Nomogram Based on Monocyte-to-Lymphocyte Ratio to Predict Survival of Unresectable Esophageal Squamous Cell Carcinoma Who Receive First-Line PD-1/PD-L1 Inhibitors Combined with Chemotherapy
Abstract
1. Introduction
2. Materials and Methods
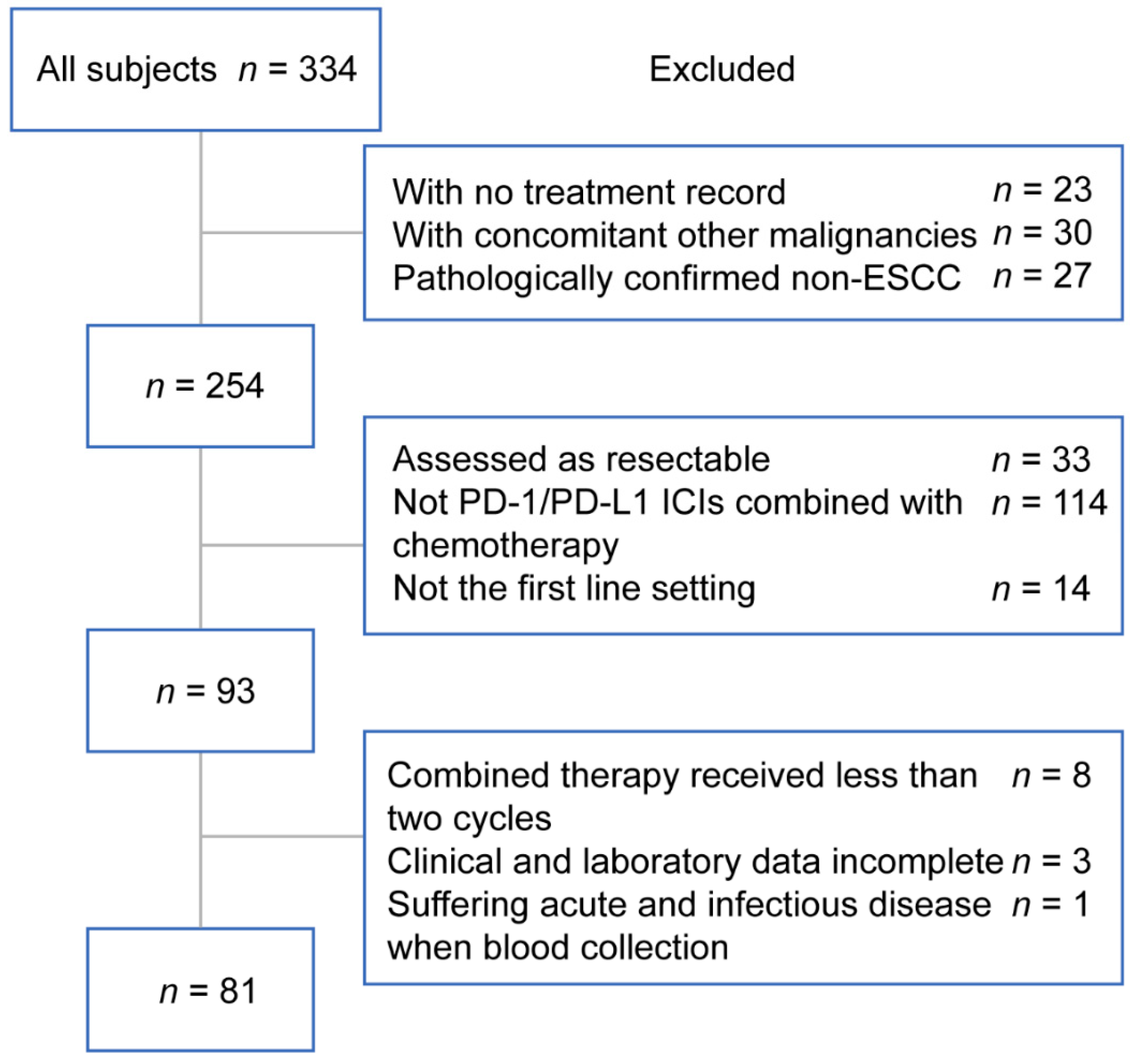
2.1. Study Population
2.2. Data Collection
2.3. Treatments
2.4. Assessment
2.5. Statistical Analysis
3. Results
3.1. Patients Characteristics
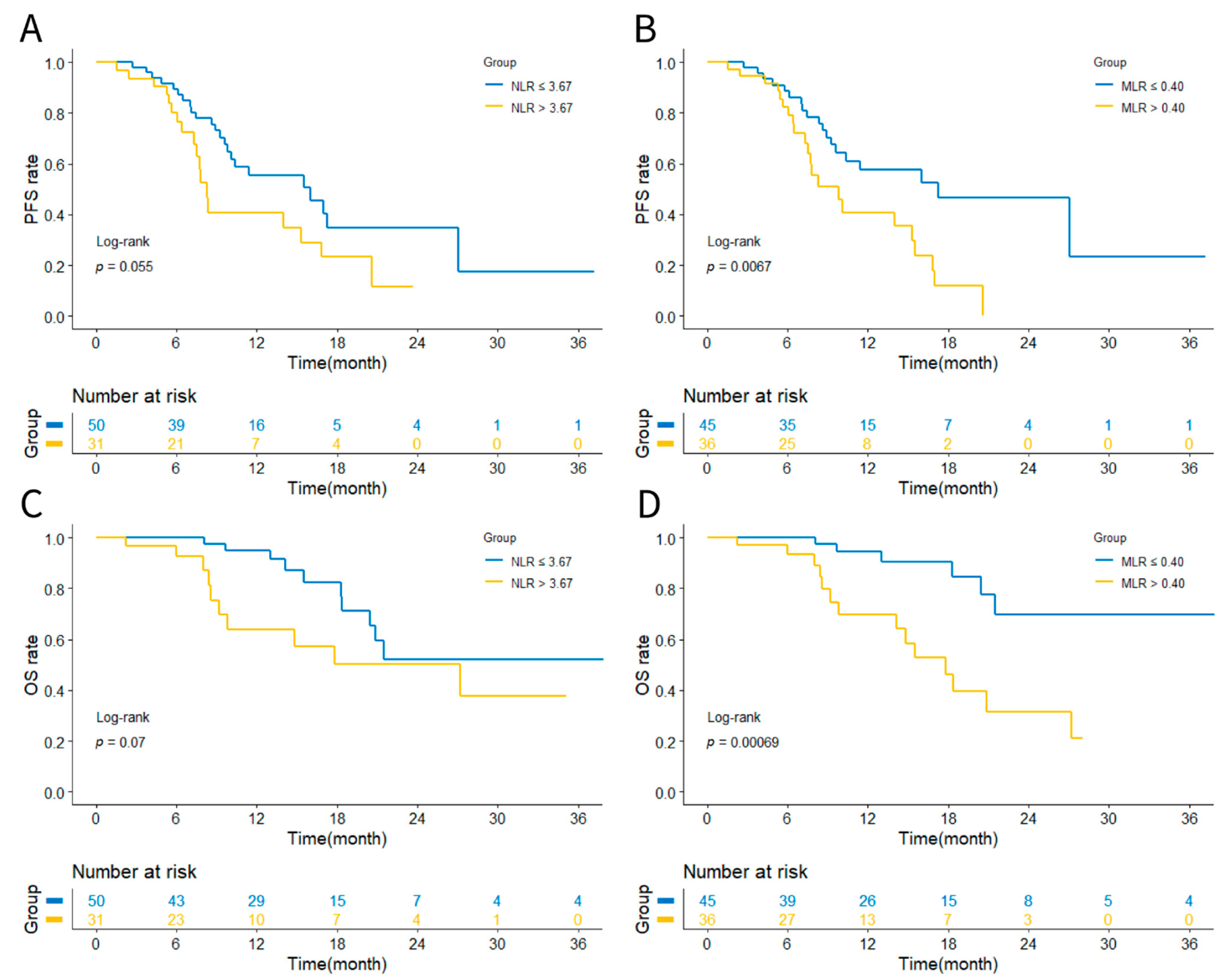
3.2. Prognostic Analysis Based on NLR or MLR
3.3. Univariate and Multivariate Cox Proportional Analyses of Prognostic Factors
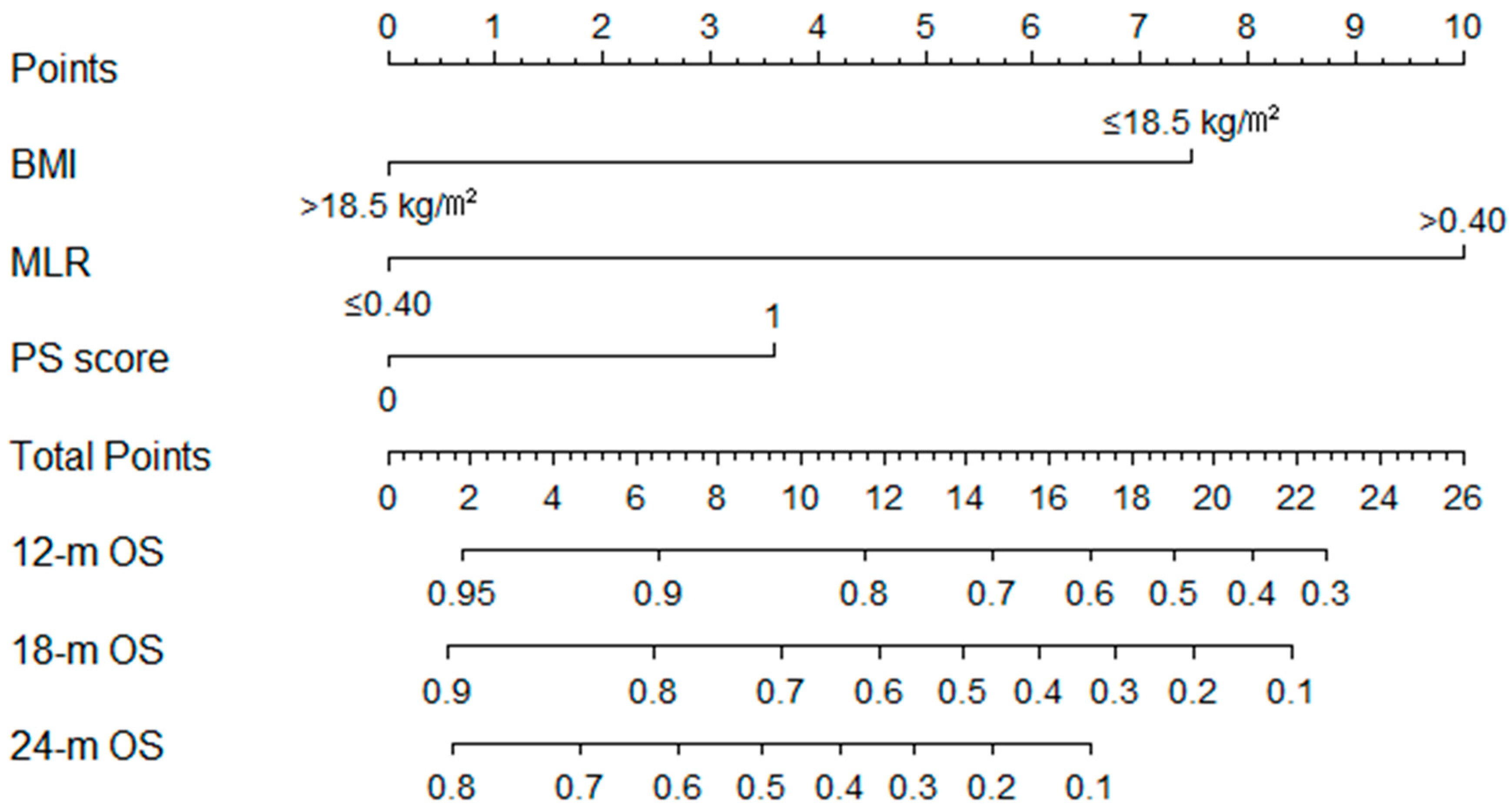
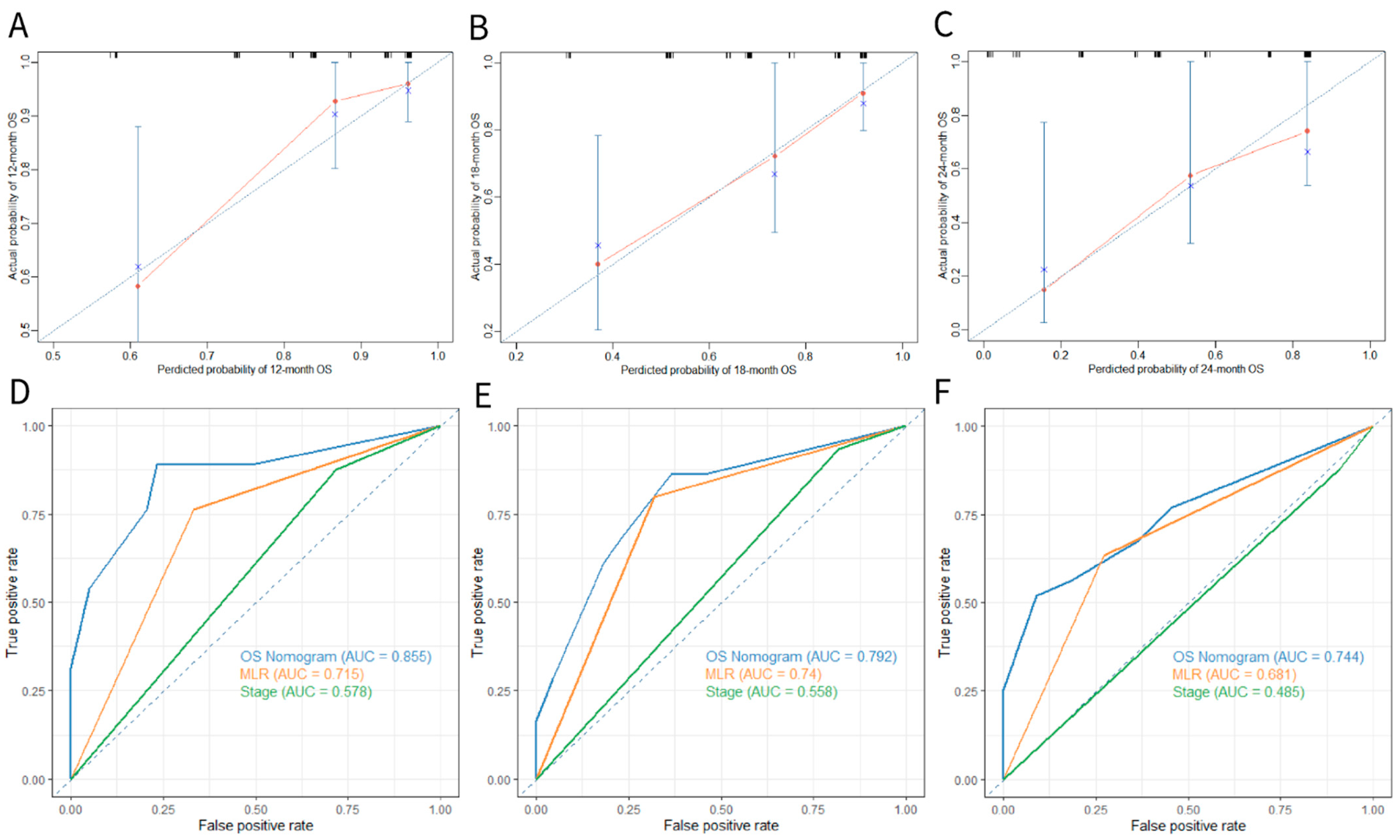
3.4. Construction and Validation of the OS Nomogram
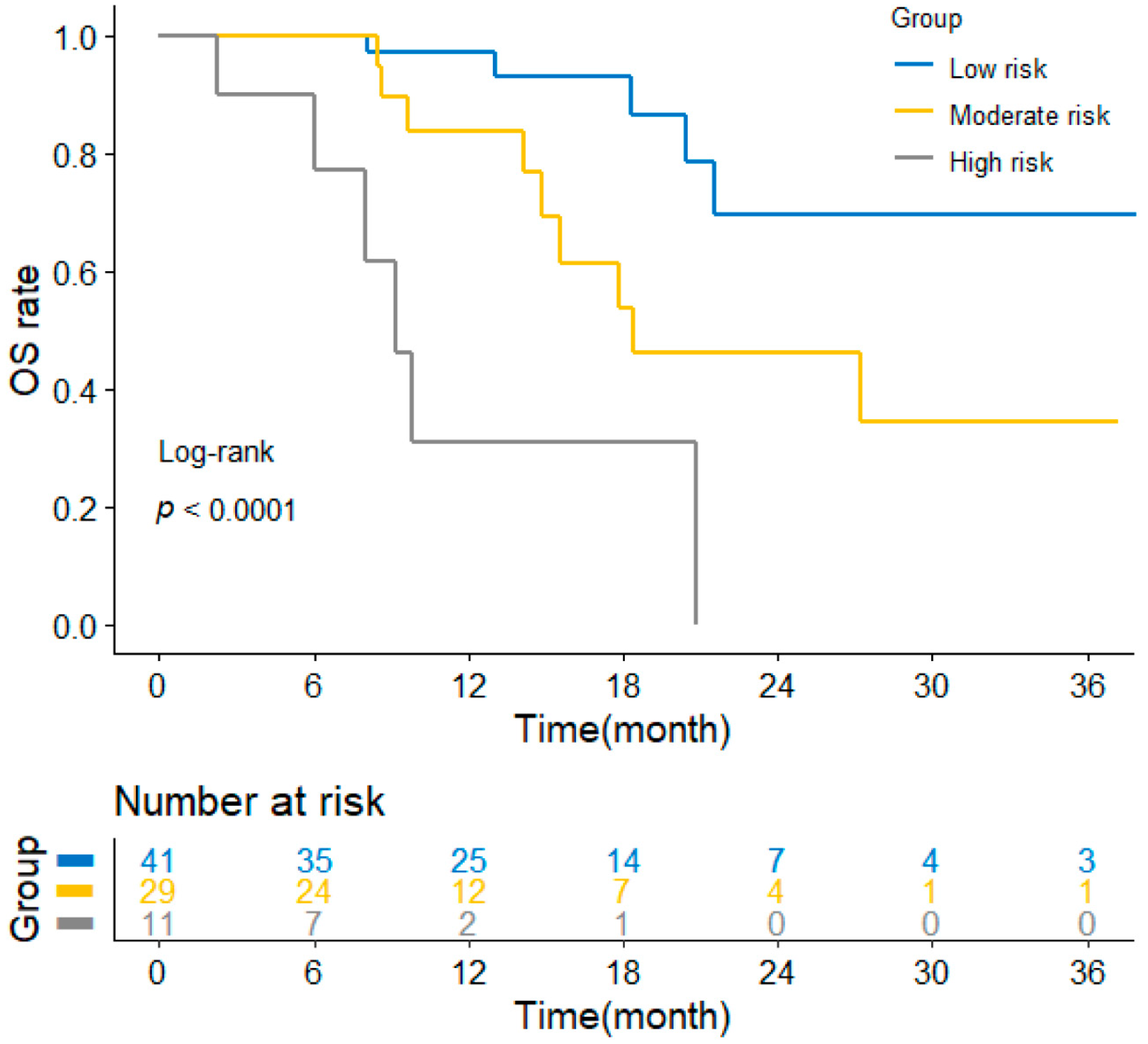
3.5. Application of the Nomogram for Risk Stratification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ESCC | esophageal squamous cell carcinoma |
| MLR | monocyte-to-lymphocyte ratio |
| PD-1 | programmed death-1 |
| PD-L1 | programmed death ligand-1 |
| OS | overall survival |
| PFS | progression free survival |
| C-index | concordance index |
| timeROC | time-dependent receiver operating characteristic |
| PS score | performance status score |
| BMI | body mass index |
| AUC | area under ROC curve |
| TNM | tumor-node-metastasis |
| ICI | immune checkpoint inhibitor |
| TMB | tumor mutational burden |
| ctDNA | circulating tumor DNA |
| NLR | neutrophil-to-lymphocyte ratio |
| NSCLC | non-small-cell lung cancer |
| GC | gastric cancer |
| AJCC | American Joint Committee on Cancer |
| ECOG | Eastern Cooperative Oncology Group |
| HB | hemoglobin |
| Nab-PTX | albumin-bound paclitaxel |
| PTX | paclitaxel |
| 5-FU | fluorouracil |
| CR | complete response |
| PR | partial response |
| SD | stable disease |
| PD | progressive disease |
| ORR | objective remission rate |
| DCR | disease control rate |
| HR | hazard ratio |
| CI | confidential interval |
| CRC | colorectal cancer |
| TTP | time to progression |
| HCC | hepatocellular carcinoma |
| TILs | tumor-infiltrating lymphocytes |
| TAMs | tumor-associated macrophages |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ye, J.; Zhu, Z.; Zhao, W.; Zhou, J.; Wu, C.; Tang, H.; Fan, M.; Li, L.; Lin, Q.; et al. Comparing Paclitaxel Plus Fluorouracil Versus Cisplatin Plus Fluorouracil in Chemoradiotherapy for Locally Advanced Esophageal Squamous Cell Cancer: A Randomized, Multicenter, Phase III Clinical Trial. J. Clin. Oncol. 2019, 37, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Imazeki, H.; Kato, K. Development of chemotherapeutics for unresectable advanced esophageal cancer. Expert Rev. Anticancer Ther. 2020, 20, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, P.S.N.; Mohammad, N.H.; Vleggaar, F.P.; Van Hillegersberg, R. Treatment for unresectable or metastatic oesophageal cancer: Current evidence and trends. Nat. Rev. Gastroenterol. Hepatol. 