Has the Practice of Radiation Oncology for Locally Advanced and Metastatic Non-Small-Cell Lung Cancer Changed in Canada?
Abstract
:1. INTRODUCTION
2. MATERIALS AND METHODS
3. RESULTS
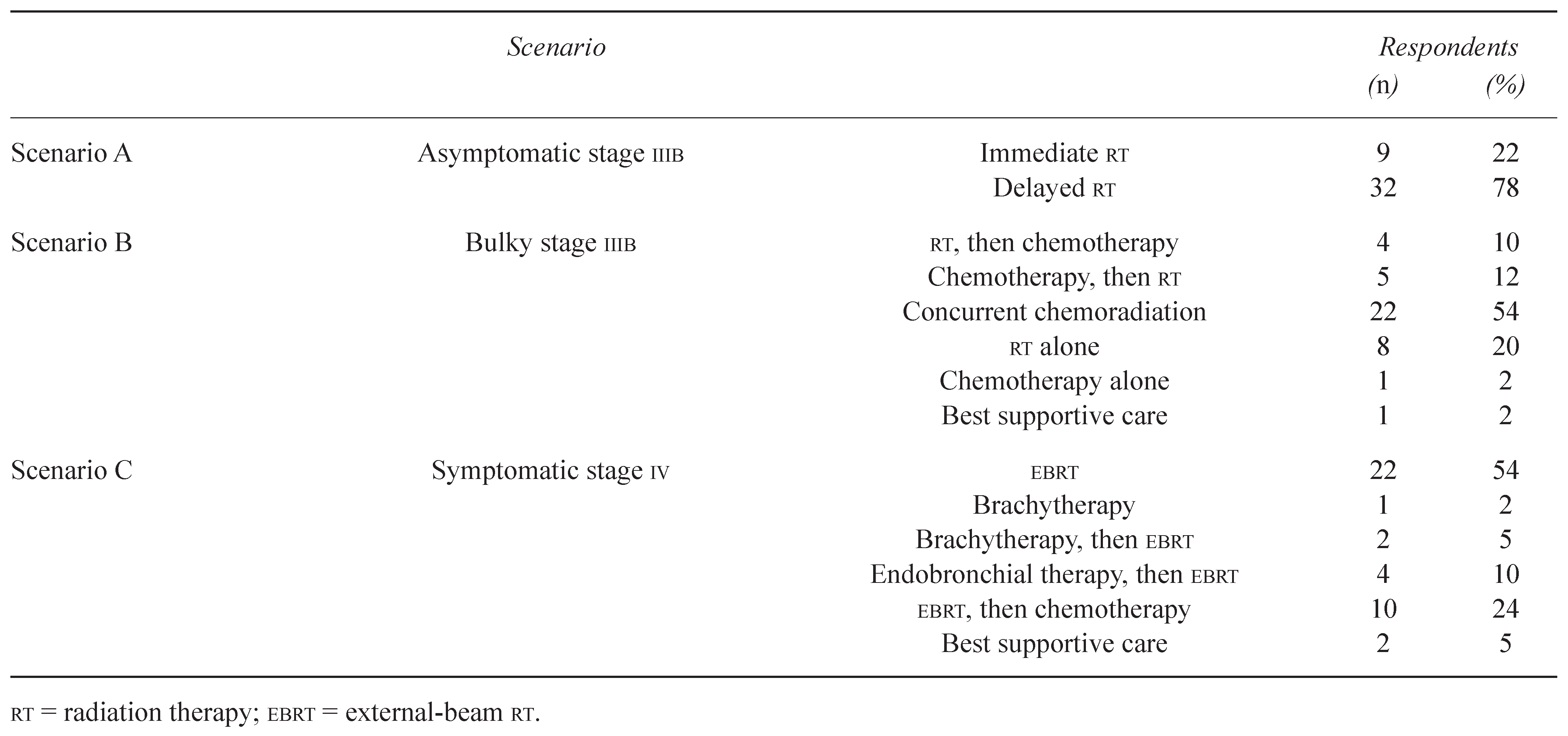
3.1. Overview
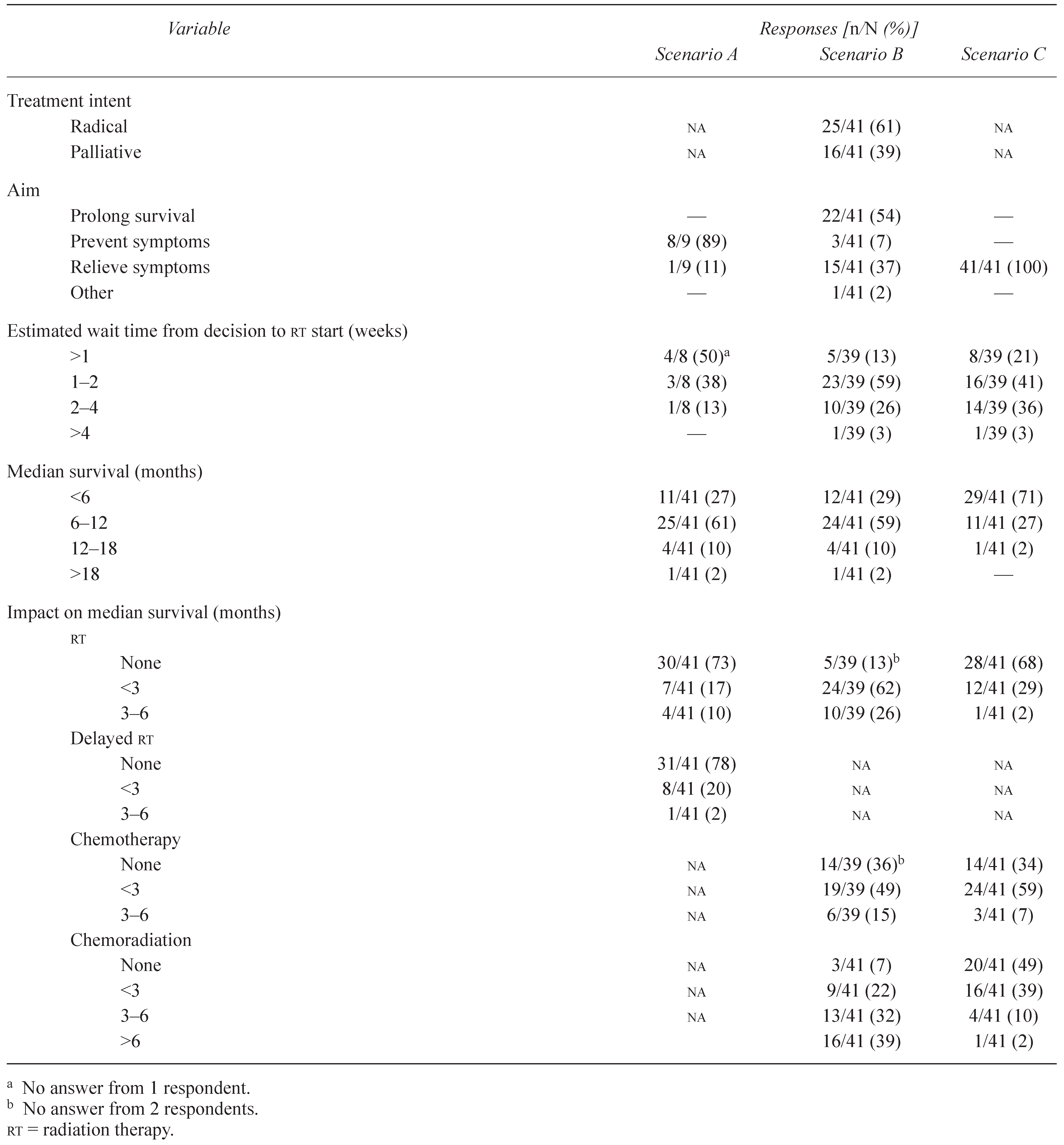
3.2.1. Radiotherapy Plan
3.2.2. Prognosis and Effect of Treatment
3.3. Scenario B: Patient with Bulky, Unresectable Stage IIIB NSCLC and Good Performance Status
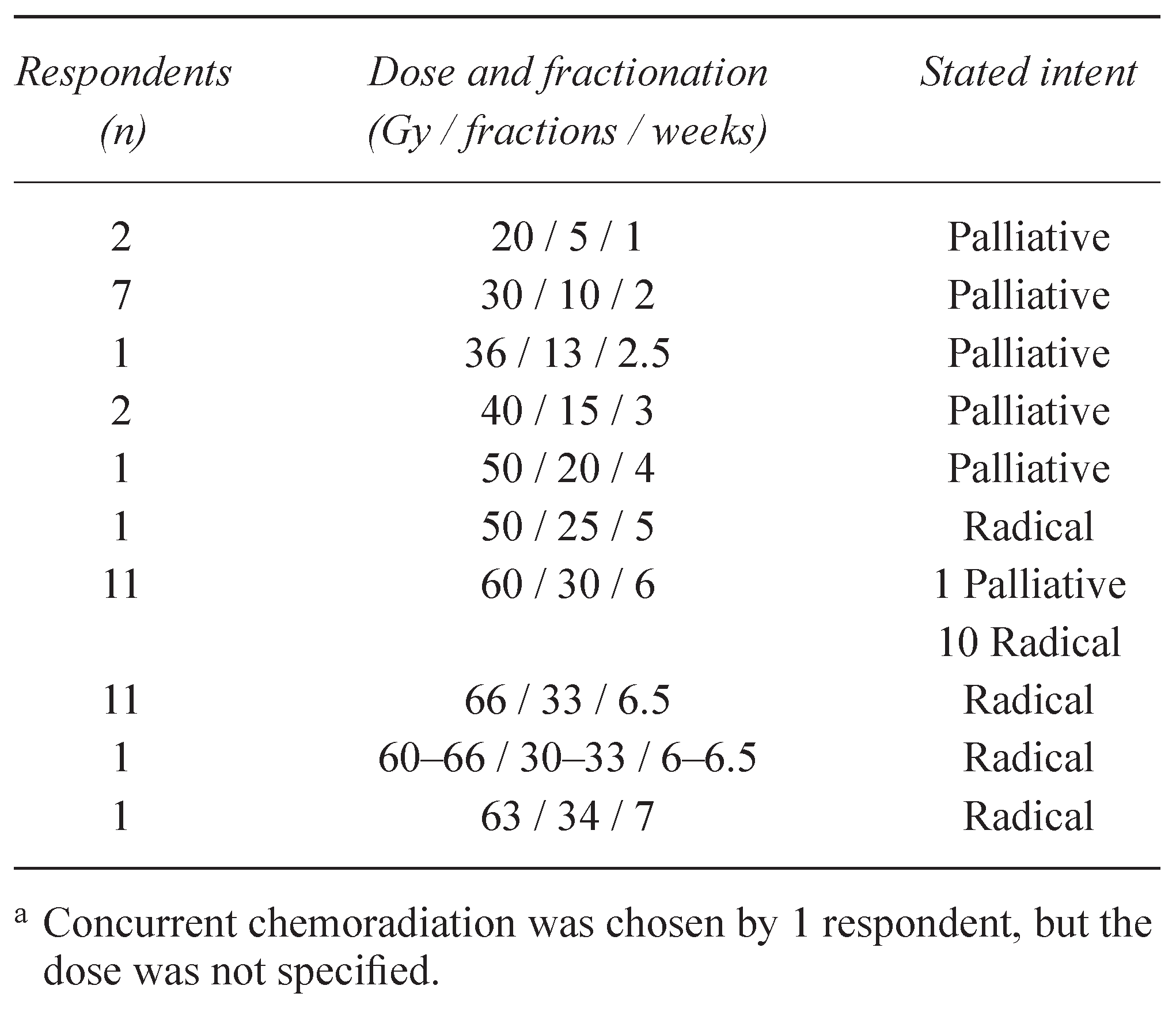
3.3.1. Radiotherapy Plan
3.3.2. Prognosis and Effect of Treatment
3.4. Scenario C: Symptomatic Stage IV Patient with Poor Performance Status
3.4.1. Radiotherapy Plan
3.4.2. Prognosis and Effect of Treatment
3.5. Correlation with Demographic Factors
4. DISCUSSION
5. ACKNOWLEDGMENTS
6. CONFLICT OF INTEREST
References
- Parkin, D.M. Global cancer statistics in the year 2000. Lancet Oncol 2001, 2, 533–543. [Google Scholar]
- Duncan, G.; Duncan, W.; Maher, E.J. Patterns of palliative radiotherapy in Canada. Clin Oncol (R Coll Radiol) 1993, 5, 92–97. [Google Scholar]
- Raby, B.; Pater, J.; Mackillop, W.J. Does knowledge guide practice? Another look at the management of non-small-cell lung cancer. J Clin Oncol 1995, 13, 1904–1911. [Google Scholar]
- Macbeth, F.R.; Bolger, J.J.; Hopwood, P.; et al. on behalf of the Medical Research Council Lung Cancer Working Party. Randomized trial of palliative two-fraction versus more intensive 13-fraction radiotherapy for patients with inoperable non-small cell lung cancer and good performance status. Clin Oncol (R Coll Radiol) 1996, 8, 167–175. [Google Scholar]
