A Primer of Bone Metastases Management in Breast Cancer Patients
Abstract
:1. Introduction
2. Bisphosphonates
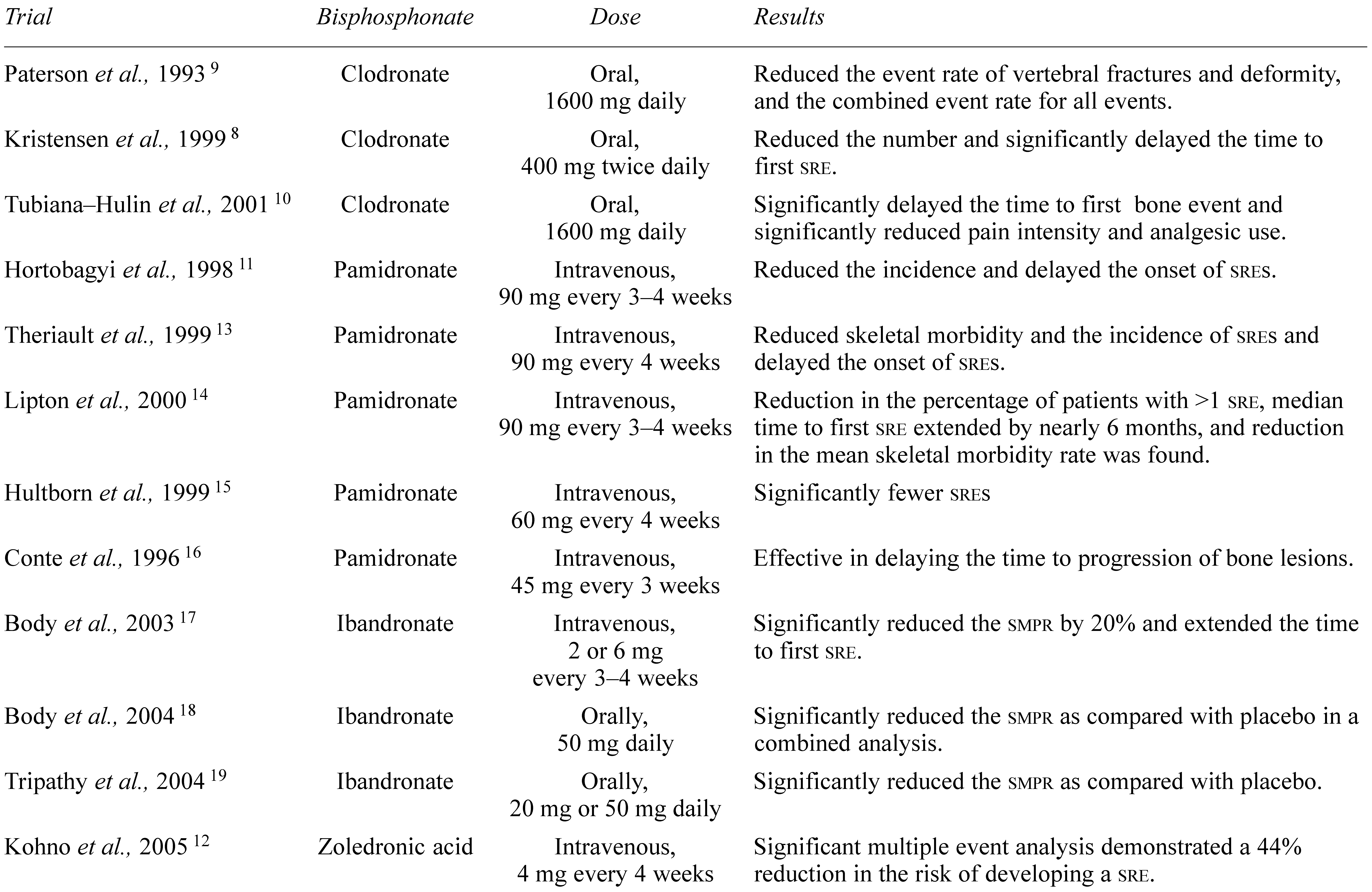
2.1. Bisphosphonate Trials and Meta-Analyses
2.2. Uncertainties About Bisphosphonate Use in Clinical Practice
2.3. Which Patients Benefit Most from Bisphosphonate Use?
2.4. When Should Bisphosphonates Be Started in Patients with Newly Diagnosed Bone Metastases?
3. Choosing a Bisphosphonate
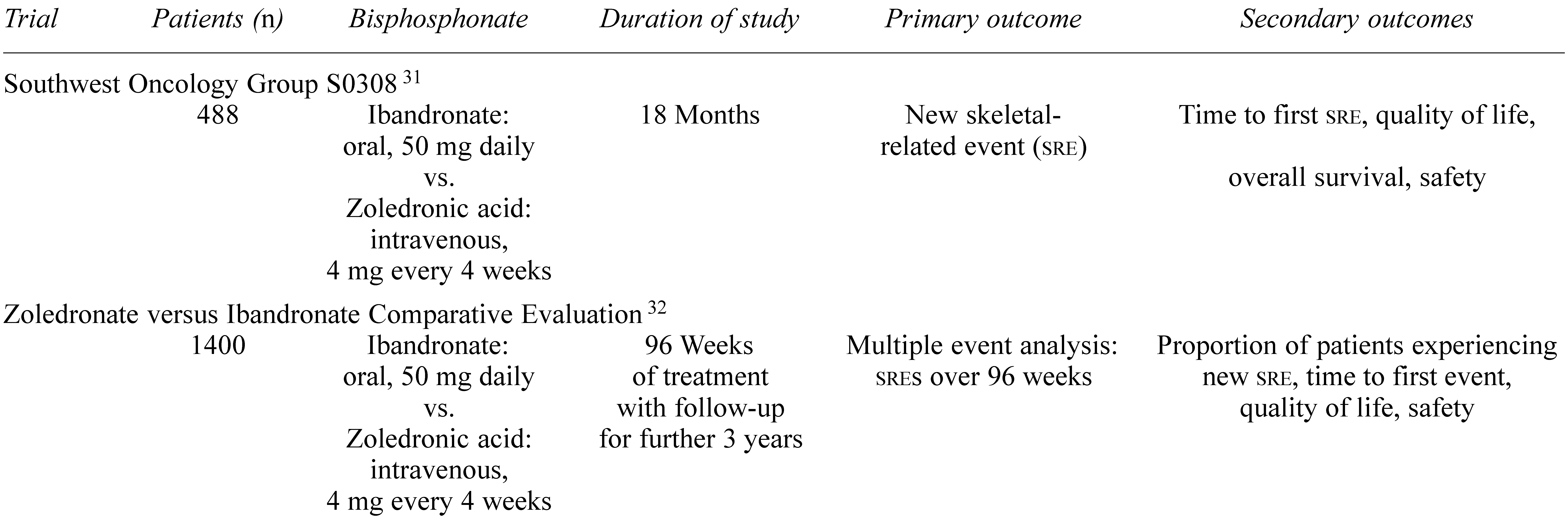
4. Optimal Duration of Bisphosphonate Treatment
5. Markers of Bone Resorption
- occurrence of SREs in breast cancer patients with bone metastases,
- response to bisphosphonates,
- pain scores, and
- patient outcomes.
6. New Agents Targeting the Mechanism of Bone Metastases
- receptor activator of nuclear factor κB (RANK),
- RANK ligand (RANKL), and
- osteoprotegerin (OPG) [49].
7. Summary
References
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Rubens, R.D. The clinical course of bone metastases from breast cancer. Br J Cancer 1987, 55, 61–66. [Google Scholar] [CrossRef]
- Warr, D.; Johnston, M. Use of bisphosphonates in women with breast cancer. Practice guideline report no. 1-11. Hamilton, ON: Cancer Care Ontario, Program in Evidence-Based Care; 2004. [Available online at: www.cancercare.on.ca/pdf/pebc1-11f.pdf; cited December 10, 2007].
