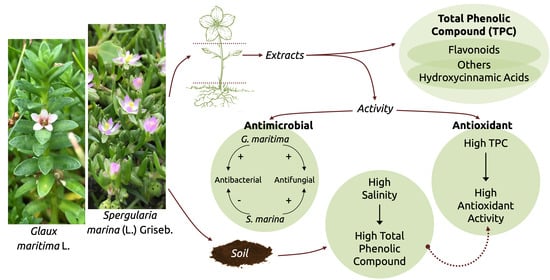
The Content of Certain Groups of Phenolic Compounds and the Biological Activity of Extracts of Various Halophyte Parts of Spergularia marina (L.) Griseb. and Glaux maritima L. at Different Levels of Soil Salinization
Abstract
:1. Introduction
2. Results
2.1. Soil Analysis
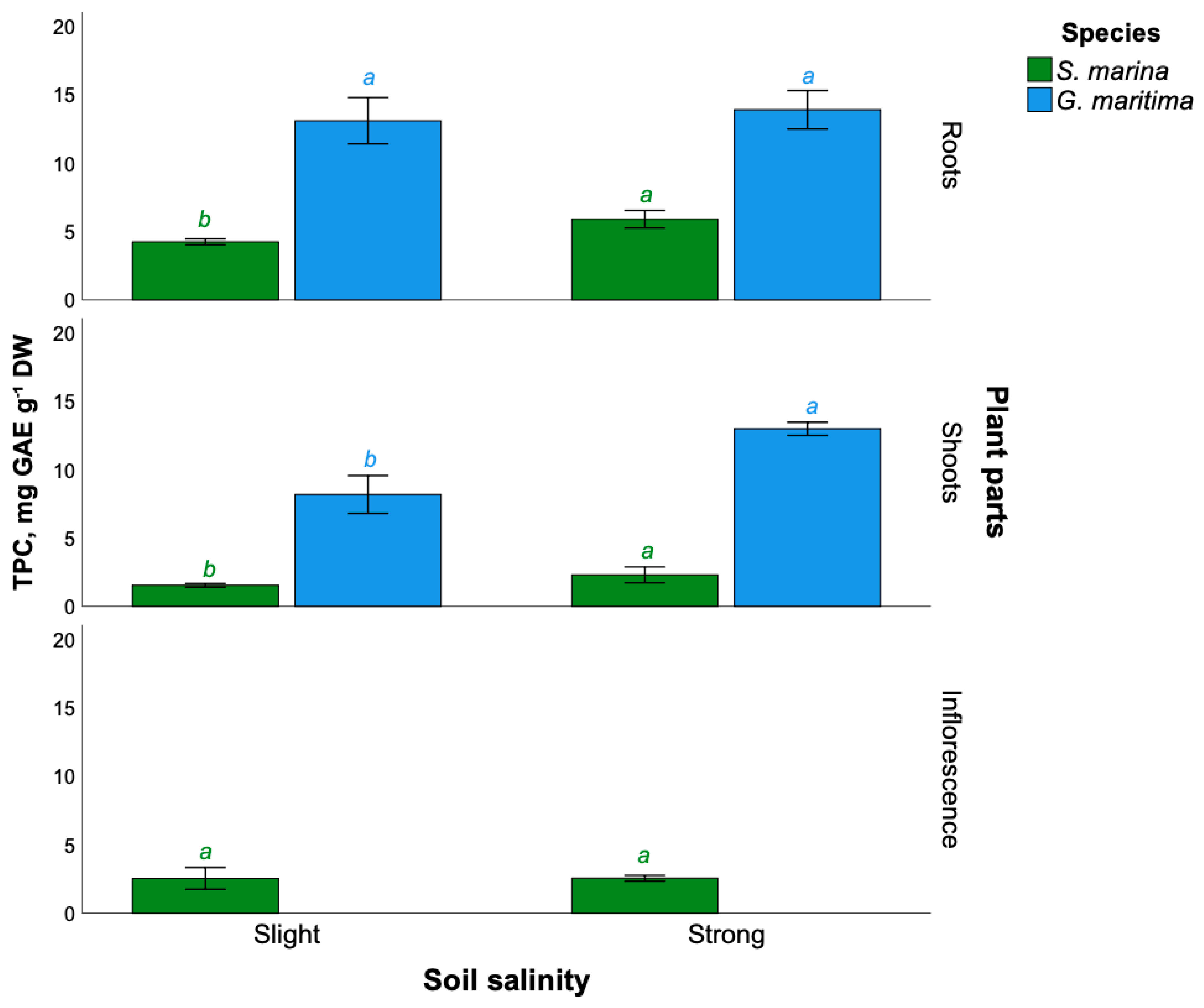
2.2. Total Phenolic Compounds Content
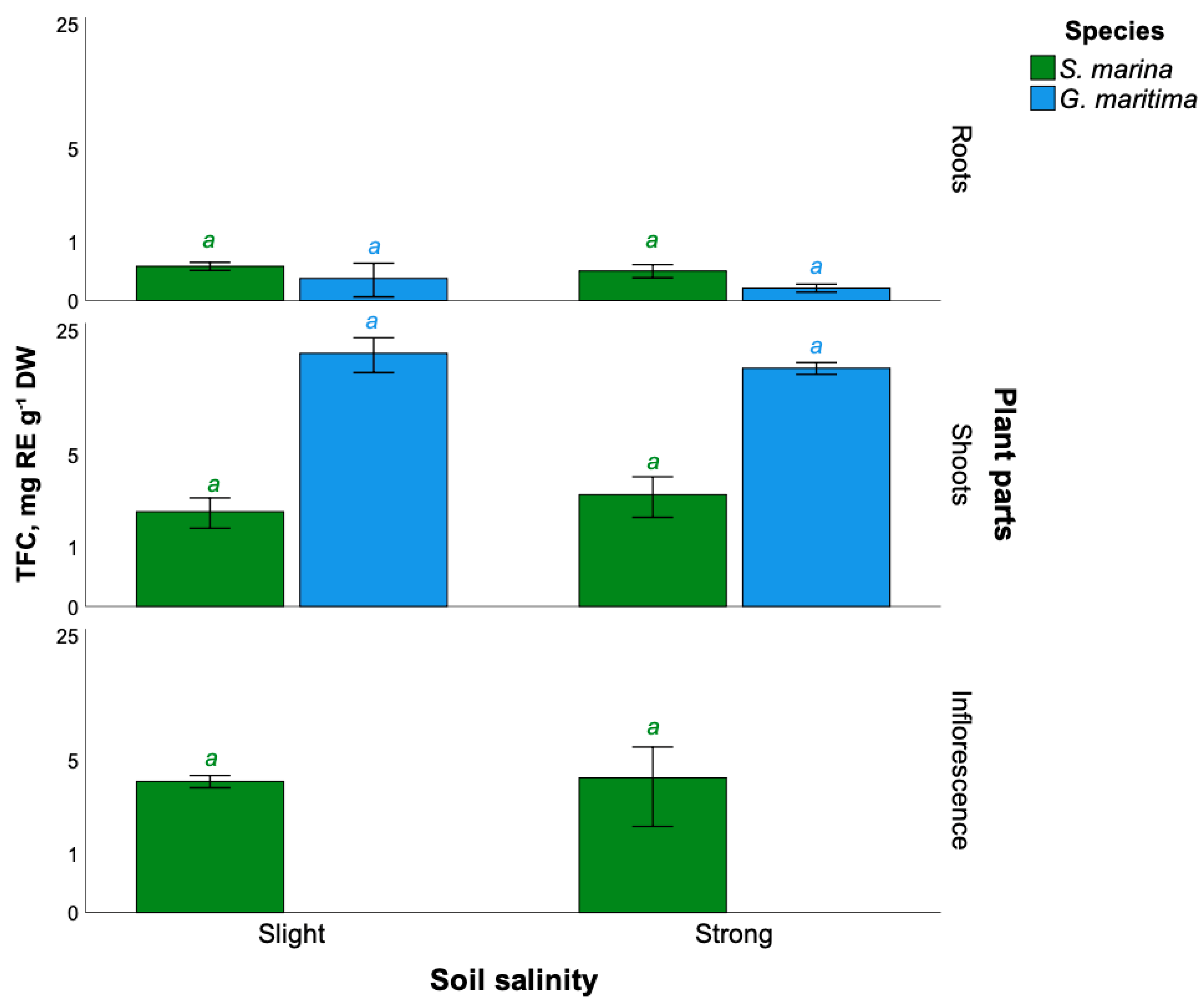
2.3. Total Flavonoids Content
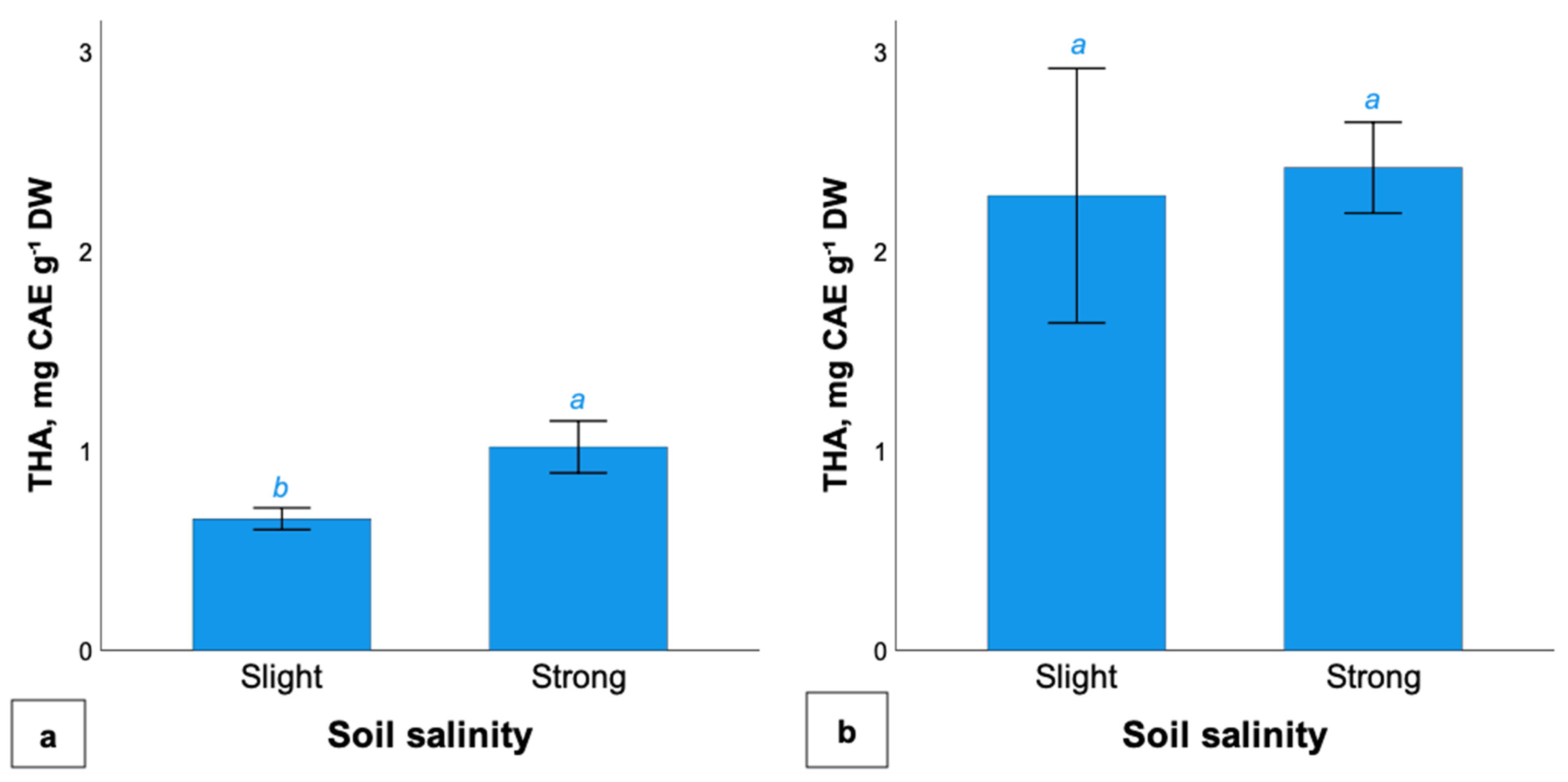
2.4. Total Hydroxycinnamic Acids Content
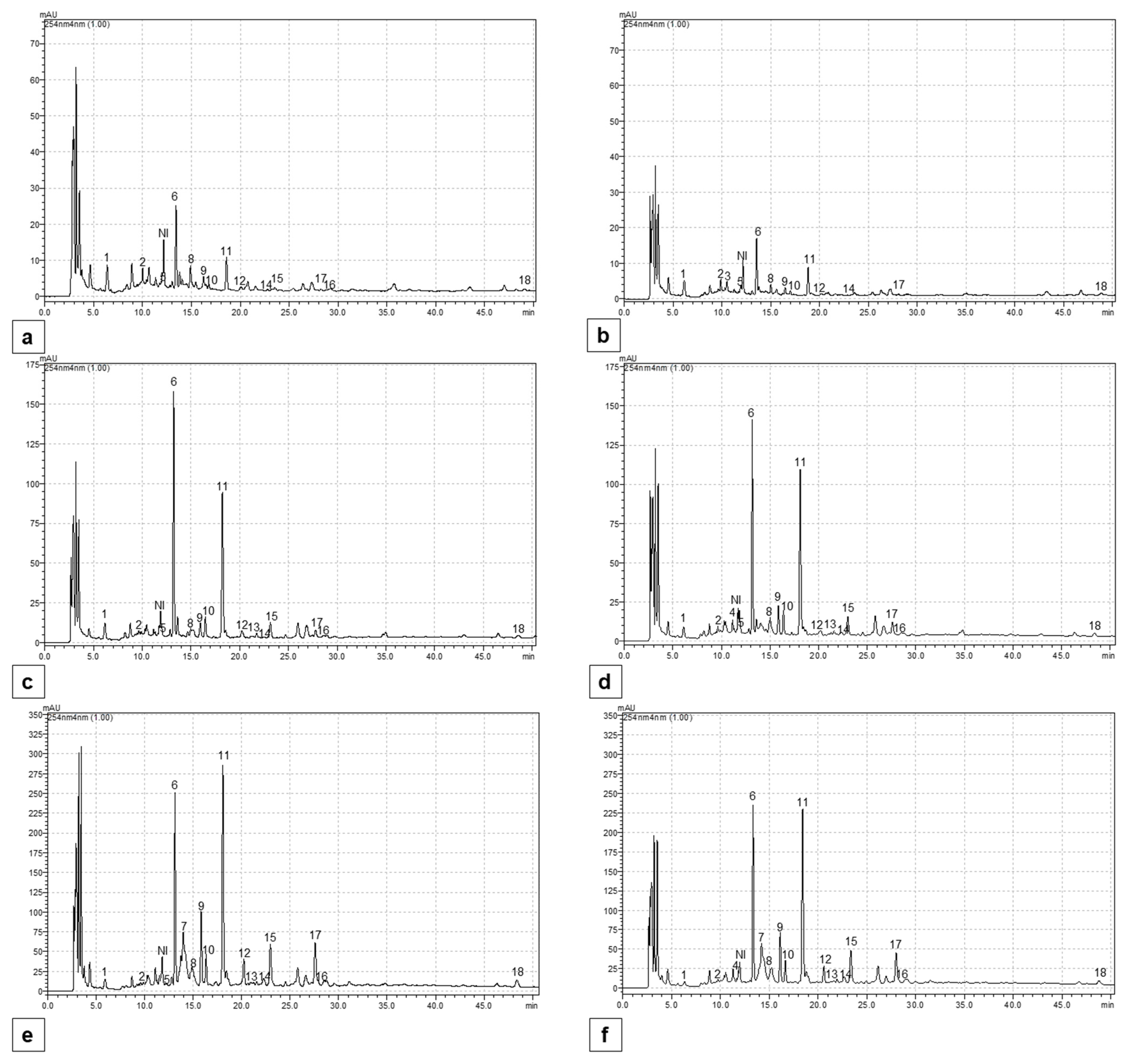
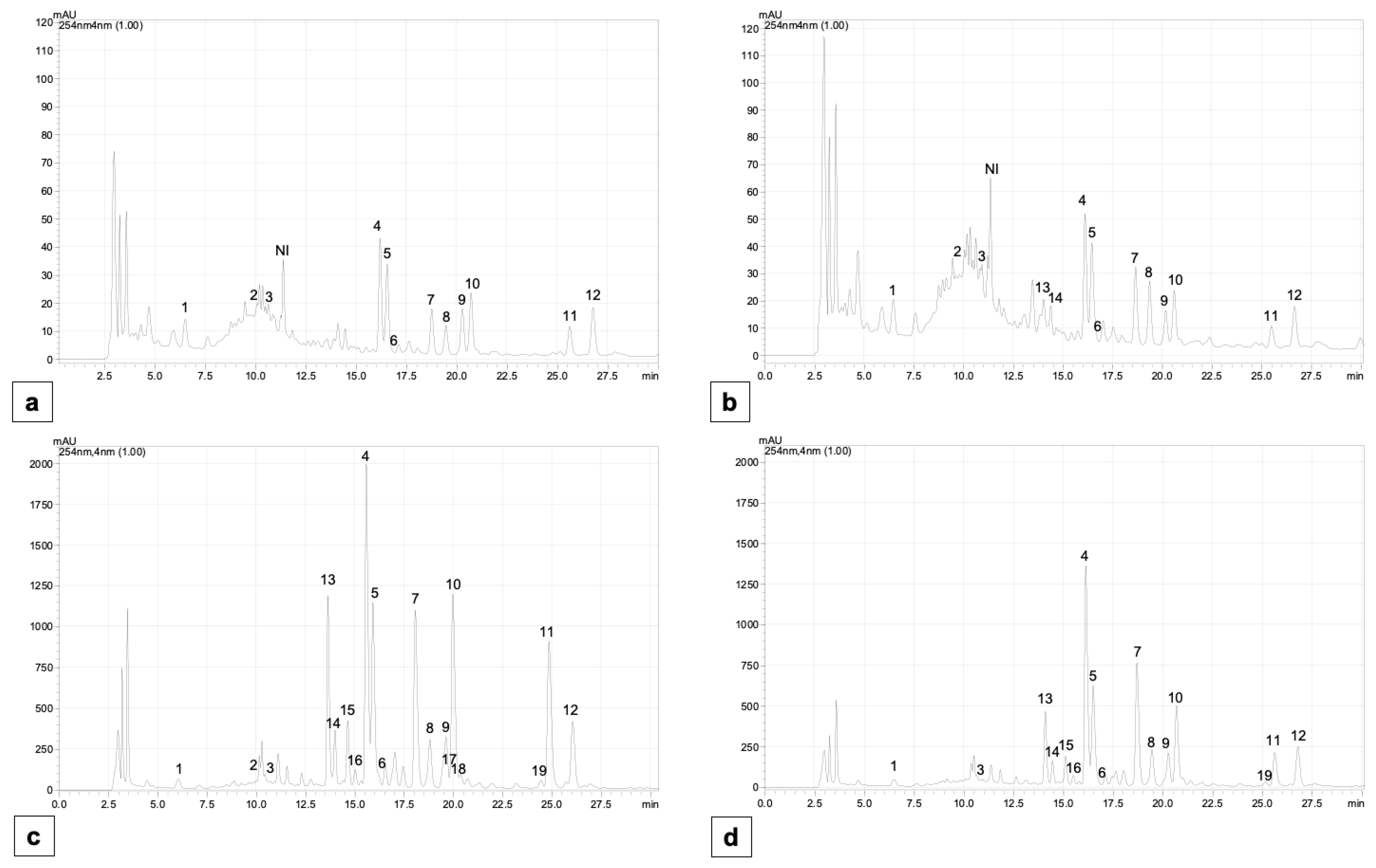
2.5. Content of Individual Phenolic Compounds
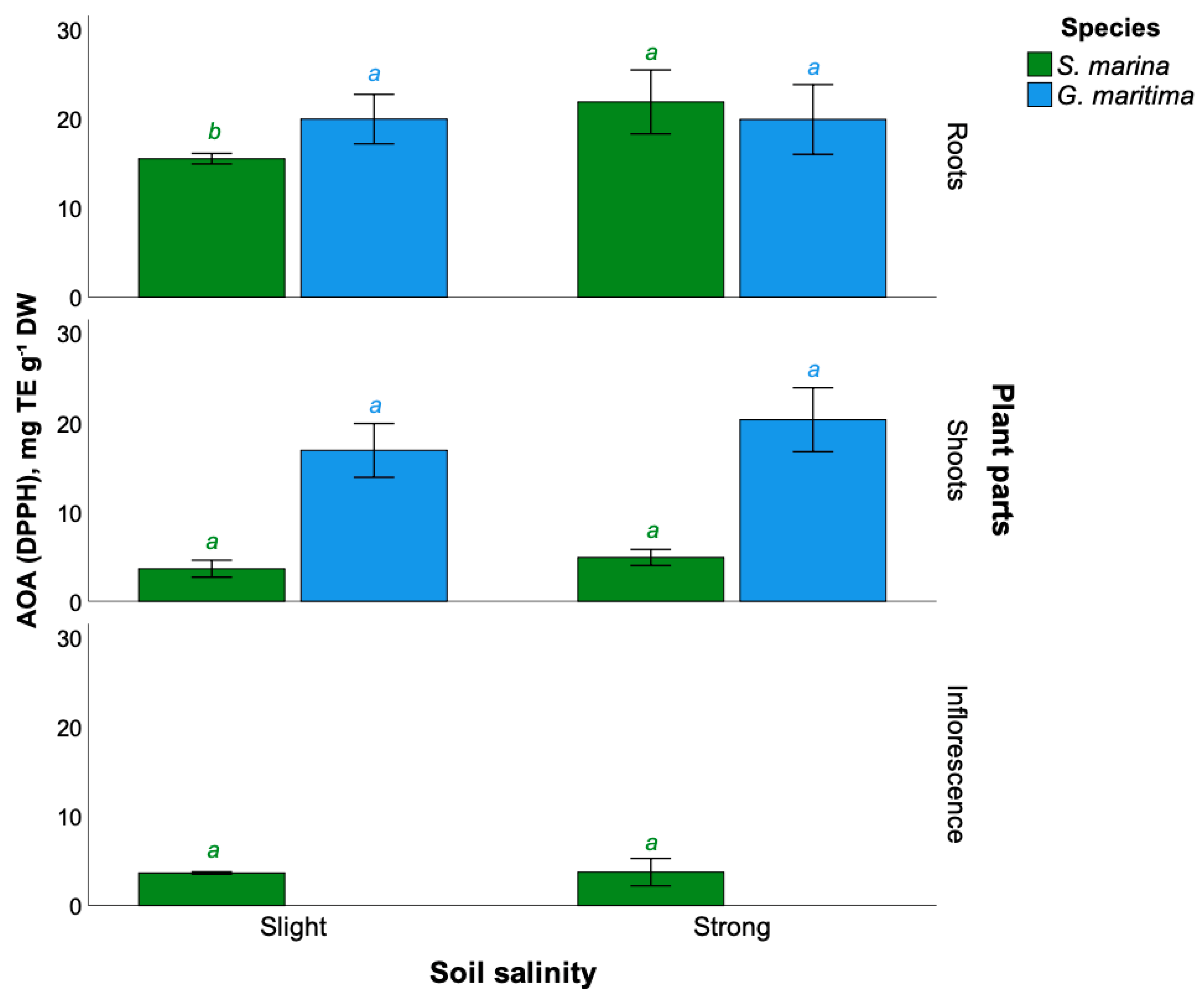
2.6. Antioxidant Activity
2.7. Correlation between Phenolic Compounds Content and Antioxidant Activity
2.8. Antibacterial and Antifungal Activity
3. Discussion
3.1. Variation in Phenolic Compounds Content, Antioxidant, Antimicrobial, and Fungicidal Activity
3.2. Effect of Soil Salinity on Phenolic Compounds Content
4. Materials and Methods
4.1. Study Area and Plant Material
4.2. Soil Analysis
4.3. Extract Preparation
4.4. The determination of Total Phenolic Content (TPC)
4.5. The Determination of Total Flavonoid Content (TFC)
4.6. The Determination of Total Content of Hydroxycinnamic Acids (THA)
4.7. The Determination of Individual Phenolic Compounds
4.8. The Determination of Antioxidant Activity (AOA)
4.9. Determination of Antimicrobial and Fungicidal Activity
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
| Method | Standard | Range, mg mL−1 | Equation | R2 |
|---|---|---|---|---|
| TPC | Gallic acid | 0.02–0.30 | y = 1.221x + 0.011 | 0.994 |
| TFC | Rutin | 0.025–0.500 | y = 1.879x + 0.004 | 0.999 |
| THA | Chlorogenic acid | 0.01–0.30 | y = 2.264x + 0.004 | 0.999 |
| AOA (DPPH) | Ascorbic acid | 0–0.34 | y = −0.624x + 0.700 | 0.999 |
References
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Azzini, E.; Zucca, P.; Varoni, E.M.; Kumar, N.V.A.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Peluso, I.; et al. Plant-Derived Bioactives and Oxidative Stress-Related Disorders: A Key Trend towards Healthy Aging and Longevity Promotion. Appl. Sci. 2020, 10, 947. [Google Scholar] [CrossRef] [Green Version]
- Zehiroglu, C.; Ozturk Sarikaya, S.B. The importance of antioxidants and place in today’s scientific and technological studies. J. Food Sci. Technol. 2019, 56, 4757–4774. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of Antioxidant Potential of Plants and its Relevance to Therapeutic Applications. Int. J. Biol. Sci. 2015, 11, 982. [Google Scholar] [CrossRef] [Green Version]
