Sintilimab-Induced Diabetic Ketoacidosis in a Patient with Radiation and Multichemorefractory Penile Cancer: A Case Report and Literature Review
Abstract
1. Background
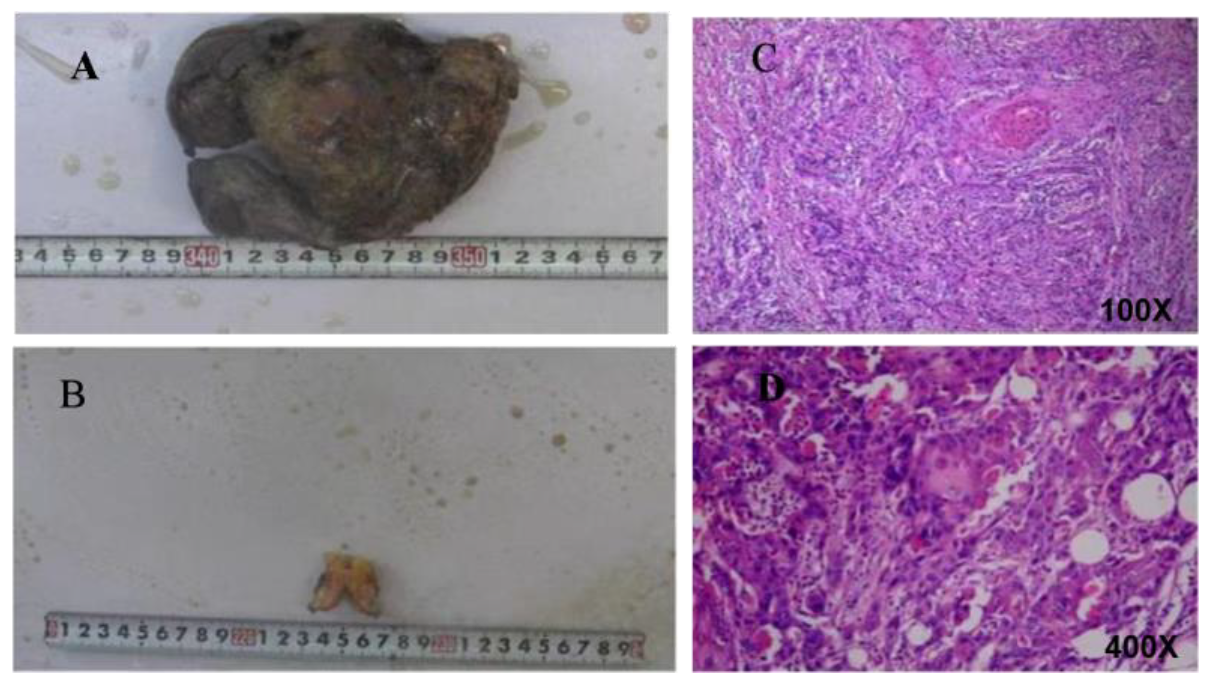
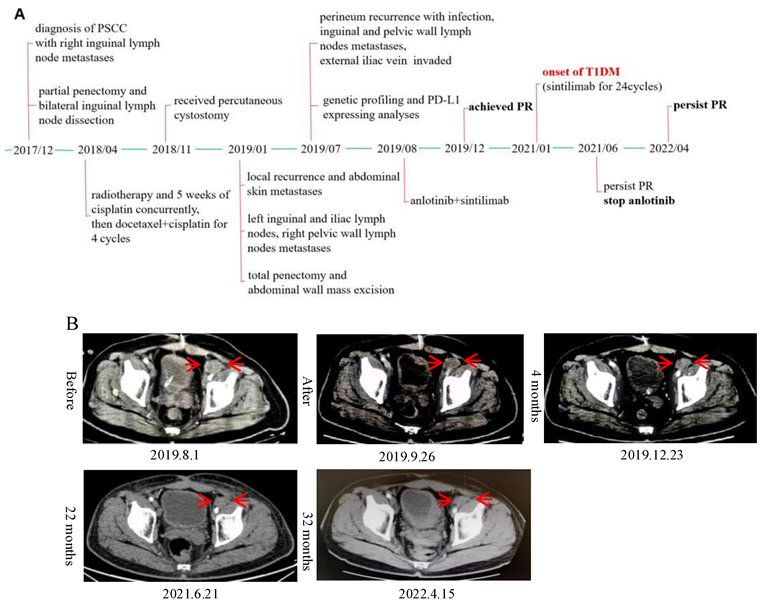
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Barone, B.; Calogero, A.; Scafuri, L.; Ferro, M.; Lucarelli, G.; Di Zazzo, E.; Sicignano, E.; Falcone, A.; Romano, L.; De Luca, L.; et al. Immune Checkpoint Inhibitors as a Neoadjuvant/Adjuvant Treatment of Muscle-Invasive Bladder Cancer: A Systematic Review. Cancers 2022, 14, 2545. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A. Releasing the Brakes on Cancer Immunotherapy. N. Engl. J. Med. 2015, 373, 1490–1492. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Paschou, S.A.; Stefanaki, K.; Psaltopoulou, T.; Liontos, M.; Koutsoukos, K.; Zagouri, F.; Lambrinoudaki, I.; Dimopoulos, M.-A. How we treat endocrine complications of immune checkpoint inhibitors. ESMO Open 2021, 6, 100011. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yi, Y. Recent updates on Sintilimab in solid tumor immunotherapy. Biomark. Res. 2020, 8, 69. [Google Scholar] [CrossRef]
- Cocks, M.; Taheri, D.; Ball, M.W.; Bezerra, S.M.; Del Carmen Rodriguez, M.; Ricardo, B.F.P.; Bivalacqua, T.J.; Sharma, R.B.; Meeker, A.; Chaux, A.; et al. Share Immune-checkpoint status in penile squamous cell carcinoma: A North American cohort. Hum. Pathol. 2017, 59, 55–61. [Google Scholar] [CrossRef]
- Stecca, C.E.; Alt, M.; Jiang, D.M.; Chung, P.; Crook, J.M.; Kulkarni, G.S.; Sridhar, S.S. Recent Advances in the Management of Penile Cancer: A Contemporary Review of the Literature. Oncol. Ther. 2021, 9, 21–39. [Google Scholar] [CrossRef]
- Stoehr, R.; Wendler, O.; Giedl, J.; Gaisa, N.T.; Richter, G.; Campean, V.; Burger, M.; Wullich, B.; Bertz, S.; Hartmann, A. No Evidence of Microsatellite Instability and Loss of Mismatch-Repair-Protein Expression in Squamous Cell Carcinoma of the Penis. Pathobiology 2019, 86, 145–151. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Yang, K.; Ma, Y.; Fu, S.; Tang, X.; Wang, Y.; Zhou, L. Endocrine Adverse Events Caused by Different Types and Different Doses of Immune Checkpoint Inhibitors in the Treatment of Solid Tumors: A Meta-Analysis and Systematic Review. J. Clin. Pharmacol. 2021, 61, 282–297. [Google Scholar] [CrossRef]