- Hillner, B.E.; Ingle, J.N.; Chlebowski, R.T.; et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol 2003, 21, 4042–4057. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, C.R.; Felsenberg, D.; Seibel, M.J. Therapy insight: The risks and benefits of bisphosphonates for the treatment of tumor-induced bone disease. Nat Clin Pract Oncol 2007, 4, 42–55. [Google Scholar] [CrossRef]
- Coleman, R.E. Bisphosphonates in breast cancer. Ann Oncol 2005, 16, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Body, J.J.; Lichinitser, M.; Tjulandin, S.; Garnero, P.; Bergström, B. Oral ibandronate is as active as intravenous zoledronic acid for reducing bone turnover markers in women with breast cancer and bone metastases. Ann Oncol 2007, 18, 1165–1171. [Google Scholar] [CrossRef]
- Kristensen, B.; Ejlertsen, B.; Groenvold, M.; Hein, S.; Loft, H.; Mouridsen, H.T. Oral clodronate in breast cancer patients with bone metastases: A randomized study. J Intern Med 1999, 246, 67–74. [Google Scholar] [CrossRef]
- Paterson, A.H.; Powles, T.J.; Kanis, J.A.; McCloskey, E.; Hanson, J.; Ashley, S. Double-blind controlled trial of oral clodronate in patients with bone metastases from breast cancer. J Clin Oncol 1993, 11, 59–65. [Google Scholar] [CrossRef]
- Tubiana-Hulin, M.; Beuzeboc, P.; Mauriac, L.; et al. Doubleblinded controlled study comparing clodronate versus placebo in patients with breast cancer bone metastases [French]. Bull Cancer 2001, 88, 701–707. [Google Scholar]
- Hortobagyi, G.N.; Theriault, R.L.; Lipton, A.; et al. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocol 19 Aredia Breast Cancer Study Group. J Clin Oncol 1998, 16, 2038–2044. [Google Scholar] [CrossRef]
- Kohno, N.; Aogi, K.; Minami, H.; et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: A randomized, placebo-controlled trial. J Clin Oncol 2005, 23, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- Theriault, R.L.; Lipton, A.; Hortobagyi, G.N.; et al. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: A randomized, placebo-controlled trial. Protocol 18 Aredia Breast Cancer Study Group. J Clin Oncol 1999, 17, 846–854. [Google Scholar] [CrossRef]
- Lipton, A.; Theriault, R.L.; Hortobagyi, G.N.; et al. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: Long term follow-up of two randomized, placebocontrolled trials. Cancer 2000, 88, 1082–1090. [Google Scholar] [CrossRef]
- Hultborn, R.; Gundersen, S.; Ryden, S.; et al. Efficacy of pamidronate in breast cancer with bone metastases: A randomized, double-blind placebo-controlled multicenter study. Anticancer Res 1999, 19, 3383–3392. [Google Scholar] [CrossRef]
- Conte, P.F.; Latreille, J.; Mauriac, L.; et al. Delay in progression of bone metastases in breast cancer patients treated with intravenous pamidronate: Results from a multinational randomized controlled trial. The Aredia Multinational Cooperative Group. J Clin Oncol 1996, 14, 2552–2559. [Google Scholar] [CrossRef]
- Body, J.J.; Diel, I.J.; Lichinitser, M.R.; et al. Intravenous ibandronate reduces the incidence of skeletal complications in patients with breast cancer and bone metastases. Ann Oncol 2003, 14, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Body, J.J.; Diel, I.J.; Lichinitzer, M.; et al. Oral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: Results from two randomised, placebo-controlled phase III studies. Br J Cancer 2004, 90, 1133–1137. [Google Scholar] [CrossRef]
- Tripathy, D.; Lichinitzer, M.; Lazarev, A.; et al. on behalf of the MF 4434 Study Group. Oral ibandronate for the treatment of metastatic bone disease in breast cancer: Efficacy and safety results from a randomized, double-blind, placebo-controlled trial. Ann Oncol 2004, 15, 743–750. [Google Scholar] [CrossRef]
- Ross, J.R.; Saunders, Y.; Edmonds, P.M.; Patel, S.; Broadley, K.E.; Johnston, S.R. Systematic review of role of bisphosphonates on skeletal morbidity in metastatic cancer. BMJ 2003, 327, 469. [Google Scholar] [CrossRef]