- Rees, G.J.; Devrell, C.E.; Barley, V.L.; Newman, H.F. Palliative radiotherapy for lung cancer: two versus five fractions. Clin Oncol (R Coll Radiol) 1997, 9, 90–95. [Google Scholar]
- Reinfuss, M.; Glinski, B.; Kowalska, T.; et al. Radiotherapy for stage III, inoperable, asymptomatic small cell lung cancer. Final results of a prospective randomized study (240 patients) [French]. Cancer Radiother 1999, 3, 475–479. [Google Scholar]
- Nestle, U.; Nieder, C.; Walter, K.; et al. A palliative accelerated irradiation regimen for advanced non-small-cell lung cancer vs. conventionally fractionated 60 Gy: results of a randomized equivalence study. Int J Radiat Oncol Biol Phys 2000, 48, 95–103. [Google Scholar]
- Bezjak, A.; Dixon, P.; Brundage, M.; et al. Randomized phase in trial of single versus fractionated thoracic radiation in the palliation of patients with lung cancer (NGC CTG SC 15). Int J Radiat Oncol Biol Phys 2002, 54, 719–728. [Google Scholar]
- Sundstrom, S.; Brennes, R.; Assebo, U.; et al. Hypofractionated palliative radiotherapy (17 Gy per two fractions) in advanced non-small-cell lung carcinoma is comparable to standard fractionation for symptom control and survival: a national phase in trial. J Clin Oncol 2004, 22, 801–810. [Google Scholar]
- Erridge, S.C.; Gaze, M.N.; Price, A.; et al. Symptom control and quality of life in people with lung cancer: a randomised trial of two palliative radiotherapy fractionation schedules. Clin Oncol (R Coll Radiol) 2005, 17, 61–67. [Google Scholar]