- Baweja, A.; Mar, N. Metastatic penile squamous cell carcinoma with dramatic response to combined checkpoint blockade with ipilimumab and nivolumab. J. Oncol. Pharm. Pract. 2021, 27, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Trafalis, D.T.; Alifieris, C.E.; Kalantzis, A.; Verigos, K.E.; Vergadis, C.; Sauvage, S. Evidence for Efficacy of Treatment With the Anti-PD-1 Mab Nivolumab in Radiation and Multichemorefractory Advanced Penile Squamous Cell Carcinoma. J. Immunother. 2018, 41, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Hui, G.; Ghafouri, S.N.; Shen, J.; Liu, S.; Drakaki, A. Treating Penile Cancer in the Immunotherapy and Targeted Therapy Era. Case Rep. Oncol. Med. 2019, 2019, 8349793. [Google Scholar] [CrossRef] [PubMed]
- Chahoud, J.; Skelton, W.P., IV; Spiess, P.E.; Walko, C.; Dhillon, J.; Gage, K.L.; Johnstone, P.A.S.; Jain, R.K. Case Report: Two Cases of Chemotherapy Refractory Metastatic Penile Squamous Cell Carcinoma With Extreme Durable Response to Pembrolizumab. Front. Oncol. 2020, 10, 615298. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.W.; Chahoud, J.; Campbell, M.T.; Karp, D.D.; Wang, J.; Stephen, B.; Tu, S.-M.; Pettaway, C.A.; Naing, A. Pembrolizumab for advanced penile cancer: A case series from a phase II basket trial. Investig. New Drugs 2021, 39, 1405–1410. [Google Scholar] [CrossRef]
- Denis, C.; Sakalihasan, S.; Frères, P.; Withofs, N.; Sautois, B. Cemiplimab for Cisplatin Resistant Metastatic Penile Cancer. Case Rep. Oncol. 2021, 14, 972–976. [Google Scholar] [CrossRef]
- Su, X.; Zhang, J.; Fu, C.; Xiao, M.; Wang, C. Recurrent Metastatic Penile Cancer Patient with Positive PD-L1 Expression Obtained Significant Benefit from Immunotherapy: A Case Report and Literature Review. OncoTargets Ther. 2020, 13, 3319–3324. [Google Scholar] [CrossRef]
- Hu, L.; Shan, X.; Han, D.; Guo, Z.; Wang, H.; Xiao, Z. Multimodal Treatment Combining Salvage Surgery-Assisted Chemotherapy and Checkpoints Blockade Immunotherapy Achieves Complete Remission on a Recurrent Penile Cancer Patient: A Case Report. OncoTargets Ther. 2021, 14, 4891–4896. [Google Scholar] [CrossRef]
- Li, N.; Xu, T.; Zhou, Z.; Li, P.; Jia, G.; Li, X. Immunotherapy Combined With Chemotherapy for Postoperative Recurrent Penile Squamous Cell Carcinoma: A Case Report and Literature Review. Front. Oncol. 2022, 12, 837547. [Google Scholar] [CrossRef]
- Mei, X.; Zhao, Y.; Zhang, Y.; Liao, J.; Jiang, C.; Qian, H.; Du, Y. Efficacy and Biomarker Exploration of Sintilimab Combined With Chemotherapy in the Treatment of Advanced Penile Squamous Cell Carcinoma—A Report of Two Cases. Front. Oncol. 2022, 12, 823459. [Google Scholar] [CrossRef]
