Response to Combination of Pembrolizumab and Axitinib in Hereditary Leyomiomatosis and Renal Cell Cancer (HLRCC)
Abstract
:1. Introduction
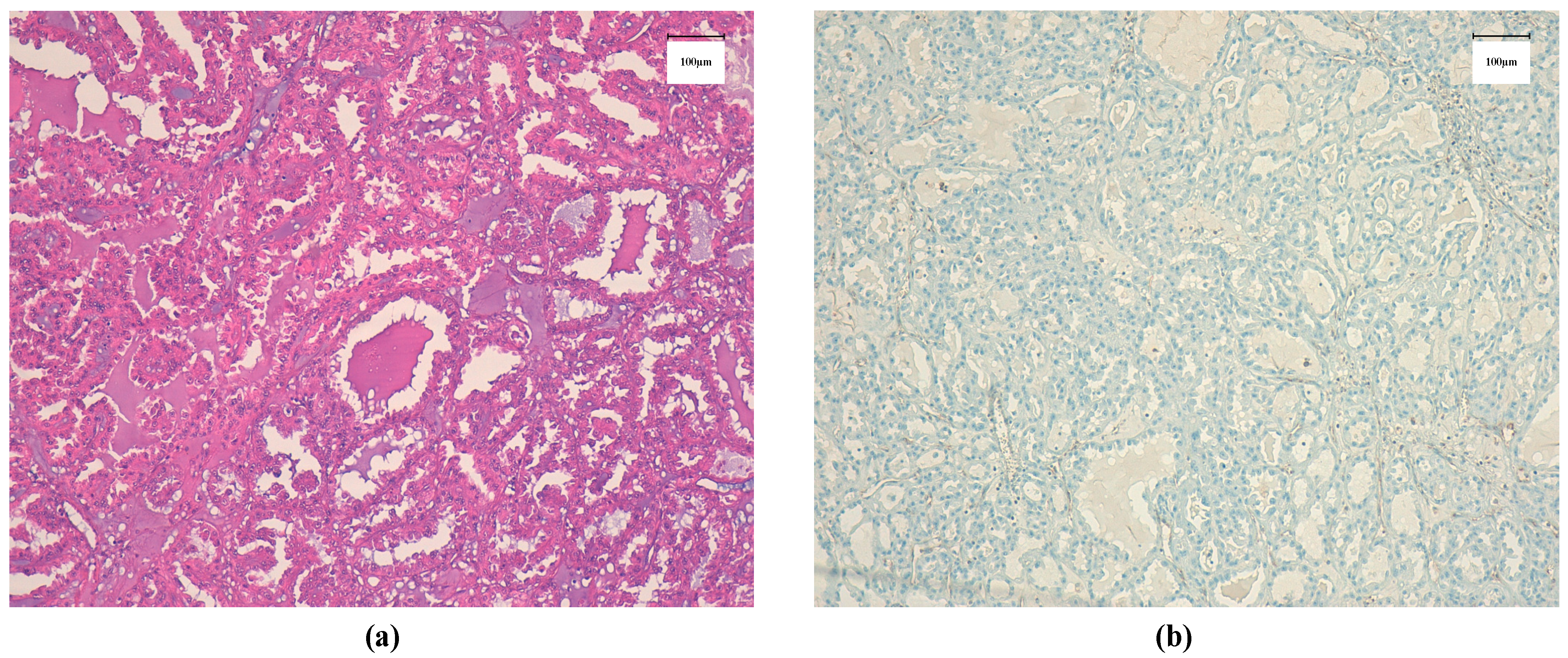
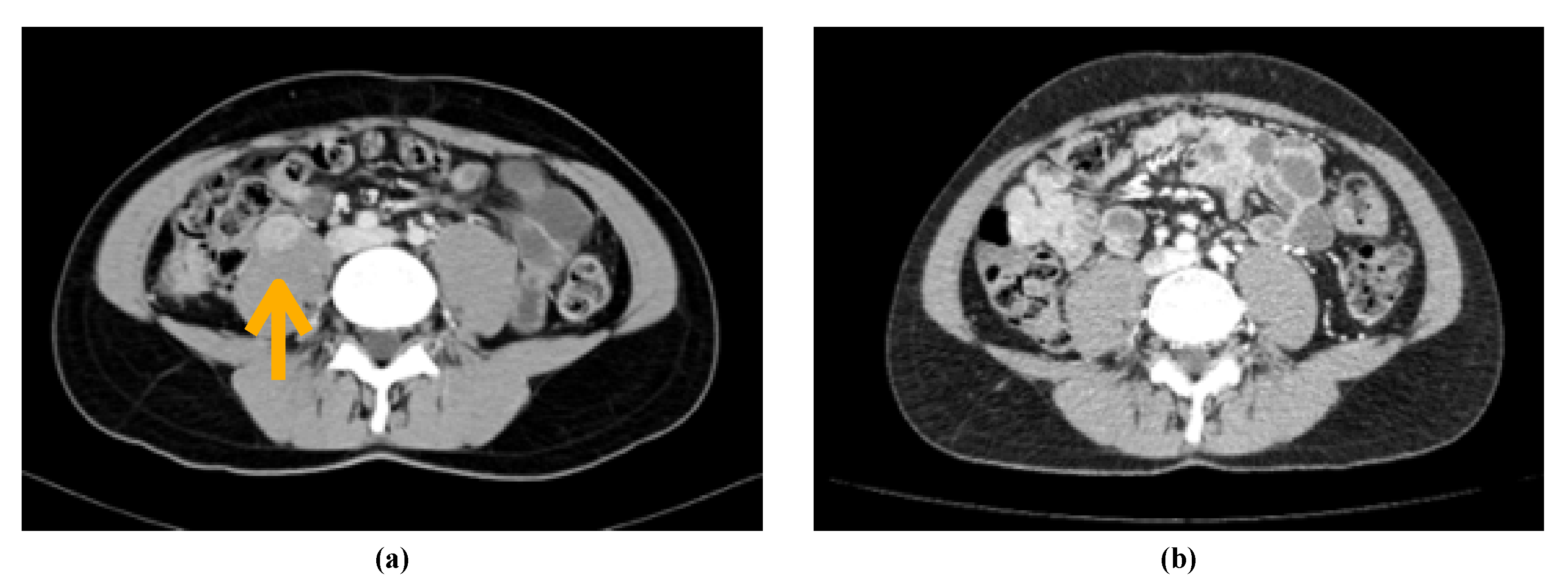
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maher, E.R. Hereditary renal cell carcinoma syndromes: Diagnosis, surveillance and management. World J. Urol. 2018, 36, 1891–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, V.M.; Handler, M.Z.; Schwartz, R.A.; Lambert, W.C. Hereditary leiomyomatosis and renal cell cancer syndrome: An update and review. J. Am. Acad. Dermatol. 2017, 77, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.A.; Barclay, E.; Rowan, A.J.; Tyrer, J.P.; Calonje, E.; Manek, S.; Kelsell, D.; Leigh, I.; Olpin, S.; Tomlinson, I.P. Clinical features of multiple cutaneous and uterine leiomyomatosis: An underdiagnosed tumor syndrome. Arch. Dermatol. 2005, 141, 199–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomlinson, I.P.M.; Alam, N.A.; Rowan, A.J.; Barclay, E.; Jaeger, E.E.M.; Kelsell, D.; Leigh, I.; Gorman, P.; Lamlum, H.; Rahman, S.; et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat. Genet. 2002, 30, 406–410. [Google Scholar] [PubMed]
- Gardie, B.; Remenieras, A.; Kattygnarath, D.; Bombled, J.; Lefèvre, S.; Perrier-Trudova, V.; Rustin, P.; Barrois, M.; Slama, A.; Avril, M.-F.; et al. Novel FH mutations in families with hereditary leiomyomatosis and renal cell cancer (HLRCC) and patients with isolated type 2 papillary renal cell carcinoma. J. Med. Genet. 2011, 48, 226–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, M.