Randomized Controlled Trials in Lung, Gastrointestinal, and Breast Cancers: An Overview of Global Research Activity
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Search Strategy
2.2. Data Abstraction and Classification
2.3. Outcomes and Statistical Analysis
3. Results
3.1. Results of the Search Strategy
3.2. Design Characteristics of RCTs
3.3. Outcomes of RCTs
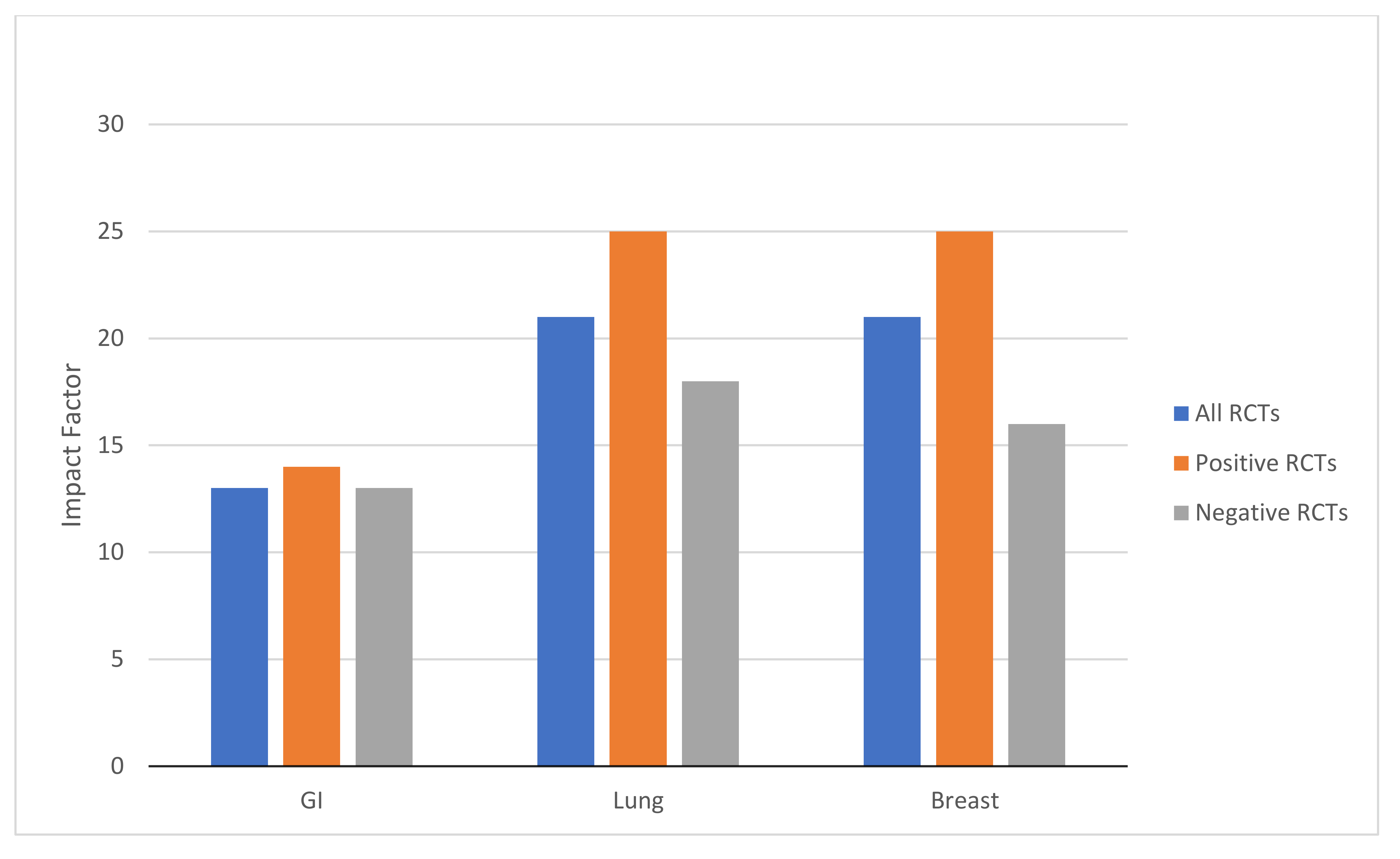
3.4. Journal Impact Factors by Disease Site
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Cancer Observatory. World Fact Sheets. Available online: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf (accessed on 10 January 2021).