- Clotman, K.; Janssens, K.; Specenier, P.; Weets, I.; De Block, C.E.M. Programmed Cell Death-1 Inhibitor–Induced Type 1 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2018, 103, 3144–3154. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.R.; Russo, A.; Li, A.Y.; McCusker, M.G.; Kroopnick, J.M.; Scilla, K.; Mehra, R.; Rolfo, C. Development of autoimmune diabetes with severe diabetic ketoacidosis and immune-related thyroiditis secondary to durvalumab: A case report. Transl. Lung Cancer Res. 2020, 9, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Muniz, T.P.; Araujo, D.V.; Savage, K.J.; Cheng, T.; Saha, M.; Song, X.; Gill, S.; Monzon, J.G.; Grenier, D.; Genta, S.; et al. CANDIED: A Pan-Canadian Cohort of Immune Checkpoint Inhibitor-Induced Insulin-Dependent Diabetes Mellitus. Cancers 2021, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- de Filette, J.M.K.; Pen, J.J.; Decoster, L.; Vissers, T.; Bravenboer, B.; Van der Auwera, B.J.; Gorus, F.K.; Roep, B.O.; Aspeslagh, S.; Neyns, B.; et al. Immune checkpoint inhibitors and type 1 diabetes mellitus: A case report and systematic review. Eur. J. Endocrinol. 2019, 181, 363–374. [Google Scholar] [CrossRef]
- Kawabata, Y.; Ikegami, H.; Awata, T.; Imagawa, A.; Maruyama, T.; Kawasaki, E.; Tanaka, S.; Shimada, A.; Osawa, H.; Kobayashi, T.; et al. Committee on Type 1 Diabetes, Japan Diabetes Society. Differential association of HLA with three subtypes of type 1 diabetes: Fulminant, slowly progressive and acute-onset. Diabetologia 2009, 52, 2513–2521. [Google Scholar] [CrossRef]
- Pociot, F.; Lernmark, Å. Genetic risk factors for type 1 diabetes. Lancet 2016, 387, 2331–2339. [Google Scholar] [CrossRef]
- Zhang, R.; Cai, X.-L.; Liu, L.; Han, X.-Y.; Ji, L.-N. Type 1 diabetes induced by immune checkpoint inhibitors. Chin. Med. J. 2020, 133, 2595–2598. [Google Scholar] [CrossRef]
- Akturk, H.K.; Kahramangil, D.; Sarwal, A.; Hoffecker, L.; Murad, M.H.; Michels, A.W. Immune checkpoint inhibitor-induced Type 1 diabetes: A systematic review and meta-analysis. Diabet. Med. 2019, 36, 1075–1081. [Google Scholar] [CrossRef]
| Author | Age | PD-L1 Status | MSI Status | TMB | Outcome |
|---|---|---|---|---|---|
| Baweja et al. [11] (2021) | 47 | TPS: 90% | MSI-high | High (24 Muts/Mb) | 12-month follow up, PR |
| Trafalis et al. [12] (2018) | 47 | TPS: ≈10% | Stable | High | 6-month follow up, PR |