2017, 15, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Miyata, H.; Sugimura, K.; Motoori, M.; Omori, T.; Yamamoto, K.; Yanagimoto, Y.; Shinno, N.; Yasui, M.; Takahashi, H.; Wada, H.; et al. Clinical Implications of Conversion Surgery after Induction Therapy for T4b Thoracic Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2019, 26, 4737–4743. [Google Scholar] [CrossRef]
- Ochi, M.; Murakami, Y.; Nishibuchi, I.; Kubo, K.; Imano, N.; Takeuchi, Y.; Kimura, T.; Hamai, Y.; Emi, M.; Okada, M.; et al. Long-term results of definitive chemoradiotherapy for unresectable locally advanced esophageal squamous cell carcinoma. J. Radiat. Res. 2020, 62, 142–148. [Google Scholar] [CrossRef]
- Kojima, T.; Shah, M.A.; Muro, K.; Francois, E.; Adenis, A.; Hsu, C.-H.; Doi, T.; Moriwaki, T.; Kim, S.-B.; Lee, S.-H.; et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J. Clin. Oncol. 2020, 38, 4138–4148. [Google Scholar] [CrossRef]
- Kato, K.; Cho, B.C.; Takahashi, M.; Okada, M.; Lin, C.-Y.; Chin, K.; Kadowaki, S.; Ahn, M.-J.; Hamamoto, Y.; Doki, Y.; et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 1506–1517. [Google Scholar] [CrossRef]
- Sun, J.-M.; Shen, L.; Shah, M.A.; Enzinger, P.; Adenis, A.; Doi, T.; Kojima, T.; Metges, J.-P.; Li, Z.; Kim, S.-B.; et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet 2021, 398, 759–771. [Google Scholar] [CrossRef]
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.-H.; Adenis, A.; et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 386, 449–462. [Google Scholar] [CrossRef]
- Luo, H.; Lu, J.; Bai, Y.; Mao, T.; Wang, J.; Fan, Q.; Zhang, Y.; Zhao, K.; Chen, Z.; Gao, S.; et al. Effect of Camrelizumab vs. Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients with Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA 2021, 326, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, J.; Shu, Y.; Liu, L.; Kong, L.; Yang, L.; Wang, B.; Sun, G.; Ji, Y.; Cao, G.; et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): Multicentre, randomised, double blind, phase 3 trial. BMJ 2022, 377, e068714. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Petrelli, F.; Ghidini, A.; Raimondi, A.; Smyth, E.; Pietrantonio, F. Efficacy and activity of PD-1 blockade in patients with advanced esophageal squamous cell carcinoma: A systematic review and meta-analysis with focus on the value of PD-L1 combined positive score. ESMO Open 2022, 7, 100380. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-X.; Cui, C.; Yao, J.; Zhang, Y.; Li, M.; Feng, J.; Yang, S.; Fan, Y.; Shi, J.; Zhang, X.; et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell 2022, 40, 277–288.e3. [Google Scholar] [CrossRef]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 Ligands, and Other Features of the Tumor Immune Microenvironment with Response to Anti–PD-1 Therapy. Clin. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef]