- Pavlakis, N.; Schmidt, R.; Stockler, M. Bisphosphonates for breast cancer. Cochrane Database Syst Rev 2005;:CD003474. [CrossRef]
- Body, J.J. Bisphosphonates for malignancy-related bone disease: Current status, future developments. Support Care Cancer 2006, 14, 408–418. [Google Scholar] [CrossRef]
- Gralow, J.; Tripathy, D. Managing metastatic bone pain: The role of bisphosphonates. J Pain Symptom Manage 2007, 33, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Jagdev, S.P.; Purohit, P.; Heatley, S.; Herling, C.; Coleman, R.E. Comparison of the effects of intravenous pamidronate and oral clodronate on symptoms and bone resorption in patients with metastatic bone disease. Ann Oncol 2001, 12, 1433–1438. [Google Scholar] [CrossRef]
- Wong, R.; Wiffen, P.J. Bisphosphonates for the relief of pain secondary to bone metastases. Cochrane Database Syst Rev 2002;CD002068. [CrossRef]
- Mancini, I.; Dumon, J.C.; Body, J.J. Efficacy and safety of ibandronate in the treatment of opioid-resistant bone pain associated with metastatic bone disease: A pilot study. J Clin Oncol 2004, 22, 3587–3592. [Google Scholar] [CrossRef] [PubMed]
- Aapro, M.; Abrahamsson, P.A.; Body, J.J.; et al. Guidance on the use of bisphosphonates in solid tumours: Recommendations of an international expert panel. Ann Oncol 2008;[in press]. [2007;(Epub ahead of print)]. [CrossRef]
- Rosen, L.S.; Gordon, D.; Kaminski, M.; et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: A phase III, double-blind, comparative trial. Cancer J 2001, 7, 377–387. [Google Scholar]
- Rosen, L.S.; Gordon, D.; Kaminski, M.; et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: A randomized, double-blind, multicenter, comparative trial. Cancer 2003, 98, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Clemons, M.; Dranitsaris, G.; Ooi, W.; Cole, D.E. A phase II trial evaluating the palliative benefit of second-line oral ibandronate in breast cancer patients with either a skeletal related event (SRE) or progressive bone metastases (BM) despite standard bisphosphonate (BP) therapy. Breast Cancer Res Treat 2008;[in press]. [2007;(Epub ahead of print)]. [CrossRef]
- Rivkin, S. Oral ibandronate versus intravenous zoledronic acid for breast cancer patients with skeletal complications: The SWOG 0308 trial [abstract]. Bone 2006, 38 (suppl 1), S82. [Google Scholar] [CrossRef]
- Barrett–Lee, P.; Murray, N. Zoledronic acid versus ibandronate comparative evaluation in breast cancer patients with bone metastases: The NCRI ZICE trial [abstract]. Bone 2006, 38 (suppl 1), S57. [Google Scholar] [CrossRef]
- Clemons, M.; Dranitsaris, G.; Cole, D.; Gainford, M.C. Too much, too little, too late to start again? Assessing the efficacy of bisphosphonates in patients with bone metastases from breast cancer. Oncologist 2006, 11, 227–233. [Google Scholar] [CrossRef]
- Plunkett, T.A.; Smith, P.; Rubens, R.D. Risk of complications from bone metastases in breast cancer. implications for management. Eur J Cancer 2000, 36, 476–482. [Google Scholar] [CrossRef]
- Clemons, M.; Cole, D.E.; Gainford, M.C. Can bone markers guide more effective treatment of bone metastases from breast cancer? Breast Cancer Res Treat 2006, 97, 81–90. [Google Scholar] [CrossRef]
- Coleman, R.E. Bisphosphonates: Clinical experience. Oncologist 2004, 9 (suppl 4), 14–27. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Kerr–Cresswell, D.; Dranitsaris, G.; et al. Bisphosphonate use for the management of breast cancer patients with bone metastases: A survey of Canadian Medical Oncologists. Support Care Cancer 2004, 12, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Clemons, M.J.; Dranitsaris, G.; Ooi, W.S.; et al. Phase II trial evaluating the palliative benefit of second-line zoledronic acid in breast cancer patients with either a skeletal-related event or progressive bone metastases despite first-line bisphosphonate therapy. J Clin Oncol 2006, 24, 4895–4900. [Google Scholar] [CrossRef] [PubMed]