- Senkus-Konefka, E.; Dziadziuszko, R.; Bednaruk-Mlyński, E.; et al. A prospective, randomised study to compare two palliative radiotherapy schedules for non-small-cell lung cancer (NSCLC). Br J Cancer 2005, 92, 1038–1045. [Google Scholar]
- Kramer, G.W.; Wanders, S.L.; Noordijk, E.M.; et al. Results of the Dutch National study of the palliative effect of irradiation using two different treatment schemes for non-small-cell lung cancer. J Clin Oncol 2005, 23, 2962–2970. [Google Scholar]
- Tai, P.; Yu, E.; Battista, J.; Van Dyk, J. Radiation treatment of lung cancer—patterns of practice in Canada. Radiother Oncol 2004, 71, 167–174. [Google Scholar]
- Lester, J.F.; Macbeth, F.R.; Toy, E.; Coles, B. Palliative radiotherapy regimens for non-small cell lung cancer. Cochrane Database Syst Rev 2006, 4, CD002143. [Google Scholar]
- Choy, H.; Shyr, Y.; Cmelak, A.J.; Mohr, P.J.; Johnson, D.H. Patterns of practice survey for nonsmall cell lung carcinoma in the U.S. Cancer 2000, 88, 1336–1346. [Google Scholar]
- Cmelak, A.J.; Choy, H.; Shyr, Y.; Mohr, P.; Glantz, M.J.; Johnson, D.H. National survey on prophylactic cranial irradiation: differences in practice patterns between medical and radiation oncologists. Int J Radiat Oncol Biol Phys 1999, 44, 157–162. [Google Scholar]
- Falk, S.J.; Girling, D.J.; White, R.J.; et al. on behalf of the Medical Research Council Lung Cancer Working Party. Immediate versus delayed palliative thoracic radiotherapy in patients with unresectable locally advanced non-small cell lung cancer and minimal thoracic symptoms: randomised controlled trial. BMJ 2002, 325, 465. [Google Scholar]
- Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995, 311, 899–909. [Google Scholar]