- Flowers, T.J.; Colmer, T.D. Salinity Tolerance in Halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Soundararajan, P.; Manivannan, A.; Jeong, B.R. Different Antioxidant Defense Systems in Halophytes and Glycophytes to Overcome Salinity Stress. In Sabkha Ecosystems; Springer: Berlin/Heidelberg, Germany, 2019; pp. 335–347. [Google Scholar] [CrossRef]
- Ozgur, R.; Uzilday, B.; Sekmen, A.H.; Turkan, I.; Ozgur, R.; Uzilday, B.; Sekmen, A.H.; Turkan, I. Reactive oxygen species regulation and antioxidant defence in halophytes. Funct. Plant Biol. 2013, 40, 832–847. [Google Scholar] [CrossRef]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Ksouri, R.; Smaoui, A.; Isoda, H.; Abdelly, C. Utilization of Halophyte Species as New Sources of Bioactive Substances. J. Arid L. Stud. 2012, 1, 41–44. [Google Scholar]
- Gubareva, I.Y. Spergularia salina J. at C. Presl. In The Red Data Book of the Kaliningrad Region; Dedkov, V.P., Grishanov, G.V., Eds.; Immanuel Kant University: Kaliningrad, Russia, 2010; p. 141. (In Russian) [Google Scholar]
- Ingeloeg, T.; Andersson, R.; Tjernberg, M. (Eds.) Red Data Book of the Baltic Region. Part 1: Lists of Threatened Vascular Plants and Vertebrates; Swedish Threatened Species Unit, Uppsala in co-Operation with Institute of Biology: Riga, Latvia, 1993; p. 96. [Google Scholar]
- GBIF. Lysimachia maritima (L.) Galasso, Banfi & Soldano in GBIF Secretariat. In GBIF Backbone Taxonomy; Checklist Dataset; GBIF: Copenhagen, Denmark, 2021. [Google Scholar]
- GBIF. Spergularia marina (L.) Griseb. in GBIF Secretariat. In GBIF Backbone Taxonomy; Checklist Dataset; GBIF: Copenhagen, Denmark, 2021. [Google Scholar]
- Pliszko, A. A new record of Spergularia marina (Caryophyllaceae) from southern Poland. Acta Musei Silesiae. Sci. Nat. 2017, 66, 49–51. [Google Scholar] [CrossRef] [Green Version]
- Banfi, E.; Galasso, G.; Soldano, A. Notes on systematics and taxonomy for the Italian vascular flora 1. Atti Soc. Ital. Sci. Nat. Mus. Civ. Stor. Nat. Milano 2005, 146, 219–244. [Google Scholar] [CrossRef] [Green Version]
- Hao, G.; Yuan, Y.M.; Hu, C.M.; Ge, X.J.; Zhao, N.X. Molecular phylogeny of Lysimachia (Myrsinaceae) based on chloroplast trnL–F and nuclear ribosomal ITS sequences. Mol. Phylogenet. Evol. 2004, 31, 323–339. [Google Scholar] [CrossRef]
- Rozema, J.; Buizer, D.A.G.; Fabritius, H.E. Population Dynamics of Glaux maritima and Ecophysiological Adaptations to Salinity and Inundation. Oikos 1978, 30, 548. [Google Scholar] [CrossRef]
- Alegro, A.; Šegota, V.; Koletic, N.; Vukovic, N.; Vilovic, T.; Rimac, A. Glaux maritima L. (Primulaceae), a new plant species in SE Europe. Acta Bot. Croat. 2019, 78, 95–98. [Google Scholar] [CrossRef] [Green Version]