- Gainford, M.C.; Dranitsaris, G.; Clemons, M. Recent developments in bisphosphonates for patients with metastatic breast cancer. BMJ 2005, 330, 769–773. [Google Scholar] [CrossRef]
- Hillner, B.E.; Weeks, J.C.; Desch, C.E.; Smith, T.J. Pamidronate in prevention of bone complications in metastatic breast cancer: A cost-effectiveness analysis. J Clin Oncol 2000, 18, 72–79. [Google Scholar] [CrossRef]
- Bamias, A.; Kastritis, E.; Bamia, C.; et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: Incidence and risk factors. J Clin Oncol 2005, 23, 8580–8587. [Google Scholar] [CrossRef]
- Dranitsaris, G.; Hsu, T. Cost utility analysis of prophylactic pamidronate for the prevention of skeletal related events in patients with advanced breast cancer. Support Care Cancer 1999, 7, 271–279. [Google Scholar] [CrossRef]
- Botteman, M.; Barghout, V.; Stephens, J.; Hay, J.; Brandman, J.; Aapro, M. Cost effectiveness of bisphosphonates in the management of breast cancer patients with bone metastases. Ann Oncol 2006, 17, 1072–1082. [Google Scholar] [CrossRef]
- Brown, J.E.; Thomson, C.S.; Ellis, S.P.; Gutcher, S.A.; Purohit, O.P.; Coleman, R.E. Bone resorption predicts for skeletal complications in metastatic bone disease. Br J Cancer 2003, 89, 2031–2037. [Google Scholar] [CrossRef]
- Vinholes, J.J.; Purohit, O.P.; Abbey, M.E.; Eastell, R.; Coleman, R.E. Relationships between biochemical and symptomatic response in a double-blind randomised trial of pamidronate for metastatic bone disease. Ann Oncol 1997, 8, 1243–1250. [Google Scholar] [CrossRef]
- Coleman, R.E. The clinical use of bone resorption markers in patients with malignant bone disease. Cancer 2002, 94, 2521–2533. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Major, P.; Lipton, A.; et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol 2005, 23, 4925–4935. [Google Scholar] [CrossRef] [PubMed]
- Lipton, A.; Cook, R.; Coleman, R.E.; et al. Normalization of bone markers and improved survival during zoledronic acid therapy [abstract]. Proc Am Soc Clin Oncol 2007, 25, 9013. [Google Scholar] [CrossRef]
- Guise, T.A. Molecular mechanisms of osteolytic bone metastases. Cancer 2000, 88 (suppl), 2892–2898. [Google Scholar] [CrossRef]
- Lipton, A.; Alvarado, A.; De Boer, R.; et al. Randomized, activecontrolled study of denosumab (AMG 162) in breast cancer patients with bone metastases not previously treated with intravenous (IV) bisphosphonates (BP) [abstract]. Proc Am Soc Clin Oncol 2006, 24, 512. [Google Scholar] [CrossRef]
- Body, J.J.; Facon, T.; Coleman, R.E.; et al. A study of the biological receptor activator of nuclear factor-κB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res 2006, 12, 1221–1228. [Google Scholar] [CrossRef]
- Lipton, A. Future treatment of bone metastases. Clin Cancer Res 2006, 12, 6305s–6308s. [Google Scholar] [CrossRef]
 |
 |
 |
 |
 |
© 2008 by the author. Multimed Inc.
Share and Cite
Petrut, B.; Trinkaus, M.; Simmons, C.; Clemons, M. A Primer of Bone Metastases Management in Breast Cancer Patients. Curr. Oncol. 2008, 15, 50-57. https://doi.org/10.3747/co.v15i0.205
Petrut B, Trinkaus M, Simmons C, Clemons M. A Primer of Bone Metastases Management in Breast Cancer Patients. Current Oncology. 2008; 15(s1):50-57. https://doi.org/10.3747/co.v15i0.205
Chicago/Turabian StylePetrut, B., M. Trinkaus, C. Simmons, and M. Clemons. 2008. "A Primer of Bone Metastases Management in Breast Cancer Patients" Current Oncology 15, no. s1: 50-57. https://doi.org/10.3747/co.v15i0.205
APA StylePetrut, B., Trinkaus, M., Simmons, C., & Clemons, M. (2008). A Primer of Bone Metastases Management in Breast Cancer Patients. Current Oncology, 15(s1), 50-57. https://doi.org/10.3747/co.v15i0.205