- Whooley, J.; Alazzawi, M.; E Donlon, N.; Bolger, J.C.; Robb, W.B. PD-1 inhibitors in esophageal cancer: A systematic review of the oncological outcomes associated with PD-1 blockade and the evolving therapeutic paradigm. Dis. Esophagus 2021, 35, doab063. [Google Scholar] [CrossRef]
- Hansen, A.R.; Siu, L.L. PD-L1 Testing in Cancer: Challenges in Companion Diagnostic Development. JAMA Oncol. 2016, 2, 15–16. [Google Scholar] [CrossRef]
- Dall’Olio, F.G.; Marabelle, A.; Caramella, C.; Garcia, C.; Aldea, M.; Chaput, N.; Robert, C.; Besse, B. Tumour burden and efficacy of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 19, 75–90. [Google Scholar] [CrossRef]
- Zhou, C.-B.; Zhou, Y.-L.; Fang, J.-Y. Gut Microbiota in Cancer Immune Response and Immunotherapy. Trends Cancer 2021, 7, 647–660. [Google Scholar] [CrossRef]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef]
- Sánchez-Gastaldo, A.; Muñoz-Fuentes, M.A.; Molina-Pinelo, S.; Alonso-García, M.; Boyero, L.; Bernabé-Caro, R. Correlation of peripheral blood biomarkers with clinical outcomes in NSCLC patients with high PD-L1 expression treated with pembrolizumab. Transl. Lung Cancer Res. 2021, 10, 2509–2522. [Google Scholar] [CrossRef] [PubMed]
- Bilen, M.A.; Ba, D.J.M.; Liu, Y.; Lewis, C.; Np, H.H.C.; Ba, J.M.S.; Akce, M.; Kissick, H.T.; Carthon, B.C.; Shaib, W.L.; et al. The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced-stage cancer treated with immunotherapy. Cancer 2018, 125, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, C.; Peng, Z.; Qi, C.; Gong, J.; Zhang, X.; Li, J.; Shen, L. Association of Lymphocyte-to-Monocyte Ratio with Survival in Advanced Gastric Cancer Patients Treated with Immune Checkpoint Inhibitor. Front. Oncol. 2021, 11, 589022. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, D.; Zhang, W.; Liu, J.; Liu, C.; Li, Q.; Ma, Z.; Li, H.; Guan, X.; Bai, Y.; et al. Inflammatory Markers Predict Survival in Patients with Advanced Gastric and Colorectal Cancers Receiving Anti–PD-1 Therapy. Front. Cell Dev. Biol. 2021, 9, 638312. [Google Scholar] [CrossRef]
- Kim, J.H.; Ahn, B.; Hong, S.-M.; Jung, H.-Y.; Kim, D.H.; Choi, K.D.; Ahn, J.Y.; Lee, J.H.; Na, H.K.; Kim, J.H.; et al. Real-World Efficacy Data and Predictive Clinical Parameters for Treatment Outcomes in Advanced Esophageal Squamous Cell Carcinoma Treated with Immune Checkpoint Inhibitors. Cancer Res. Treat. 2022, 54, 505–516. [Google Scholar] [CrossRef]
- Guo, J.-C.; Lin, C.-C.; Lin, C.-Y.; Hsieh, M.-S.; Kuo, H.-Y.; Lien, M.-Y.; Shao, Y.-Y.; Huang, T.-C.; Hsu, C.-H. Neutrophil–to–lymphocyte Ratio and Use of Antibiotics Associated with Prognosis in Esophageal Squamous Cell Carcinoma Patients Receiving Immune Checkpoint Inhibitors. Anticancer Res. 2019, 39, 5675–5682. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Z.; Li, Y.; Chen, S.; Lu, J.; Wu, L.; Ma, Z.; Hu, Y.; Zhang, G. Pretreatment Neutrophil-to-Lymphocyte Ratio as a Prognostic Biomarker in Unresectable or Metastatic Esophageal Cancer Patients with Anti-PD-1 Therapy. Front. Oncol. 2022, 12, 834564. [Google Scholar] [CrossRef]
