Does Systemic Chemotherapy Influence Skeletal Growth of Young Osteosarcoma Patients as a Treatment-Related Late Adverse Effect?
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analyses
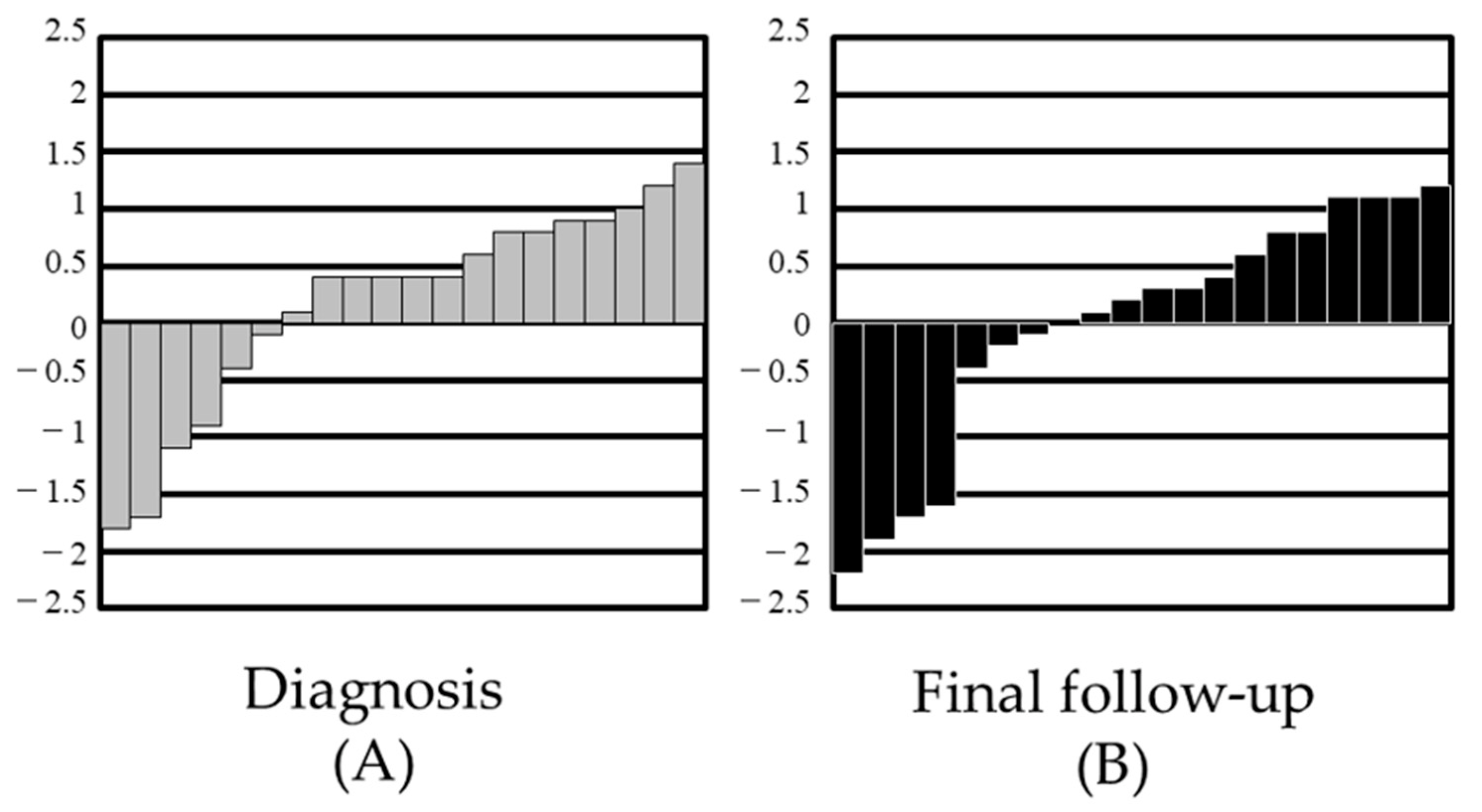
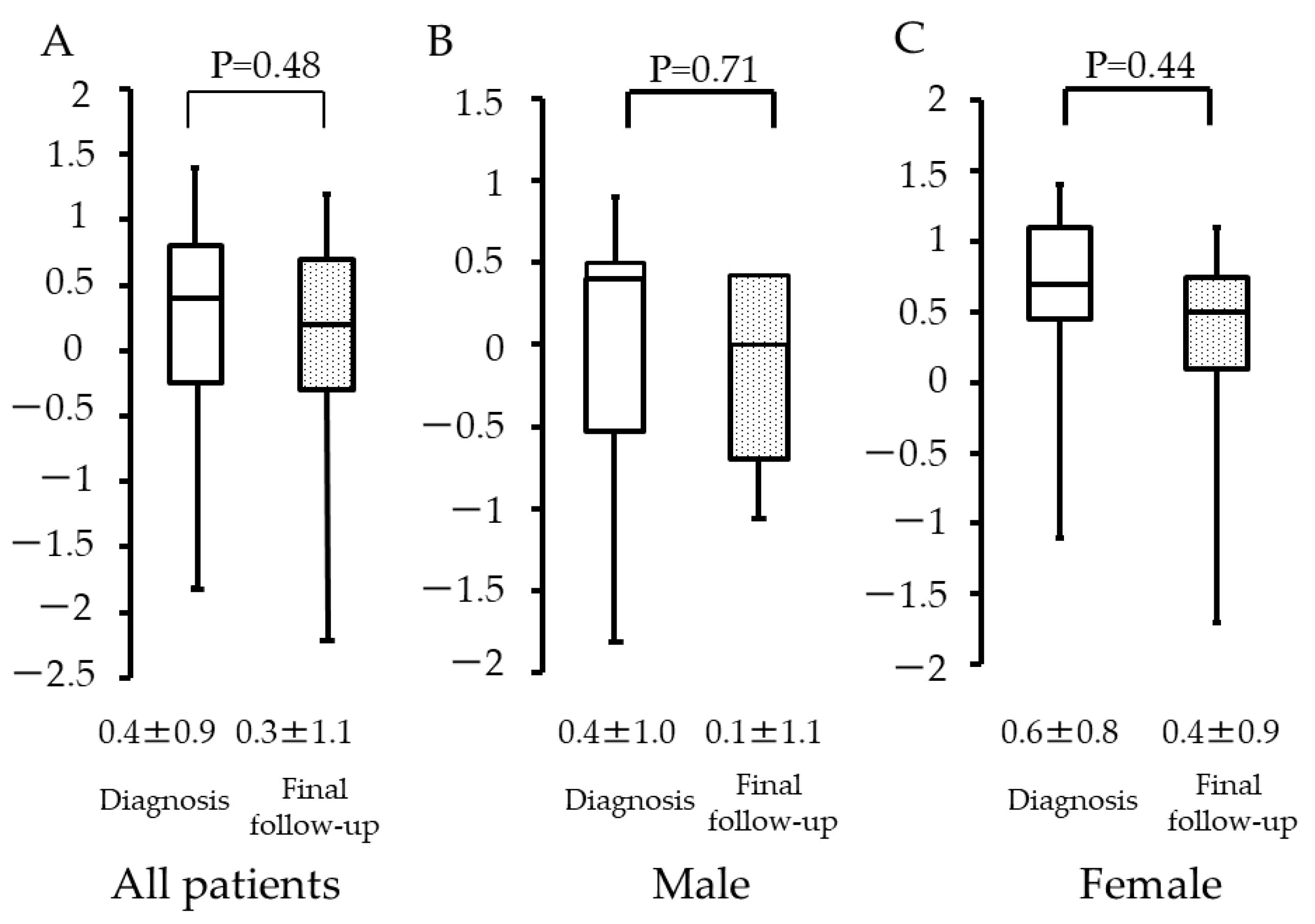
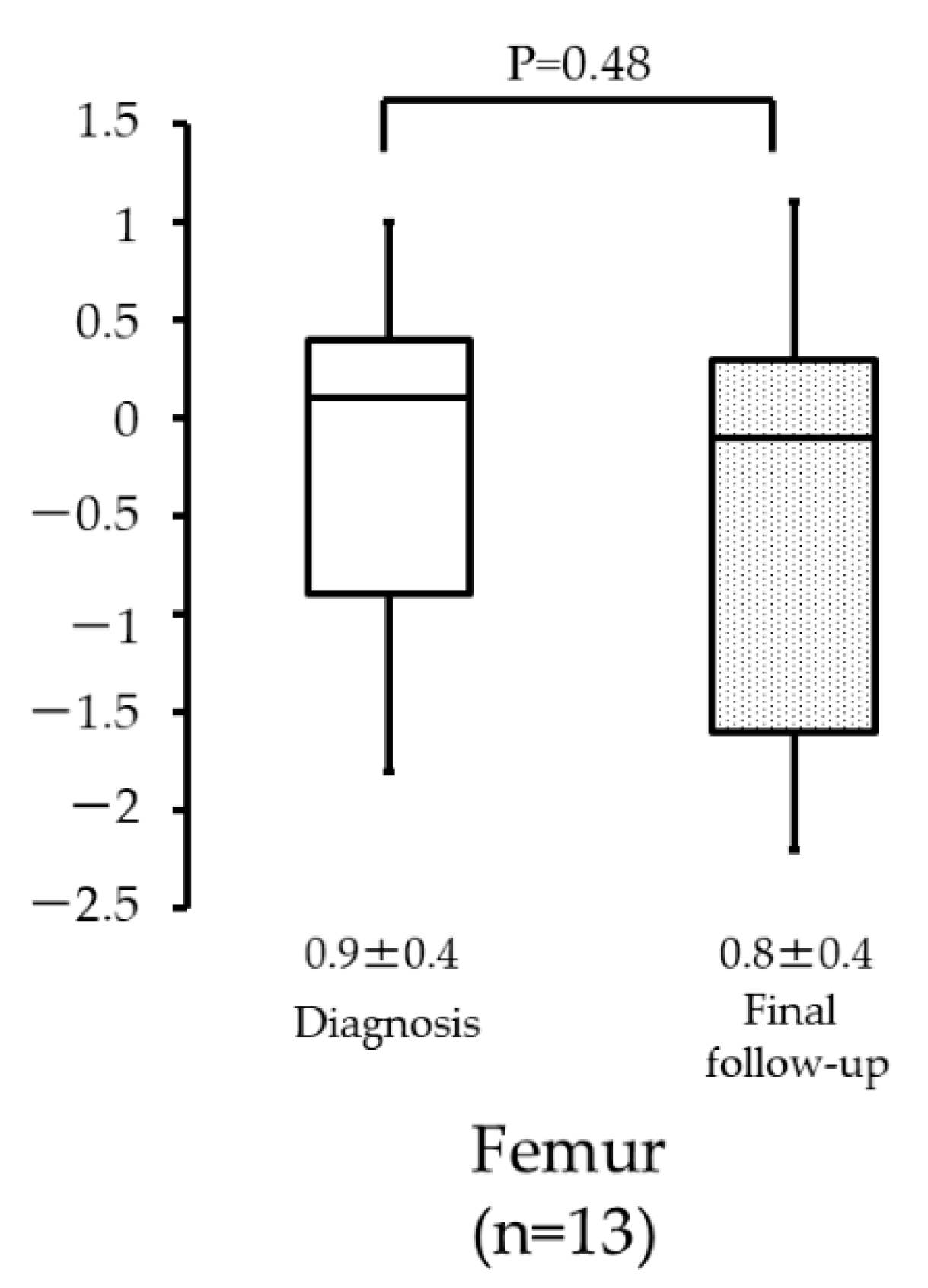
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosen, G.; Nirenberg, A. Neoadjuvant chemotherapy for osteogenic sarcoma: A five year follow-up (T-10) and preliminary report of new studies (T-12). Prog. Clin. Biol. Res. 1985, 201, 39–51. [Google Scholar] [PubMed]
- Link, M.P.; Goorin, A.M.; Horowitz, M.; Meyer, W.H.; Belasco, J.; Baker, A.; Ayala, A.; Shuster, J. Adjuvant chemotherapy of high-grade osteosarcoma of the extremity. Updated results of the Multi-Institutional Osteosarcoma Study. Clin. Orthop. Relat. Res. 1991, 270, 8–14. [Google Scholar] [CrossRef]
- Bielack, S.S.; Kempf-Bielack, B.; Delling, G.; Exner, G.U.; Flege, S.; Helmke, K.; Kotz, R.; Salzer-Kuntschik, M.; Werner, M.; Winkelmann, W.; et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002, 20, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Meyers, P.A.; Heller, G.; Healey, J.; Huvos, A.; Lane, J.; Marcove, R.; Applewhite, A.; Vlamis, V.; Rosen, G. Chemotherapy for nonmetastatic osteogenic sarcoma: The Memorial Sloan-Kettering experience. J. Clin. Oncol. 1992, 10, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Longhi, A.; Ferrari, S.; Tamburini, A.; Luksch, R.; Fagioli, F.; Bacci, G.; Ferrari, C. Late effects of chemotherapy and radiotherapy in osteosarcoma and Ewing sarcoma patients: The Italian Sarcoma Group Experience (1983–2006). Cancer 2012, 118, 5050–5059. [Google Scholar] [CrossRef] [Green Version]