-H.; Toure, O.; Glenn, G.M.; Pithukpakorn, M.; Neckers, L.; Stolle, C.; Choyke, P.; Grubb, R.; Middelton, L.; Turner, M.L.; et al. Novel mutations in FH and expansion of the spectrum of phenotypes expressed in families with hereditary leiomyomatosis and renal cell cancer. J. Med. Genet. 2006, 43, 18–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuch, B.; Li, S.; Risch, H.; Bindra, R.S.; McGillivray, P.D.; Gerstein, M. Estimation of the carrier frequency of fumarate hydratase alterations and implications for kidney cancer risk in hereditary leiomyomatosis and renal cancer. Cancer 2020, 126, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Gurram, S.; Al Harthy, M.; Singer, E.A.; Sidana, A.; Shuch, B.M.; Ball, M.W.; Friend, J.C.; Mac, L.; Purcell, E.; et al. Results from a phase II study of bevacizumab and erlotinib in subjects with advanced hereditary leiomyomatosis and renal cell cancer (HLRCC) or sporadic papillary renal cell cancer. J. Clin. Oncol. 2020, 38, 5004. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Walter, B.; Gil, S.; Naizhen, X.; Kruhlak, M.J.; Linehan, W.M.; Srinivasan, R.; Merino, M.J. Determination of the Expression of PD-L1 in the Morphologic Spectrum of Renal Cell Carcinoma. J. Cancer 2020, 11, 3596–3603. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Yang, Y.; Han, P.; Wei, X. The preliminary outcome of the combination of immunotherapy and targeted therapy after recurrence and metastasis for hereditary leiomyomatosis and renal cell cancer-a case report. Transl. Androl. Urol. 2020, 9, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Yonese, I.; Ito, M.; Takemura, K.; Kamai, T.; Koga, F. A