- Nishijima, T.F.; Muss, H.B.; Shachar, S.S.; Tamura, K.; Takamatsu, Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat. Rev. 2015, 41, 971–978. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, D.; Song, H.; Qiu, B.; Tian, D.; Li, Z.; Ji, Y.; Wang, J. Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: A systematic review and meta-analysis. BMJ Open 2021, 11, e048324. [Google Scholar] [CrossRef]
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. Immunother. Cancer 2018, 6, 8. [Google Scholar] [CrossRef]
- Zhu, Z.-F.; Zhuang, L.-P.; Zhang, C.-Y.; Ning, Z.-Y.; Wang, D.; Sheng, J.; Hua, Y.-Q.; Xie, J.; Xu, L.-T.; Meng, Z.-Q. Predictive role of the monocyte-to-lymphocyte ratio in advanced hepatocellular carcinoma patients receiving anti-PD-1 therapy. Transl. Cancer Res. 2022, 11, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Han, R.; Zhong, Y.; Weng, N.; Zhang, A. Post treatment NLR is a predictor of response to immune checkpoint inhibitor therapy in patients with esophageal squamous cell carcinoma. Cancer Cell Int. 2021, 21, 356. [Google Scholar] [CrossRef] [PubMed]
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell. Mol. Immunol. 2020, 18, 842–859. [Google Scholar] [CrossRef] [PubMed]
- Ménétrier-Caux, C.; Ray-Coquard, I.; Blay, J.-Y.; Caux, C. Lymphopenia in Cancer Patients and its Effects on Response to Immunotherapy: An opportunity for combination with Cytokines? J. Immunother. Cancer 2019, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.M.; Lee, J.; Bae, S.J.; Baik, S.J.; Ji, J.; Kim, D.; Ahn, S.G.; Jeong, J. Body mass index and absolute lymphocyte count predict disease-free survival in Korean breast cancer patients. Br. J. Cancer 2021, 125, 119–125. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pagès, F.; Sautes-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Jakubowska, K.; Koda, M.; Grudzińska, M.; Lomperta, K.; Famulski, W. Tumor-infiltrating lymphocytes in tissue material combined with systemic lymphocyte inflammation in patients with colorectal cancer. Mol. Clin. Oncol. 2021, 14, 97. [Google Scholar] [CrossRef]
- Yin, T.; Wang, P.; Yu, J.; Teng, F. Treatment-related lymphopenia impairs the treatment response of anti-PD-1 therapy in esophageal squamous cell carcinoma. Int. Immunopharmacol. 2022, 106, 108623. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Vera Aguilera, J.; Chintakuntlawar, A.; et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 1008–1019. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Baba, Y.; Nomoto, D.; Okadome, K.; Ishimoto, T.; Iwatsuki, M.; Miyamoto, Y.; Yoshida, N.; Baba, H. Tumor immune microenvironment and immune checkpoint inhibitors in esophageal squamous cell carcinoma. Cancer Sci. 2020, 111, 3132–3141. [Google Scholar] [CrossRef] [PubMed]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-Associated Macrophages as Major Players in the Tumor Microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Pollard, J.W. Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018, 17, 887–904. [Google Scholar] [CrossRef] [PubMed]
- Balta, E.; Wabnitz, G.; Samstag, Y. Hijacked Immune Cells in the Tumor Microenvironment: Molecular Mechanisms of Immunosuppression and Cues to Improve T Cell-Based Immunotherapy of Solid Tumors. Int. J. Mol. Sci. 2021, 22, 5736. [Google Scholar] [CrossRef] [PubMed]
- Peranzoni, E.; Lemoine, J.; Vimeux, L.; Feuillet, V.; Barrin, S.; Kantari-Mimoun, C.; Bercovici, N.; Guérin, M.; Biton, J.; Ouakrim, H.; et al. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti–PD-1 treatment. Proc. Natl. Acad. Sci. USA 2018, 115, E4041–E4050. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Yoon, D.H.; Suh, C.; Huh, J. Body mass index as a prognostic factor in Asian patients treated with chemoimmunotherapy for diffuse large B cell lymphoma, not otherwise specified. Ann. Hematol. 2015, 94, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Minami, S.; Ihara, S.; Tanaka, T.; Komuta, K. Sarcopenia and Visceral Adiposity Did Not Affect Efficacy of Immune-Checkpoint Inhibitor Monotherapy for Pretreated Patients with Advanced Non-Small Cell Lung Cancer. World J. Oncol. 2020, 11, 9–22. [Google Scholar] [CrossRef]
- Flint, T.R.; Janowitz, T.; Connell, C.M.; Roberts, E.W.; Denton, A.E.; Coll, A.P.; Jodrell, D.I.; Fearon, D.T. Tumor-Induced IL-6 Reprograms Host Metabolism to Suppress Anti-tumor Immunity. Cell Metab. 2016, 24, 672–684. [Google Scholar] [CrossRef]
- Bertrand, F.; Montfort, A.; Marcheteau, E.; Imbert, C.; Gilhodes, J.; Filleron, T.; Rochaix, P.; Andrieu-Abadie, N.; Levade, T.; Meyer, N.; et al. TNFα blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat. Commun. 2017, 8, 2256. [Google Scholar] [CrossRef]
- Tanaka, K.; Nakamura, S.; Narimatsu, H. Nutritional Approach to Cancer Cachexia: A Proposal for Dietitians. Nutrients 2022, 14, 345. [Google Scholar] [CrossRef]
- Yao, X.; Huang, J.; Zhong, H.; Shen, N.; Faggioni, R.; Fung, M.; Yao, Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol. Ther. 2013, 141, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Barone, I.; Giordano, C. Leptin and Beyond: Actors in Cancer. Biomolecules 2021, 11, 1836. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Cortegana, C.; López-Saavedra, A.; Sánchez-Jiménez, F.; Pérez-Pérez, A.; Castiñeiras, J.; Virizuela-Echaburu, J.; de la Cruz-Merino, L.; Sánchez-Margalet, V. Leptin, Both Bad and Good Actor in Cancer. Biomolecules 2021, 11, 913. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Tanabe, H.; Sato, H.; Ishikawa, C.; Goto, M.; Yanagida, N.; Akabane, H.; Yokohama, S.; Hasegawa, K.; Kitano, Y.; et al. Prognostic factors to predict the survival in patients with advanced gastric cancer who receive later-line nivolumab monotherapy—The Asahikawa Gastric Cancer Cohort Study (AGCC). Cancer Med. 2021, 11, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhou, X.; Li, Y.; Tian, X.; Wang, Y.; Huang, M.; Ren, L.; Zhou, L.; Ding, Z.; Zhu, J.; et al. A Novel Nomogram and Risk Classification System Predicting the Cancer-Specific Survival of Patients with Initially Diagnosed Metastatic Esophageal Cancer: A SEER-Based Study. Ann. Surg. Oncol. 2018, 26, 321–328. [Google Scholar] [CrossRef]
- Duan, F.; Song, C.; Ma, Y.; Jiang, K.; Xu, F.; Bi, X.; Huang, J.; Hong, R.; Huang, Z.; Lu, Q.; et al. Establishment of Prognostic Nomograms for Predicting the Survival of HR-Positive, HER2-Negative Metastatic Breast Cancer Patients Treated with Everolimus. Drug Des. Dev. Ther. 2021, 15, 3463–3473. [Google Scholar] [CrossRef]
| Characteristics | Total (n = 81) | (%) |
|---|---|---|
| Gender | ||
| Male | 74 | (91.36) |
| Female | 7 | (8.64) |
| Age (years) | ||
| ≤60 | 28 | (34.57) |
| >60 | 53 | (65.43) |
| Smoking history | ||
| Yes | 63 | (77.78) |
| None | 18 | (22.22) |
| Drinking history | ||
| Yes | 58 | (71.60) |
| None | 23 | (28.40) |
| BMI (kg/m²) | ||
| ≤18.5 | 19 | (23.46) |
| >18.5 | 62 | (76.54) |
| Location | ||
| Cervical | 9 | (11.11) |
| Upper | 8 | (9.88) |
| Middle | 39 | (48.15) |
| Lower | 25 | (30.86) |
| Distant metastasis | ||
| None | 61 | (75.31) |
| Yes | 20 | (24.69) |
| Liver metastasis | ||
| None | 74 | (91.36) |
| Yes | 7 | (8.64) |
| Bone metastasis | ||
| None | 73 | (90.12) |
| Yes | 8 | (9.88) |
| Lung metastasis | ||
| None | 75 | (92.59) |
| Yes | 6 | (7.41) |
| TNM stage | ||
| III | 19 | (23.46) |
| IV | 62 | (76.54) |
| PS score | ||
| 0 | 50 | (61.73) |
| 1 | 31 | (38.27) |
| PD-1 or PD-L1 | ||
| PD-1 ICIs + chemotherapy | 76 | (93.83) |
| PD-L1 ICIs + chemotherapy | 5 | (6.17) |
| Chemotherapy | ||
| Nab-PTX/PTX + platinum | 57 | (70.37) |
| 5-FU + platinum | 15 | (18.52) |
| Other regimens | 9 | (11.11) |
| HB (g/L) | ||
| ≤120 | 20 | (24.69) |
| >120 | 61 | (75.31) |
| Neutrophil (×109/L) | ||
| Median (range) | 4.45 (1.71–9.06) | |
| Lymphocyte (×109/L) | ||
| Median (range) | 1.39 (0.45–3.23) | |
| Monocyte (×109/L) | ||
| Median (range) | 0.54 (0.23–1.68) | |
| Platelet (×109/L) | ||
| Median (range) | 260 (94–542) | |
| Variables | NLR ≤ 3.67 | NLR > 3.67 | p-Value a | MLR ≤ 0.40 | MLR > 0.40 | p-Value a |
|---|---|---|---|---|---|---|
| (n = 50) (%) | (n = 31) (%) | (n = 45) (%) | (n = 36) (%) | |||
| Gender | 0.040 | 0.015 | ||||
| Male | 43 (86.0) | 31 (100.0) | 38 (84.4) | 36 (100.0) | ||