- Hashim, D.; Boffetta, P.; La Vecchia, C.; Rota, M.; Bertuccio, P.; Malvezzi, M.; Negri, E. The global decrease in cancer mortality: Trends and disparities. Ann. Oncol. 2016, 27, 926–933. [Google Scholar] [CrossRef]
- Siegel, R.; Miller, K.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Ma, J.; Rosenberg, P.S.; Siegel, R.; Anderson, W.F. Increasing lung cancer death rates among young women in southern and midwestern states. J. Clin. Oncol. 2012, 30, 2739–2744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howlader, N.; Forjaz, G.; Mooradian, M.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The effect of advances in lung-cancer treatment on population mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef] [PubMed]
- The National Lung Cancer Screening Trial Research Team. Reduced-lung cancer mortality with low dose-computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, C.M.; Cescon, D.W.; Wang, L.; Tannock, I.F.; Krzyzanowska, M.K. Evolution of the randomized controlled trial in oncology over three decades. J. Clin. Oncol. 2008, 26, 5458–5464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, A.; Higgins, J.; Day, A.G.; Meyer, R.M.; Booth, C.M. Randomized controlled trials in the era of molecular oncology: Methodology, biomarkers, and end points. Ann. Oncol. 2012, 23, 1646–1651. [Google Scholar] [CrossRef]
- Seruga, B.; Hertz, P.C.; Wang, L.; Booth, C.M.; Cescon, D.W.; Krzyzanowska, M.; Tannock, I.F. Absolute benefits of medical therapies in phase III clinical trials for breast and colorectal cancer. Ann. Oncol. 2010, 21, 1411–1418. [Google Scholar] [CrossRef]
- Sacher, A.G.; Le, L.W.; Leighl, N.B. Shifting patterns in the interpretation of phase III clinical trial outcomes in advanced non-small-cell lung cancer: The bar is dropping. J. Clin. Oncol. 2014, 32, 1407–1411. [Google Scholar] [CrossRef] [Green Version]
- Ocana, A.; Amir, E.; Vera-Badillo, F.; Seruga, B.; Tannock, I.F. Phase III trials of targeted anticancer therapies: Redesigning the concept. Clin. Cancer Res. 2013, 19, 4931–4940. [Google Scholar] [CrossRef] [Green Version]
- Mailankody, S.; Prasad, V. Five years of cancer drug approvals. Innovation, efficacy, and costs. JAMA Oncol. 2015, 1, 539–540. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, C.; Bremner, K.E.; Pataky, R.; Gunraj, N.; Haq, M.; Chan, K.; Cheung, W.Y.; Hoch, J.S.; Peacock, S.; Krahn, M. Trends in use and cost of initial cancer treatment in Ontario: A population-based descriptive study. CMAJ Open 2013, 1, E151–E158. [Google Scholar] [CrossRef] [Green Version]
- Fojo, T.; Mailankody, S.; Lo, A. Unintended consequences of expensive cancer therapeutics-the pursuit of marginal indications and a me-too mentality that stifles innovation and creativity: The John Conley Lecture. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 1225–1236. [Google Scholar] [CrossRef]
- Wells, J.C.; Sharma, S.; Del Paggio, J.C.; Hopman, W.M.; Gyawali, B.; Mukherji, D.; Hammad, N.; Pramesh, C.S.; Aggarwal, A.; Sullivan, R.; et al. An analysis of contemporary oncology randomized clinical trials from low/middle-income vs. high income countries. JAMA Oncol. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Cherny, N.I.; Dafni, U.; Bogaerts, J.; Latino, N.J.; Pentheroudakis, G.; Douillard, J.-Y.; Tabernero, J.; Zielinski, C.; Piccart, M.J.; De Vries, E.G.E. ESMO-Magnitude of clinical benefit scale version 1.1. Ann. Oncol. 2017, 28, 2340–2366. [Google Scholar] [CrossRef]
- The World Bank. World Bank Country and Lending Groups. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 9 July 2020).
- Clarivate Analytics. Journal Citation Reports: Impact Factor. Available online: https://jcr-clarivate-com.proxy.queensu.ca/JCRJournalHomeAction.action? (accessed on 10 July 2020).