- Ottaviani, G.; Jaffe, N. The etiology of osteosarcoma. Cancer Treat Res. 2009, 152, 15–32. [Google Scholar] [PubMed]
- Viana, M.B.; Vilela, M.I. Height deficit during and many years after treatment for acute lymphoblastic leukemia in children: A review. Pediatr. Blood Cancer 2008, 50, 509–516. [Google Scholar] [CrossRef]
- Gurney, J.G.; Ness, K.K.; Stovall, M.; Wolden, S.; Punyko, J.A.; Neglia, J.P.; Mertens, A.C.; Packer, R.J.; Robison, L.L.; Sklar, C.A. Final Height and Body Mass Index Among Adult Survivors of Childhood Brain Cancer: Childhood Cancer Survivor Study. J. Clin. Endocrinol. Metab. 2003, 88, 4731–4739. [Google Scholar] [CrossRef] [PubMed]
- Paley, J.; Talor, J.; Levin, A.; Bhave, A.; Paley, D.; Herzenberg, J.E. The multiplier method for prediction of adult height. J. Pediatr. Orthop. 2004, 24, 732–737. [Google Scholar] [CrossRef] [PubMed]
- The Japanese Society for Pediatric Endocrinology. Cross-Sectional Growth Chart for Boys (0–18 yrs) and Cross-Sectional Growth Chart for Girls (0–18 yrs). Clinical Growth Charts. Available online: http://jspe.umin.jp/medical/chart_dl.html (accessed on 10 February 2022).
- Kudawara, I.; Aoki, Y.; Ueda, T.; Araki, N.; Naka, N.; Nakanishi, H.; Matsumine, A.; Ieguchi, M.; Mori, S.; Myoui, A.; et al. Neoadjuvant and adjuvant chemotherapy with high-dose ifosfamide, doxorubicin, cisplatin and high-dose methotrexate in non-metastatic osteosarcoma of the extremities: A phase II trial in Japan. J. Chemother. 2013, 25, 41–48. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Tomita, K.; Mori, Y.; Asada, N.; Yamamoto, N. Marginal excision for osteosarcoma with caffeine assisted chemotherapy. Clin. Orthop. Relat. Res. 1999, 358, 27–35. [Google Scholar] [CrossRef]