- Vinholes, J.; Grosso, C.; Andrade, P.B.; Gil-Izquierdo, A.; Valentão, P.; De Pinho, P.G.; Ferreres, F. In vitro studies to assess the antidiabetic, anti-cholinesterase and antioxidant potential of Spergularia rubra. Food Chem. 2011, 129, 454–462. [Google Scholar] [CrossRef]
- Tegin, I.; Canpolat, G.; Fidan, M. The Antioxidant Capacity, Total Phenolic Content and Phenolic Compounds of Plantago coronopus L. subsp. coronopus in Naturally Distributed in Akdoǧmuş-Siirt. In Proceedings of the 2018 2nd International Symposium on Multidisciplinary Studies and Innovative Technologies (ISMSIT), Ankara, Turkey, 19–21 October 2018. [Google Scholar] [CrossRef]
- Lee, J.-J.; Jung, H.-O. Changes in Physicochemical Properties of Spergularia marina Griseb by Blanching. Korean J. Food Preserv. 2012, 19, 866–872. [Google Scholar] [CrossRef]
- Son, H.-K.; Kong, H.-M.; Cha, S.-S.; Choi, Y.-J.; Lee, J.-J. Quality characteristics of cookies added with Spergularia marina Griseb powder. Korean J. Food Preserv. 2015, 22, 211–217. [Google Scholar] [CrossRef]
- Chang, H.; Kim, M.; Kim, M.; Lee, J.; Kim, Y.; Sim, K.H. Quality Characteristics and Antioxidant Activities of Noodles added with Spergularia marina L. Griseb Powder. J. East Asian Soc. Diet. Life 2017, 27, 50–60. [Google Scholar] [CrossRef]
- Cho, J.Y.; Huang, Z.; Park, S.Y.; Park, K.H.; Pai, T.K.; Kim, S.Y.; Kim, H.R.; Ham, K.S. The Effects of Several Halophytes on Insulin Resistance in Otsuka Long-evans Tokushima Fatty Rats. Korean J. Food Sci. Technol. 2014, 46, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.H.; Lee, J.J.; Son, H.K.; Kim, B.H.; Byun, J.; Ha, J.H. Antiobesity Effects of Extract from Spergularia marina Griseb in Adipocytes and High-Fat Diet-Induced Obese Rats. Nutrients 2020, 12, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karadeniz, F.; Kim, J.-A.; Ahn, B.-N.; Kim, M.; Kong, C.-S. Anti-adipogenic and Pro-osteoblastogenic Activities of Spergularia marina Extract. Prev. Nutr. Food Sci. 2014, 19, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Kong, C.S. Anti-Inflammatory Activity of the Solvent-Partitioned Fractions from Spergularia marina in LPS-Stimulated RAW 264.7 Cells. Prev. Nutr. Food Sci. 2014, 19, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miri, A.; Shahraki, E.; Tabrizian, K.; Bilimleri Dergisi, F.; Mïrï, A.; Shahrakï, E.; Tabrïzïan, K.; Oudï, S. Anti-Nociceptive and Antiinflammatory Effects of the Hydroalcoholic Extract of the Spergularia marina (L). Griseb in Male Mice. Cumhur. Univ. Fac. Sci. Sci. J. 2015, 36, 1732–1738. [Google Scholar]
