50%) | 20 (77%) | |
| HER-2 | 8 (18%) | 1 (3.5%) | |
| TNBC | 6 (14%) | 1 (3.5%) | |
| pT stage | |||
| pT1 | 10 (22%) | 7 (27%) | 0.47 |
| pT2 | 24 (53%) | 16 (61.5%) | |
| pT3 | 11 (25%) | 3 (12.5%) | |
| Pathologic stage (TNM 8th) | |||
| Stage 1 | 29 (64.4%) | 18 (69.2%) | 0.19 |
| Stage 2 | 8 (17.8%) | 7 (26.9%) | |
| Stage 3 | 8 (17.8%) | 1 (3.8%) | |
| Type of axillary surgery | |||
| ALND | 24 (54%) | 10 (38.5%) | 0.26 |
| SLNB | 21 (46%) | 16 (61.5%) | |
| Radiotherapy | |||
| Yes | 34 (89.5%) | 18 (95%) | 0.71 |
| No | 4 (10.5%) | 1 (5%) | |
| Taxane-based regimen | |||
| Taxane | 21 (47%) | 10 (38.5%) | |
| Without taxane | 24 (53%) | 16 (61.5%) | 0.50 |
| Type of ChT | |||
| AC | 15 (65%) | 8 (61.5%) | 0.82 |
| TC | 8 (35%) | 5 (38.5%) | |
| ChT cycle number | |||
| >6 | 26 (58%) | 5 (42%) | 0.38 |
| ≤6 | 19 (42%) | 21 (58%) |
| Age ≤ 35 Years (n = 23) | Age > 35 Years (n = 48) | p Score | |
|---|---|---|---|
| AMH (1st) | 1.84 (0.03–5.50) | 0.71 (0.01–6.20) | 0.001 |
| AMH (5th) | 0.27 (0.01–4.30) | 0.01 (0.01–4.76) | 0.002 |
| AFC (1st) | 7 (0–24) | 3 (0–21) | 0.007 |
| AFC (5th) | 1 (0–8) | 0 (0–8) | 0.05 |
| MOV (1st) | 6.46 (0.93–42.0) | 6.27 (1.01–27.6) | 0.26 |
| MOV (5th) | 3.47 (0.58–15.3) | 3.73 (1.10–15.6) | 0.80 |
| Age ≤ 40 years (n = 44) | Age > 40 years (n = 27) | p score | |
| AMH (1st) | 1.52 (0.03–6.20) | 0.36 (0.01–3.91) | <0.001 |
| AMH (5th) | 0.06 (0.01–4.76) | 0.01 (0.01–0.23) | 0.001 |
| AFC (1st) | 4.5 (0–24) | 3 (0–13) | 0.09 |
| AFC (5th) | 1 (0–8) | 0 (0–5) | 0.08 |
| MOV (1st) | 6.24(0. 39–42.0) | 6.33 (2.08–23.3) | 0.60 |
| MOV (5th) | 3.78 (0.58–15.6) | 3.20 (1.10–10.1) | 0.20 |
| Univariate Analysis | Multivariate Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |||||
| ER −/+ | 0.19 | 0.038 | - | 0.891 | 0.03 | 0.96 | 0.94 | - | 0.98 | 0.002 |
| HER2 −/+ | 0.80 | 0.435 | - | 2.936 | 1.13 | |||||
| ALND −/+ | 1.75 | 0.653 | - | 4.702 | 0.26 | |||||
| AMH(pre-chemo) | 0.64 | 0.454 | - | 0.914 | 0.01 | 0.58 | 0.353 | - | 0.952 | 0.03 |
| AFC (pre-chemo) | 0.87 | 0.780 | - | 0.961 | 0.007 | 0.75 | 0.574 | - | 0.958 | 0.02 |
| MOV (pre-chemo) | 1.00 | 0.987 | - | 1.021 | 0.68 | |||||
| Taxane (+/−) | 0.71 | 0.267 | - | 1.910 | 0.50 | |||||
| TC/AC | 0.85 | 0.209 | - | 3.491 | 0.82 | |||||
| Age ≤35/>35 | 4.66 | 1.613 | - | 13.498 | 0.004 | |||||
| BMI ≤25/>25 | 2.57 | 0.929 | - | 7.118 | 0.06 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ünal, Ç.; Ordu, Ç.; Özmen, T.; İlgun, A.S.; Çelebi, F.; Baysal, B.; Özkurt, E.; Duymaz, T.; Erdoğan İyigün, Z.; Kurt, S.; et al. Evaluation of Anti-Mullerian Hormone Levels, Antral Follicle Counts, and Mean Ovarian Volumes in Chemotherapy-Induced Amenorrhea among Breast Cancer Patients: A Prospective Clinical Study. Curr. Oncol. 2023, 30, 9217-9229. https://doi.org/10.3390/curroncol30100666
Ünal Ç, Ordu Ç, Özmen T, İlgun AS, Çelebi F, Baysal B, Özkurt E, Duymaz T, Erdoğan İyigün Z, Kurt S, et al. Evaluation of Anti-Mullerian Hormone Levels, Antral Follicle Counts, and Mean Ovarian Volumes in Chemotherapy-Induced Amenorrhea among Breast Cancer Patients: A Prospective Clinical Study. Current Oncology. 2023; 30(10):9217-9229. https://doi.org/10.3390/curroncol30100666
Chicago/Turabian StyleÜnal, Çağlar, Çetin Ordu, Tolga Özmen, Ahmet Serkan İlgun, Filiz Çelebi, Bülent Baysal, Enver Özkurt, Tomris Duymaz, Zeynep Erdoğan İyigün, Sevgi Kurt, and et al. 2023. "Evaluation of Anti-Mullerian Hormone Levels, Antral Follicle Counts, and Mean Ovarian Volumes in Chemotherapy-Induced Amenorrhea among Breast Cancer Patients: A Prospective Clinical Study" Current Oncology 30, no. 10: 9217-9229. https://doi.org/10.3390/curroncol30100666
APA StyleÜnal, Ç., Ordu, Ç., Özmen, T., İlgun, A. S., Çelebi, F., Baysal, B., Özkurt, E., Duymaz, T., Erdoğan İyigün, Z., Kurt, S., Öztürk, M. A., Pilancı, K. N., Alço, G., Yararbaş, K., Kayan Tapan, T., Güven, D. C., Soybir, G., & Özmen, V. (2023). Evaluation of Anti-Mullerian Hormone Levels, Antral Follicle Counts, and Mean Ovarian Volumes in Chemotherapy-Induced Amenorrhea among Breast Cancer Patients: A Prospective Clinical Study. Current Oncology, 30(10), 9217-9229. https://doi.org/10.3390/curroncol30100666