| Female | 7 (14.0) | 0 (0.0) | 7 (15.6) | 0 (0.0) | ||
| Age (years) | 0.537 | 0.834 | ||||
| ≤60 | 16 (32.0) | 12 (38.7) | 16 (35.6) | 12 (33.3) | ||
| >60 | 34 (68.0) | 19 (61.3) | 29 (64.4) | 24 (66.7) | ||
| Smoking history | 0.625 | 0.591 | ||||
| Yes | 38 (76.0) | 25 (80.7) | 34 (75.6) | 29 (80.6) | ||
| None | 12 (24.0) | 6 (19.4) | 11 (24.4) | 7 (19.4) | ||
| Drinking history | 0.155 | 0.544 | ||||
| Yes | 33 (66.0) | 25 (80.7) | 31 (68.9) | 27 (75.0) | ||
| None | 17 (34.0) | 6 (19.4) | 14 (31.1) | 9 (25.0) | ||
| BMI (kg/m²) | 0.351 | 0.177 | ||||
| ≤18.5 | 10 (20.0) | 9 (29.0) | 8 (17.8) | 11 (30.6) | ||
| >18.5 | 40 (80.0) | 22 (71.0) | 37 (82.2) | 25 (69.4) | ||
| Distant Metastasis | 0.214 | 0.033 | ||||
| None | 40 (80.0) | 21 (67.7) | 38 (84.4) | 23 (63.9) | ||
| Yes | 10 (20.0) | 10 (32.3) | 7 (15.6) | 13 (36.1) | ||
| Liver Metastasis | 0.100 | 0.041 | ||||
| None | 48 (96.0) | 26 (83.9) | 44 (97.8) | 30 (83.3) | ||
| Yes | 2 (4.0) | 5 (16.1) | 1 (2.2) | 6 (16.7) | ||
| Bone Metastasis | 0.145 | 0.290 | ||||
| None | 43 (86.0) | 30 (96.8) | 39 (86.7) | 34 (94.4) | ||
| Yes | 7 (14.0) | 1 (3.2) | 6 (13.3) | 2 (5.6) | ||
| Lung Metastasis | 0.196 | 0.083 | ||||
| None | 48 (96.0) | 27 (87.1) | 44 (97.8) | 31 (86.1) | ||
| Yes | 2 (4.0) | 4 (12.9) | 1 (2.2) | 5 (13.9) | ||
| TNM stage | 0.220 | 0.019 | ||||
| III | 14 (28.0) | 5 (16.1) | 15 (33.3) | 4 (11.1) | ||
| IV | 36 (72.0) | 26 (83.9) | 30 (66.7) | 32 (88.9) | ||
| PS score | 0.052 | 0.004 | ||||
| 0 | 35 (70.0) | 15 (48.4) | 34 (75.6) | 16 (44.4) | ||
| 1 | 15 (30.0) | 16 (51.6) | 11 (24.4) | 20 (55.6) | ||
| HB (g/L) | 0.021 | 0.107 | ||||
| ≤120 | 8 (16.0) | 12 (38.7) | 8 (17.8) | 12 (33.3) | ||
| >120 | 42 (84.0) | 19 (61.3) | 37 (82.2) | 24 (66.7) | ||
| PD-1 or PD-L1 | 1.000 | 0.375 | ||||
| PD-1 ICIs + chemotherapy | 47 (94.0) | 29 (93.5) | 41 (91.1) | 35 (97.2) | ||
| PD-L1 ICIs + chemotherapy | 3 (6.0) | 2 (6.5) | 4 (8.9) | 1 (2.8) | ||
| Chemotherapy | 0.576 | 0.278 | ||||
| Nab-PTX/PTX + platinum | 34 (68.0) | 23 (74.2) | 32 (71.1) | 25 (69.4) | ||
| 5-FU + platinum | 11 (22.0) | 4 (12.9) | 10 (22.2) | 5 (13.9) | ||
| Other regimens | 5 (10.0) | 4 (12.9) | 3 (6.7) | 6 (16.7) |
| PFS | OS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||||||
| Variables | HR | 95%CI | p-Value a | HR | 95%CI | p-Value a | HR | 95%CI | p-Value a | HR | 95%CI | p-Value a |
| Gender | ||||||||||||
| Male | Reference | Reference | ||||||||||
| Female | 0.245 | 0.034–1.789 | 0.166 | 1.009 | 0.130–7.811 | 0.993 | ||||||
| Age (years) | ||||||||||||
| ≤60 | Reference | Reference | ||||||||||
| >60 | 0.678 | 0.365–1.260 | 0.219 | 1.732 | 0.662–4.533 | 0.263 | ||||||
| BMI (kg/m²) | ||||||||||||
| ≤18.5 | Reference | Reference | Reference | Reference | ||||||||