- Gamal El-Dien, O.; Shawky, E.; Aly, A.H.; Abdallah, R.M.; Abdel-Salam, N.A. Phytochemical and Biological Investigation of Spergularia marina (L.) Griseb. Growing in Egypt. Nat. Prod. Sci. 2014, 20, 152–159. [Google Scholar]
- Lee, J.; Kim, M.; Kim, S.; Kim, Y.; Kim, J.; Yang, Y.; Seo, Y.; Ju, J. Changes in Antioxidant and Cancer Cell Growth Inhibitory Activities of Spergularia marina Griseb Extract according to Different Cooking Methods. Korean J. Food Cook. Sci. 2017, 33, 673–681. [Google Scholar] [CrossRef]
- Ismail, C.A.; Baraka, A.M.; Abdallah, R.M.; El-Dien, O.G.; Mostafa, D.K. Spergularia marina: A potential source of a novel calcium channel blocker with antihypertensive and diuretic activities. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 506–517. [Google Scholar] [CrossRef]
- Lellau, T.F.; Liebezeit, G. Alkaloids, saponins and phenolic compounds in salt marsh plants from the Lower Saxonian Wadden Sea. Senckenbergiana Marit. 2001, 31, 1–9. [Google Scholar] [CrossRef]
- Lellau, T.F.; Liebezeit, G. Activity of ethanolic extracts of salt marsh plants from the Lower Saxonian Wadden Sea Coast against microorganisms. Senckenbergiana Marit. 2003, 32, 177–181. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, J.Y.; Jhin, C.; Shin, J.M.; Kim, M.; Ahn, H.R.; Yoo, G.; Son, Y.J.; Jung, S.H.; Nho, C.W. Reduction of Hepatic Lipogenesis by Loliolide and Pinoresinol from Lysimachia vulgaris via Degrading Liver X Receptors. J. Agric. Food Chem. 2019, 67, 12419–12427. [Google Scholar] [CrossRef]
- Tóth, A.; Riethmüller, E.; Alberti, Á.; Végh, K.; Kéry, Á. Comparative phytochemical screening of phenoloids in Lysimachia species. Eur. Chem. Bull 2012, 1, 27–30. [Google Scholar]
- Podolak, I.; Koczurkiewicz, P.; Galanty, A.; Michalik, M. Cytotoxic triterpene saponins from the underground parts of six Lysimachia L. species. Biochem. Syst. Ecol. 2013, 47, 116–120. [Google Scholar] [CrossRef]
- Li, H.; Hao, Z.; Wang, X.; Huang, L.; Li, J. Antioxidant activities of extracts and fractions from Lysimachia foenum-graecum Hance. Bioresour. Technol. 2009, 100, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Toth, A.; Toth, G.; Kery, A. Polyphenol Composition and Antioxidant Capacity of Three Lysimachia Species. Nat. Prod. Commun. 2014, 9, 1473–1478. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.; Ke, Z.; Wu, S.; Yang, X.; Chen, Q.; Huang, S.; Liu, C. Evaluation of the antioxidant and endothelial protective effects of Lysimachia christinae Hance (Jin Qian Cao) extract fractions. BMC Complement. Altern. Med. 2018, 18, 128. [Google Scholar] [CrossRef]
- Challam, M.; Roy, B.; Tandon, V. Effect of Lysimachia ramosa (Primulaceae) on helminth parasites: Motility, mortality and scanning electron microscopic observations on surface topography. Vet. Parasitol. 2010, 169, 214–218. [Google Scholar] [CrossRef]