- Dalton, V.K.; Rue, M.; Silverman, L.B.; Gelber, R.D.; Asselin, B.L.; Barr, R.D.; Clavell, L.A.; Hurwitz, C.A.; Moghrabi, A.; Samson, Y.; et al. Height and weight in children treated for acute lymphoblastic leukemia: Relationship to CNS treatment. J. Clin. Oncol. 2003, 21, 2953–2960. [Google Scholar] [CrossRef] [PubMed]
- Vilela, M.I.; Viana, M.B. Longitudinal growth and risk factors for growth deficiency in children treated for acute lymphoblastic leukemia. Pediatr. Blood Cancer 2007, 48, 86–92. [Google Scholar] [CrossRef]
- Glasser, D.B.; Duane, K.; Lane, J.M.; Healey, J.H.; Caparros-Sison, B. The effect of chemotherapy on growth in the skeletally immature individual. Clin. Orthop. Relat. Res. 1991, 262, 93–100. [Google Scholar] [CrossRef]
- Cool, W.P.; Grimer, R.J.; Carter, S.R.; Tillman, R.M.; Davies, A.M. Longitudinal growth following treatment for osteosarcoma. Sarcoma 1998, 2, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Gilg, M.M.; Wibmer, C.; Andreou, D.; Avian, A.; Sovinz, P.; Maurer-Ertl, W.; Tunn, P.U.; Leithner, A. Paley’s multiplier method does not accurately predict adult height in children with bone sarcoma. Clin. Orthop. Relat. Res. 2014, 472, 2506–2513. [Google Scholar] [CrossRef] [Green Version]
- Bayley, N.; Pinneau, S. Tables for predicting adult height from skeletal age. J. Pediatr. 1952, 14, 432. [Google Scholar]
- Tanner, J.M.; Whitehouse, R.H.; Marshall, W.A. Prediction of adult height from height, bone age, and occurrence of menarche, at ages 4 to 16 with allowance for midparent height. Arch. Dis. Child 1975, 50, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Roche, A.F.; Wainer, H.; Thissen, D. The RWT method for the prediction of adult stature. Pediatrics 1975, 56, 1027–1033. [Google Scholar] [CrossRef]