- Cherny, N.I.; Sullivan, R.; Dafni, U.; Kerst, J.M.; Sobrero, A.; Zielinski, C.; De Vries, E.G.; Piccart, M.J. A standardized, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: The European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann. Oncol. 2015, 26, 1547–1573. [Google Scholar] [CrossRef]
- Booth, C.M.; Eisenhauer, E.A. Progression-free survival: Meaningful or simply measurable? J. Clin. Oncol. 2012, 30, 1030–1033. [Google Scholar] [CrossRef]
- Gyawali, B.; Hey, S.P.; Kesselheim, A.S. Evaluating the evidence behind the surrogate measures included in the FDA’s table of surrogate endpoints as supporting approval of cancer drugs. eClinicalMedicine 2020, 21, 100332. [Google Scholar] [CrossRef]
- Gyawali, B.; D’Andrea, E.; Franklin, J.M.; Kesselheim, A.S. A correlation analysis to assess event-free survival as a trial-level surrogate for overall survival in early breast cancer. eClinicalMedicine 2021. online ahead of print. [Google Scholar] [CrossRef]
- Mauguen, A.; Pignon, J.P.; Burdett, S.; Domerg, C.; Fisher, D.; Paulus, R.; Mandrekar, S.J.; Belani, C.; Shepherd, F.A.; Eisen, T.; et al. Surrogate endpoints for overall survival in chemotherapy and radiotherapy trials in operable and locally advanced lung cancer: A re-analysis of meta-analyses of individual patients’ data. Lancet Oncol. 2013, 14, 619–626. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Table of Surrogate Endpoints That Were the Basis of Drug Approval or Licensure. Available online: https://www.fda.gov/drugs/development-resources/table-surrogate-endpoints-were-basis-drug-approvalor-licensure (accessed on 10 January 2021).
- Tannock, I.F.; Amir, E.; Booth, C.M.; Niraula, S.; Ocana, A.; Seruga, B.; Templeton, A.J.; Vera-Badillo, F. Relevance of randomised controlled trials in oncology. Lancet Oncol. 2016, 17, e560–e567. [Google Scholar] [CrossRef]
- Ranganathan, P.; Pramesh, C.S.; Buyse, M. Common pitfalls in statistical analysis: Clinical versus statistical significance. Perspect. Clin. Res. 2015, 6, 169–170. [Google Scholar] [CrossRef]
- Del Paggio, J.C.; Azariah, B.; Sullivan, R.; Hopman, W.M.; James, F.; Roshni, S.; Tannock, I.F.; Booth, C.M. Do contemporary randomized controlled trials meet ESMO thresholds for meaningful clinical benefit. Ann. Oncol. 2017, 28, 157–162. [Google Scholar] [CrossRef]
- Hida, T.; Nokihara, H.; Kondo, M.; Kim, Y.H.; Azuma, K.; Seto, T.; Takiguchi, Y.; Nishio, M.; Yoshioka, H.; Imamura, F.; et al. Alectinib versus crizotinib in patients with ALK-positive NSCLC (J-ALEX). Lancet 2017, 390, 29–39. [Google Scholar] [CrossRef]
- Mok, T.; Wu, Y.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.; Lee, C.K. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Reck, M.; Rodriguez, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab vs. chemotherapy for PDL1 positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Antonia, S.C.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [Green Version]
- Meropol, N.J.; Schrag, D.; Smith, T.J.; Mulvey, T.M.; Langdon, R.M., Jr.; Blum, D.; Ubel, P.A.; Schnipper, L.E. American Society of Clinical Oncology guidance statement: The cost of cancer care. J. Clin. Oncol. 2009, 27, 3868–3874. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, N.A.; Spyropoulous, A.C.; Halperin, J.L.; Kessler, C.M.; Schulman, S.; Turpie, A.G.G.; Skene, A.M.; Cutler, N.R.; Hiatt, W.R. Improving academic leadership and oversight in large industry-sponsored clinical trials: The ARO-CRO model. Blood 2011, 177, 2089–2092. [Google Scholar] [CrossRef] [Green Version]
- Krzyzanowska, M.K.; Pintilie, M.; Tannock, I.F. Factors associated with failure to publish large randomized trials presented at an oncology meeting. JAMA 2003, 290, 495–501. [Google Scholar] [CrossRef] [Green Version]