- Csepregi, R.; Temesfői, V.; Das, S.; Alberti, Á.; Tóth, C.A.; Herczeg, R.; Papp, N.; Kőszegi, T. Cytotoxic, Antimicrobial, Antioxidant Properties and Effects on Cell Migration of Phenolic Compounds of Selected Transylvanian Medicinal Plants. Antioxidants 2020, 9, 166. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.X.; Liu, X.F.; Ke, Z.Q.; Wu, N.H.; Chen, H.G.; Liu, C. The Effects of Lysimachia christinae Hance Extract Fractions on Endothelium-Dependent Vasodilatation. Pharmacology 2019, 104, 36–42. [Google Scholar] [CrossRef]
- Özgen, U.; Şener, S.Ö.; Šmejkal, K.; Vaclavik, J.; Şenol Deniz, F.S.; Erdoğan Orhan, İ.; Svajdlenka, E.; Gören, A.C.; Žemlička, M. Cholinesterase and Tyrosinase Inhibitory Potential and Antioxidant Capacity of Lysimachia verticillaris L. and Isolation of the Major Compounds. Turkish J. Pharm. Sci. 2020, 17, 528. [Google Scholar] [CrossRef]
- Adam, P. Saltmarsh Ecology; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Silvestri, S.; Defina, A.; Marani, M. Tidal regime, salinity and salt marsh plant zonation. Estuar. Coast. Shelf Sci. 2005, 62, 119–130. [Google Scholar] [CrossRef]
- Richards, L. Diagnosis and Improvement of Saline and Alkali Soils; LWW: Philadelphia, PA, USA, 1954. [Google Scholar]
- Manzhina, S.A. On the issue of chemical mechanism and soil salinity degree determination: Russian and foreign practices. L. Reclam. Hydraul. Eng. 2021, 11, 163–181. [Google Scholar] [CrossRef]
- Pappalardo, H.D.; Toscano, V.; Puglia, G.D.; Genovese, C.; Raccuia, S.A. Cynara cardunculus L. as a Multipurpose Crop for Plant Secondary Metabolites Production in Marginal Stressed Lands. Front. Plant Sci. 2020, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Feduraev, P.; Skrypnik, L.; Nebreeva, S.; Dzhobadze, G.; Vatagina, A.; Kalinina, E.; Pungin, A.; Maslennikov, P.; Riabova, A.; Krol, O.; et al. Variability of Phenolic Compound Accumulation and Antioxidant Activity in Wild Plants of Some Rumex Species (Polygonaceae). Antioxidants 2022, 11, 311. [Google Scholar] [CrossRef] [PubMed]
- Jakimiuk, K.; Wink, M.; Tomczyk, M. Flavonoids of the Caryophyllaceae. Phytochem. Rev. 2022, 21, 179–218. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, M.S.; Lee, Y.G.; Jeong, H.Y.; Lee, H.J.; Ham, K.S.; Moon, J.H. A phenyl lipid alkaloid and flavone C-diglucosides from Spergularia marina. Food Sci. Biotechnol. 2016, 25, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Kraa, O.; Ghodbane, H.; Saadi, R.; Ayad, M.; Becherif, M.; Bahri, M.; Aboubou, A. Evaluation of antioxidant and antimicrobial activity of Spergularia marina (L.) Griseb extract. J. Fundam. Appl. Sci. 2016, 8, 501–517. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.M.; Ibrahim, R.K. Tricin—A potential multifunctional nutraceutical. Phytochem. Rev. 2009, 9, 413–424. [Google Scholar] [CrossRef]