- Pritchard, R.S.; Anthony, S.P. Chemotherapy plus radiotherapy compared with radiotherapy alone in the treatment of locally advanced, unresectable, non-small-cell lung cancer. A meta-analysis. Ann Intern Med 1996, 125, 723–729. [Google Scholar]
- Rowell, N.P.; O’rourke, N.P. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev 2004, 4, CD002140. [Google Scholar]
- Gridelli, C.; Ardizzoni, A.; Le Chevalier, T.; et al. Treatment of advanced non-small-cell lung cancer patients with ECO performance status 2: results of an European Experts Panel. Ann Oncol 2004, 15, 419–426. [Google Scholar]
- Pfister, D.G.; Johnson, D.H.; Azzoli, C.G.; et al. on behalf of the American Society of Clinical Oncology. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol 2004, 22, 330–353. [Google Scholar]
- Langer, C.J. Neglected and underrepresented subpopulations: elderly and performance status 2 patients with advanced-stage non-small-cell lung cancer. Clin Lung Cancer 2006, 7 (Suppl. S4), S126–S137. [Google Scholar]
- Ung, V.C.; Yu, E.; Falkson, C.; Haynes, A.E.; Stys-Norman, D.; Evans, W.K. on behalf of the Lung Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-Based Care. The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small-cell lung cancer: a systematic review. Brachytherapy 2006, 5, 189–202. [Google Scholar]
- Cardona, A.F.; Revelz, L.; Ospina, E.G.; Ospina, V.; Yepes, A. Palliative endobronchial brachytherapy for non-small cell lung cancer. Cochrane Database Syst Rev 2008, 2, CD004284. [Google Scholar]