- Begg, C.B.; Berlin, J.A. Publication bias and dissemination of clinical research. J. Natl. Cancer Inst. 1989, 81, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Patafio, F.M.; Brooks, S.C.; Wei, X.; Peng, Y.; Biagi, J.; Booth, C.M. Research output and the public health burden of cancer: Is there any relationship? Curr. Oncol. 2016, 23, 75–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, C.M.; Dranitsaris, G.; Gainford, M.C.; Berry, S.; Fralick, M.; Fralick, J.; Sue, J.; Clemons, M. External influences and priority-setting for anti-cancer agents: A case study of media coverage in adjuvant trastuzumab for breast cancer. BMC Cancer 2007, 7, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| All RCTs | GI | Lung | Breast | p- Value | |
|---|---|---|---|---|---|
| n = 352 | n = 127 | n = 104 | n = 121 | ||
| n (%) | |||||
| Treatment intent | |||||
| Palliative | 223 (63) | 82 (65) | 90 (87) | 51 (42) | <0.001 |
| Curative | 14 (4) | 7 (6) | 3 (3) | 4 (3) | |
| Neoadjuvant/adjuvant | 115 (33) | 38 (30) | 11 (11) | 66 (55) | |
| Experimental arm | |||||
| Systemic | 312 (89) | 110 (87) | 91 (88) | 111 (92) | 0.009 |
| Radiation | 18 (5) | 4 (3) | 6 (6) | 8 (7) | |
| Surgery | 9 (3) | 7 (6) | 0 (0) | 2 (2) | |
| Other/Combinations | 13 (4) | 6 (5) | 7 (7) | 0 (0) | |
| Study design | |||||
| Superiority | 297 (84) | 107 (84) | 94 (90) | 96 (79) | 0.075 |
| NI/equivalence | 55 (16) | 20 (16) | 10 (10) | 25 (21) | |
| Primary endpoint | |||||
| OS | 122 (35) | 55 (43) | 53 (51) | 14 (12) | <0.001 |
| DFS/EFS/RFS | 84 (24) | 28 (22) | 7 (7) | 49 (40) | |
| PFS/TTF | 108 (31) | 32 (25) | 38 (37) | 38 (31) | |
| Other | 38 (11) | 12 (9) * | 6 (6) # | 20 (17) ^ | |
| Country of origin @ | |||||
| HIC | 315 (89) | 116 (91) | 84 (81) | 115 (95) | 0.002 |
| LMIC | 37 (11) | 11 (9) | 20 (19) | 6 (5) | |
| Industry funding ~ | 263 (75) | 85 (67) | 82 (79) | 96 (79) | 0.037 |
| All RCTs | GI | Lung | Breast | p-Value | |
|---|---|---|---|---|---|
| n = 352 | n = 127 | n = 104 | n = 121 | ||
| n (%) | |||||
| Total sample size | |||||
| Median (IQR) | 494 (259–845) | 438 (244–700) | 348 (212–627) | 666 (393–1505) | <0.001 |
| Primary endpoint met | |||||
| Yes | 160 (45) | 52 (41) | 43 (41) | 65 (54) | 0.079 |
| No | 192 (55) | 75 (59) | 61 (59) | 56 (46) | |
| HR for + RCTs | n = 118 * | n = 38 a | n = 36 b | n = 44 c | |
| Median (IQR) | 0.69 (0.65–0.75) | 0.71 (0.66–0.75) | 0.67 (0.62–0.72) | 0.69 (0.67–0.75) | 0.137 |
| ESMO-MCBS grade | n = 81 | n = 23 | n = 30 | n = 28 | |
| Substantial benefit (A, B, 4, 5) | 29 (36) | 7 (30) | 15 (50) | 7 (25) | 0.114 |
| Not substantial benefit (C,1,2,3) | 52 (64) | 16 (70) | 15 (50) | 21 (75) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wells, J.C.; Fundytus, A.; Sharma, S.; Hopman, W.M.; Del Paggio, J.C.; Gyawali, B.; Mukherji, D.; Hammad, N.; Pramesh, C.S.; Aggarwal, A.; et al. Randomized Controlled Trials in Lung, Gastrointestinal, and Breast Cancers: An Overview of Global Research Activity. Curr. Oncol. 2022, 29, 2530-2538. https://doi.org/10.3390/curroncol29040207
Wells JC, Fundytus A, Sharma S, Hopman WM, Del Paggio JC, Gyawali B, Mukherji D, Hammad N, Pramesh CS, Aggarwal A, et al. Randomized Controlled Trials in Lung, Gastrointestinal, and Breast Cancers: An Overview of Global Research Activity. Current Oncology. 2022; 29(4):2530-2538. https://doi.org/10.3390/curroncol29040207
Chicago/Turabian StyleWells, J. Connor, Adam Fundytus, Shubham Sharma, Wilma M. Hopman, Joseph C. Del Paggio, Bishal Gyawali, Deborah Mukherji, Nazik Hammad, C. S. Pramesh, Ajay Aggarwal, and et al. 2022. "Randomized Controlled Trials in Lung, Gastrointestinal, and Breast Cancers: An Overview of Global Research Activity" Current Oncology 29, no. 4: 2530-2538. https://doi.org/10.3390/curroncol29040207
APA StyleWells, J. C., Fundytus, A., Sharma, S., Hopman, W. M., Del Paggio, J. C., Gyawali, B., Mukherji, D., Hammad, N., Pramesh, C. S., Aggarwal, A., Sullivan, R., & Booth, C. M. (2022). Randomized Controlled Trials in Lung, Gastrointestinal, and Breast Cancers: An Overview of Global Research Activity. Current Oncology, 29(4), 2530-2538. https://doi.org/10.3390/curroncol29040207