- El-Dien, O.G.; Shawky, E.; Aly, A.H.; Abdallah, R.M.; Abdel-Salam, N.A. A Validated High-Performance Thin-Layer Chromatography (Hptlc) Method for the Quantitative Determination of Tricin in Two Spergularia Species. Am. J. Anal. Chem. 2013, 4, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [Green Version]
- El-Amier, Y.A.; Soufan, W.; Almutairi, K.F.; Zaghloul, N.S.; Abd-Elgawad, A.M. Proximate Composition, Bioactive Compounds, and Antioxidant Potential of Wild Halophytes Grown in Coastal Salt Marsh Habitats. Molecules 2021, 27, 28. [Google Scholar] [CrossRef]
- Chepel, V.; Lisun, V.; Skrypnik, L. Changes in the Content of Some Groups of Phenolic Compounds and Biological Activity of Extracts of Various Parts of Heather (Calluna vulgaris (L.) Hull) at Different Growth Stages. Plants 2020, 9, 926. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Shiwa, Y.; Ishige, T.; Sakamoto, H.; Tanaka, K.; Uchino, M.; Tanaka, N.; Oguri, S.; Saitoh, H.; Tsushima, S. Bacterial diversity associated with the rhizosphere and endosphere of two halophytes: Glaux maritima and Salicornia europaea. Front. Microbiol. 2018, 9, 2878. [Google Scholar] [CrossRef]
- Druva-Lūsīte, I.; Karlsons, A.; Osvalde, A.; Ņečajeva, J.; Ievinsh, G. Photosynthetic performance and mycorrhizal symbiosis of a coastal marsh plant, Glaux maritima, in conditions of fluctuating soil salinity. Acta Univ. Latv. 2014, 745, 155–164. [Google Scholar]
- Sonjak, S.; Udovič, M.; Wraber, T.; Likar, M.; Regvar, M. Diversity of halophytes and identification of arbuscular mycorrhizal fungi colonising their roots in an abandoned and sustained part of Sečovlje salterns. Soil Biol. Biochem. 2009, 41, 1847–1856. [Google Scholar] [CrossRef]
- Munakata, R.; Larbat, R.; Duriot, L.; Olry, A.; Gavira, C.; Mignard, B.; Bourgaud, F. Polyphenols from Plant Roots. Recent Adv. Polyphen. Res. 2019, 6, 207–236. [Google Scholar]
- Lopes, M.; Sanches-Silva, A.; Castilho, M.; Cavaleiro, C.; Ramos, F. Halophytes as source of bioactive phenolic compounds and their potential applications. Crit. Rev. Food Sci. Nutr. 2021, 1–21. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B.; Sanada, Y.; Mohanty, P. Effects of salinity on biochemical components of the mangrove, Aegiceras corniculatum. Aquat. Bot. 2004, 80, 77–87. [Google Scholar] [CrossRef]