| >18.5 | 0.537 | 0.276–1.041 | 0.066 | 0.572 | 0.293–1.119 | 0.103 | 0.383 | 0.156–0.939 | 0.036 | 0.323 | 0.128–0.818 | 0.017 |
| Location | ||||||||||||
| Cervical | Reference | Reference | ||||||||||
| Upper | 0.922 | 0.200–4.253 | 0.917 | 1.273 | 0.253–6.410 | 0.770 | ||||||
| Middle | 1.217 | 0.408–3.631 | 0.725 | 0.640 | 0.165–2.481 | 0.518 | ||||||
| Lower | 2.740 | 0.915–8.201 | 0.072 | 1.154 | 0.294–4.523 | 0.837 | ||||||
| Metastasis | ||||||||||||
| None | Reference | Reference | ||||||||||
| Yes | 1.703 | 0.876–3.309 | 0.116 | 0.698 | 0.249–1.958 | 0.494 | ||||||
| TNM stage | ||||||||||||
| III | Reference | Reference | Reference | |||||||||
| IV | 2.178 | 0.912–5.199 | 0.080 | 1.806 | 0.747–4.368 | 0.190 | 2.588 | 0.597–11.219 | 0.204 | |||
| PS score | ||||||||||||
| 0 | Reference | Reference | Reference | |||||||||
| 1 | 1.256 | 0.663–2.379 | 0.485 | 2.493 | 1.016–6.119 | 0.046 | 1.718 | 0.687–4.301 | 0.247 | |||
| HB (g/L) | ||||||||||||
| ≤120 | Reference | Reference | ||||||||||
| >120 | 0.564 | 0.284–1.118 | 0.101 | 0.545 | 0.207–1.432 | 0.218 | ||||||
| MLR | ||||||||||||
| ≤0.40 | Reference | Reference | Reference | Reference | ||||||||
| >0.40 | 2.341 | 1.245–4.404 | 0.008 | 2.254 | 1.188–4.276 | 0.013 | 4.557 | 1.744–11.906 | 0.002 | 4.524 | 1.681–12.175 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Ding, Y.; Qian, J.; Wan, M.; Li, N.; Mao, C.; Xiao, C.; Jiang, H.; Zheng, Y.; Wu, L.; et al. Nomogram Based on Monocyte-to-Lymphocyte Ratio to Predict Survival of Unresectable Esophageal Squamous Cell Carcinoma Who Receive First-Line PD-1/PD-L1 Inhibitors Combined with Chemotherapy. Curr. Oncol. 2022, 29, 8937-8954. https://doi.org/10.3390/curroncol29110702
Ma X, Ding Y, Qian J, Wan M, Li N, Mao C, Xiao C, Jiang H, Zheng Y, Wu L, et al. Nomogram Based on Monocyte-to-Lymphocyte Ratio to Predict Survival of Unresectable Esophageal Squamous Cell Carcinoma Who Receive First-Line PD-1/PD-L1 Inhibitors Combined with Chemotherapy. Current Oncology. 2022; 29(11):8937-8954. https://doi.org/10.3390/curroncol29110702
Chicago/Turabian StyleMa, Xiaolu, Yongfeng Ding, Jiong Qian, Mingyu Wan, Ning Li, Chenyu Mao, Cheng Xiao, Haiping Jiang, Yulong Zheng, Luntao Wu, and et al. 2022. "Nomogram Based on Monocyte-to-Lymphocyte Ratio to Predict Survival of Unresectable Esophageal Squamous Cell Carcinoma Who Receive First-Line PD-1/PD-L1 Inhibitors Combined with Chemotherapy" Current Oncology 29, no. 11: 8937-8954. https://doi.org/10.3390/curroncol29110702
APA StyleMa, X., Ding, Y., Qian, J., Wan, M., Li, N., Mao, C., Xiao, C., Jiang, H., Zheng, Y., Wu, L., Chen, X., & Xu, N. (2022). Nomogram Based on Monocyte-to-Lymphocyte Ratio to Predict Survival of Unresectable Esophageal Squamous Cell Carcinoma Who Receive First-Line PD-1/PD-L1 Inhibitors Combined with Chemotherapy. Current Oncology, 29(11), 8937-8954. https://doi.org/10.3390/curroncol29110702