- Sklar, C.; Mertens, A.; Walter, A.; Mitchell, D.; Nesbit, M.; O’Leary, M.; Hutchinson, R.; Meadows, A.; Robison, L. Final height after treatment for childhood acute lymphoblastic leukemia: Comparison of no cranial irradiation with 1800 and 2400 centigrays of cranial irradiation. J. Pediatr. 1993, 123, 59–64. [Google Scholar] [CrossRef]
- van Leeuwen, B.L.; Kamps, W.A.; Jansen, H.W.; Hoekstra, H.J. The effect of chemotherapy on the growing skeleton. Cancer Treat Rev. 2000, 26, 363–376. [Google Scholar] [CrossRef]
- van Leeuwen, B.L.; Kamps, W.A.; Hartel, R.M.; Veth, R.P.; Sluiter, W.J.; Hoekstra, H.J. Effect of single chemotherapeutic agents on the growing skeleton of the rat. Ann. Oncol. 2000, 11, 1121–1126. [Google Scholar] [CrossRef]
- van Leeuwen, B.L.; Hartel, R.M.; Jansen, H.W.; Kamps, W.A.; Hoekstra, H.J. The effect of chemotherapy on the morphology of the growth plate and metaphysis of the growing skeleton. Eur. J. Surg. Oncol. 2003, 29, 49–58. [Google Scholar] [CrossRef]
- Roman, J.; Villaizán, C.J.; García-Foncillas, J.; Salvador, J.; Sierrasesúmaga, L. Growth and growth hormone secretion in children with cancer treated with chemotherapy. J. Pediatr. 1997, 131, 105–112. [Google Scholar] [CrossRef]



| Author | Year | Pre-Operative Chemotherapy | Post-Operative Chemotherapy |
|---|---|---|---|
| Kudawara, et al. [11] | 2013 | DOX 80–90 mg/m2 + CDDP 120 mg/m2: 2 courses IFM 15 g/m2: 2 courses | MTX 10–12 g/m2: 4 courses |
| DOX 80–90 mg/m2 + CDDP 120 mg/m2: 2 courses | |||
| IFM 15 g/m2: 2 courses | |||
| Tsuchiya, et al. [12] | 1999 | DOX 60 mg/m2 + CDDP 100–120mg/m2: 3 courses | MTX 10–12 g/m2: 3 courses |
| IFM 9g/m2 + ETP 180 mg/m2: 2 courses | DOX 60 mg/m2 + CDDP 100–120 mg/m2: 3 courses |