- Castillo, J.M.; Mancilla-Leytón, J.M.; Martins-Noguerol, R.; Moreira, X.; Moreno-Pérez, A.J.; Muñoz-Vallés, S.; Pedroche, J.J.; Figueroa, M.E.; García-González, A.; Salas, J.J.; et al. Interactive effects between salinity and nutrient deficiency on biomass production and bio-active compounds accumulation in the halophyte Crithmum maritimum. Sci. Hortic. 2022, 301, 111136. [Google Scholar] [CrossRef]
- Falcinelli, B.; Sileoni, V.; Marconi, O.; Perretti, G.; Quinet, M.; Lutts, S.; Benincasa, P. Germination under Moderate Salinity Increases Phenolic Content and Antioxidant Activity in Rapeseed (Brassica napus var oleifera Del.) Sprouts. Molecules 2017, 22, 1377. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [Green Version]
- López-Berenguer, C.; Martínez-Ballesta, M.D.C.; Moreno, D.A.; Carvajal, M.; García-Viguera, C. Growing Hardier Crops for Better Health: Salinity Tolerance and the Nutritional Value of Broccoli. J. Agric. Food Chem. 2009, 57, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B. Salinity effects on phenolic content and antioxidant activity of Salvia macrosiphon. Iran. J. Sci. Technol. Trans. A Sci. 2017, 41, 295–300. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [Green Version]
- Saha, B.; Chowardhara, B.; Awasthi, J.P.; Panda, S.K.; Panigrahi, K.C.S. Salinity Stress and Plant Secondary Metabolite Enhancement: An Overview. In Biotechnological Approaches to Enhance Plant Secondary Metabolites; CRC Press: Boca Raton, FL, USA, 2021; pp. 49–60. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, H.; Chen, D.; Li, Z.; Peng, R.; Yao, Q. A Grape BHLH Transcription Factor Gene, VvbHLH1, Increases the Accumulation of Flavonoids and Enhances Salt and Drought Tolerance in Transgenic Arabidopsis thaliana. Plant Cell. Tissue Organ Cult. 2016, 125, 387–398. [Google Scholar] [CrossRef]
- Chen, S.; Wu, F.; Li, Y.; Qian, Y.; Pan, X.; Li, F.; Wang, Y.; Wu, Z.; Fu, C.; Lin, H.; et al. NTMYB4 and NTCHS1 Are Critical Factors in the Regulation of Flavonoid Biosynthesis and Are Involved in Salinity Responsiveness. Front. Plant Sci. 2019, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, L.; Majd, A.; Nematzadeh, G.; Shokri, M.; Kelij, S.; Irian, S. Salt-induced changes in cell wall peroxidase (CWPRX) and phenolic content of “Aeluropus littoralis” (willd) parl. Aust. J. Crop Sci. 2014, 8, 296–300. [Google Scholar]
- Chubarenko, B.; Margoński, P. The Vistula Lagoon. Ecol. Stud. 2008, 197, 167–195. [Google Scholar] [CrossRef]
- Packham, J.R.; Willis, A.J. Ecology of Dunes, Salt Marsh and Shingle; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- ISO 11465; Soil Quality–Determination of Dry Matter and Water Content on a Mass Basis-Gravimetric Method. ISO-International Organization for Standardization: Geneva, Switzerland, 1993.