Case of Metastatic Hereditary Leiomyomatosis and Renal Cell Cancer Syndrome-Associated Renal Cell Carcinoma Treated with a Sequence of Axitinib and Nivolumab Following Cytoreductive Nephrectomy. J. Kidney Cancer VHL 2020, 7, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Oomen, L.; Leijte, E.; Shilhan, D.E.; Battye, M. Members of ERN eUROGEN, Feitz WFJ. Rare and Complex Urology: Clinical Overview of ERN eUROGEN. Eur. Urol. 2021, 1–9. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurruchaga Sotés, I.; Alves, A.N.; Arregui, S.V.; Santander Lobera, C. Response to Combination of Pembrolizumab and Axitinib in Hereditary Leyomiomatosis and Renal Cell Cancer (HLRCC). Curr. Oncol. 2021, 28, 2346-2350. https://doi.org/10.3390/curroncol28040216
Gurruchaga Sotés I, Alves AN, Arregui SV, Santander Lobera C. Response to Combination of Pembrolizumab and Axitinib in Hereditary Leyomiomatosis and Renal Cell Cancer (HLRCC). Current Oncology. 2021; 28(4):2346-2350. https://doi.org/10.3390/curroncol28040216
Chicago/Turabian StyleGurruchaga Sotés, Ibon, Ana Nuño Alves, Sandra Vicente Arregui, and Carmen Santander Lobera. 2021. "Response to Combination of Pembrolizumab and Axitinib in Hereditary Leyomiomatosis and Renal Cell Cancer (HLRCC)" Current Oncology 28, no. 4: 2346-2350. https://doi.org/10.3390/curroncol28040216
APA StyleGurruchaga Sotés, I., Alves, A. N., Arregui, S. V., & Santander Lobera, C. (2021). Response to Combination of Pembrolizumab and Axitinib in Hereditary Leyomiomatosis and Renal Cell Cancer (HLRCC). Current Oncology, 28(4), 2346-2350. https://doi.org/10.3390/curroncol28040216