| Author | Year | Diagnosis | N | Reference | Outcome |
|---|---|---|---|---|---|
| Glasser, et al. [15] | 1990 | Osteosarcoma | 68 | United Kingdim cross sectional reference data | The final height was not affected. |
| Ewing sarcoma | 54 | ||||
| Cool, et al. [16] | 1998 | Osteosarcoma | 72 | National Cancer for Health Statistic data | The final height was not affected. |
| This study | 2020 | Osteosacoma | 24 | Paley’s multiplier method | The final height was not affected. |
| Japanese national growth curve data |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoshi, M.; Oebisu, N.; Iwai, T.; Ban, Y.; Nakamura, H. Does Systemic Chemotherapy Influence Skeletal Growth of Young Osteosarcoma Patients as a Treatment-Related Late Adverse Effect? Curr. Oncol. 2022, 29, 4081-4089. https://doi.org/10.3390/curroncol29060325
Hoshi M, Oebisu N, Iwai T, Ban Y, Nakamura H. Does Systemic Chemotherapy Influence Skeletal Growth of Young Osteosarcoma Patients as a Treatment-Related Late Adverse Effect? Current Oncology. 2022; 29(6):4081-4089. https://doi.org/10.3390/curroncol29060325
Chicago/Turabian StyleHoshi, Manabu, Naoto Oebisu, Tadashi Iwai, Yoshitaka Ban, and Hiroaki Nakamura. 2022. "Does Systemic Chemotherapy Influence Skeletal Growth of Young Osteosarcoma Patients as a Treatment-Related Late Adverse Effect?" Current Oncology 29, no. 6: 4081-4089. https://doi.org/10.3390/curroncol29060325
APA StyleHoshi, M., Oebisu, N., Iwai, T., Ban, Y., & Nakamura, H. (2022). Does Systemic Chemotherapy Influence Skeletal Growth of Young Osteosarcoma Patients as a Treatment-Related Late Adverse Effect? Current Oncology, 29(6), 4081-4089. https://doi.org/10.3390/curroncol29060325