| Hui et al. [13] (2019) | 64 | UK | UK | UK | 6-month follow up, Stable |
| Hui et al. [13] (2019) | 79 | UK | UK | UK | 24-month follow up, CR |
| Chahoud et al. [14] (2020) | 64 | UK | UK | High (14 Muts/Mb) | 38-month follow up, CR |
| Chahoud et al. [14] (2020) | 85 | CPS: 130 | Stable | Low (3 Muts/Mb) | 18-month follow up, PR |
| Hahn et al. [15] (2021) | 76 | TPS: 10% | MSI-high | UK | 38.7-month follow up, PR |
| Hahn et al. [15] (2021) | 72 | TPS: 80% | Stable | UK | 8.3-month follow up, PD |
| Hahn et al. [15] (2021) | 66 | TPS: 1% | Stable | UK | 3.8-month follow up, PD, |
| Denis et al. [16] (2021) | 75 | TPS: >95% | UK | UK | 15.1-month follow up, CR |
| Su et al. [17] (2020) | 46 | TPS: ≥10% | Stable | High (8.87 Muts/Mb) | 10.5-month follow up, CR |
| Hu et al. [18] (2021) | 49 | TPS: 20~30% | Stable | Low (2.25 Muts/Mb) | 19-month follow up, CR |
| Li et al. [19] (2022) | 76 | TPS: ≈10% | UK | UK | 5-month follow up, CR |
| Mei et al. [20] (2022) | 63 | TPS: 50~60% | Stable | High (17.95 Muts/Mb) | 28-month follow up, PR |
| Mei et al. [20] (2022) | 39 | TPS: <1% | Stable | Low (0 Muts/Mb) | 24-month follow up, CR |
| Present case (2022) | 52 | TPS: 90% | Stable | High (14.40 Muts/Mb) | 32-month follow up, PR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, C.; Wu, C.; Zhang, Y.; Li, W.; Wang, X.; Liang, L. Sintilimab-Induced Diabetic Ketoacidosis in a Patient with Radiation and Multichemorefractory Penile Cancer: A Case Report and Literature Review. Curr. Oncol. 2022, 29, 7987-7993. https://doi.org/10.3390/curroncol29110632
Lv C, Wu C, Zhang Y, Li W, Wang X, Liang L. Sintilimab-Induced Diabetic Ketoacidosis in a Patient with Radiation and Multichemorefractory Penile Cancer: A Case Report and Literature Review. Current Oncology. 2022; 29(11):7987-7993. https://doi.org/10.3390/curroncol29110632
Chicago/Turabian StyleLv, Chuan, Can Wu, Yan Zhang, Wendong Li, Xuesong Wang, and Li Liang. 2022. "Sintilimab-Induced Diabetic Ketoacidosis in a Patient with Radiation and Multichemorefractory Penile Cancer: A Case Report and Literature Review" Current Oncology 29, no. 11: 7987-7993. https://doi.org/10.3390/curroncol29110632
APA StyleLv, C., Wu, C., Zhang, Y., Li, W., Wang, X., & Liang, L. (2022). Sintilimab-Induced Diabetic Ketoacidosis in a Patient with Radiation and Multichemorefractory Penile Cancer: A Case Report and Literature Review. Current Oncology, 29(11), 7987-7993. https://doi.org/10.3390/curroncol29110632