- Maziak, D.E.; Markman, B.R.; Mackay, J.A.; Evans, W.K. on behalf of the Lung Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-Based Care. The Role of Photodynamic Therapy (PDT) in Patients with Non-Small Cell Lung Cancer: A Clinical Practice Guideline. Toronto, ON: Cancer Care Ontario; 2005. [Available online at: www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=14190; cited November 1, 2008].
- Graham, I.D.; Brouwers, M.; Davies, C.; Tetroc, J. Ontario doctors’ attitudes toward and use of clinical practice guidelines in oncology. J Eval Clin Pract 2007, 13, 607–615. [Google Scholar]


© 2010 by the author. Multimed Inc.
Share and Cite
Han, K.; Bezjak, A.; Xu, W.; Kane, G. Has the Practice of Radiation Oncology for Locally Advanced and Metastatic Non-Small-Cell Lung Cancer Changed in Canada? Curr. Oncol. 2010, 17, 33-40. https://doi.org/10.3747/co.v17i1.387
Han K, Bezjak A, Xu W, Kane G. Has the Practice of Radiation Oncology for Locally Advanced and Metastatic Non-Small-Cell Lung Cancer Changed in Canada? Current Oncology. 2010; 17(1):33-40. https://doi.org/10.3747/co.v17i1.387
Chicago/Turabian StyleHan, K., A. Bezjak, W. Xu, and G. Kane. 2010. "Has the Practice of Radiation Oncology for Locally Advanced and Metastatic Non-Small-Cell Lung Cancer Changed in Canada?" Current Oncology 17, no. 1: 33-40. https://doi.org/10.3747/co.v17i1.387
APA StyleHan, K., Bezjak, A., Xu, W., & Kane, G. (2010). Has the Practice of Radiation Oncology for Locally Advanced and Metastatic Non-Small-Cell Lung Cancer Changed in Canada? Current Oncology, 17(1), 33-40. https://doi.org/10.3747/co.v17i1.387