- Padhi, E.M.T.; Liu, R.; Hernandez, M.; Tsao, R.; Ramdath, D.D. Total polyphenol content, carotenoid, tocopherol and fatty acid composition of commonly consumed Canadian pulses and their contribution to antioxidant activity. J. Funct. Foods 2017, 38, 602–611. [Google Scholar] [CrossRef]
- FS.2.5.0033.15 Artemisiae absinthii herba. In State Pharmacopoeia of the Russian Federation, 14th ed.; Ministry of Health of the Russian Federation: Moscow, Russia, 2018; Volume 4, pp. 6343–6350.
- Štefan, M.B.; Vuković Rodríguez, J.; Blažeković, B.; Kindl, M.; Vladimir-Knežević, S. Total Hydroxycinnamic Acids Assay: Prevalidation and Application on Lamiaceae Species. Food Anal. Methods 2013, 7, 326–336. [Google Scholar] [CrossRef]
- Skrypnik, L.; Grigorev, N.; Michailov, D.; Antipina, M.; Danilova, M.; Pungin, A. Comparative study on radical scavenging activity and phenolic compounds content in water bark extracts of alder (Alnus glutinosa (L.) Gaertn.), oak (Quercus robur L.) and pine (Pinus sylvestris L.). Eur. J. Wood Wood Prod. 2019, 77, 879–890. [Google Scholar] [CrossRef]
- Klančnik, A.; Piskernik, S.; Jeršek, B.; Možina, S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.H.; Hinton, J. A Protein-Free Medium for Primary Isolation of the Gonococcus and Meningococcus. Exp. Biol. Med. 2016, 48, 330–333. [Google Scholar] [CrossRef]

| Place of Growth | EC, dSm−1 | pH | Dry Residue, % | Content of Soluble Cations and Anions, cmolc kg−1 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cl− | Na+ | K+ | Ca2+ | SO42− | HCO3− | ||||
| Lower level of salt marsh | 1.38 ± 0.02 b* | 7.74 ± 0.02 a | 0.50 ± 0.05 b | 6.9 ± 0.2 b | 4.3 ± 0.1 b | 0.16 ± 0.01 b | 0.50 ± 0.04 b | 0.45 ± 0.08 b | 0.61 ± 0.06 a |
| Upper level of salt marsh | 4.02 ± 0.23 a | 7.56 ± 0.10 b | 1.42 ± 0.45 a | 16.5 ± 6.2 a | 9.1 ± 2.6 a | 0.25 ± 0.04 a | 3.00 ± 0.30 a | 0.67 ± 0.13 a | 0.64 ± 0.10 a |
| Content of Individual Phenolic Compounds, µg g−1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S. marina | G. maritima | |||||||||
| Plant Parts | Roots | Shoots | Inflorescence | Roots | Shoots | |||||
| Soil Salinity | Slight | Strong | Slight | Strong | Slight | Strong | Slight | Strong | Slight | Strong |
| Compounds | Flavonoids | |||||||||
| Catechin | 190.1 ± 9.7 a** | 244.6 ± 47.4 a | + *** | + | + | + | + | + | + | - |
| Hyperoside | - | - | - | - | - | - | - | - | 97.5 ± 10.9 | - |
| Quercetin 3-O-rutinoside (rutin) | - | - | - | - | - | - | - | - | + | - |
| Quercetin 3-β-d-glucoside (isoquercitrin) | - | - | - | - | - | - | 52.2 ± 9.4 a | 47.1 ± 5.6 a | 1711.0 ± 323.4 a | 587.5 ± 42.6 b |
| Quercetin derivative * | - | - | - | - | - | - | 273.9 ± 2.4 a | 165.4 ± 8.0 b | 5203.5 ± 1809.3 a | 2294.0 ± 240.4 b |
| Kaempferol 3-O-glucoside (astragalin) | - | - | - | - | - | - | - | - | 88.2 ± 22.9 a | 55.6 ± 6.0 b |
| Kaempferol derivative | - | - | - | - | - | - | 108.2 ± 14.4 b | 151.6 ± 17.0 a | 4248.0 ± 622.1 a | 2522.3 ± 329.2 b |
| Hesperetin | 32.9 ± 1.1 b | 51.9 ± 10.5 a | 48.9 ± 10.4 b | 71.9 ± 10.1 a | 111.0 ± 11.1 a | 100.6 ± 23.1 a | - | - | - | - |
| Epicatechin | 742.8 ± 42.4 b | 1012.6 ± 50.4 a | + | 77.1 ± 27.2 | + | + | - | - | - | - |
| Tricin derivative | + | + | + | + | + | + | - | - | - | - |
| Apigenin derivative | 311.3 ± 43.1 b | 410.7 ± 9.0 a | 1593.9 ± 327.6 b | 2170.9 ± 205.2 a | 2911.7 ± 176.9 a | 3541.4 ± 1340.4 a | - | - | - | - |
| Luteolin derivative | - | - | - | 102.9 ± 40.4 | - | 61.8 ± 19.9 | - | - | - | - |
| Phenolic acids | ||||||||||
| 3,4-Dihydroxybenzoic acid (protocatechuic acid) | 35.9 ± 1.7 b | 52.4 ± 8.3 a | 31.4 ± 1.8 a | 24.6 ± 2.6 b | 27.1 ± 4.2 a | 21.4 ± 3.9 a | 21.7 ± 1.1 a | 19.6 ± 1.9 a | 46.8 ± 12.8 a | 38.6 ± 2.0 a |
| p-Coumaric acid | - | - | - | - | - | - | - | - | 16.9 ± 3.2 a | 21.7 ± 6.3 a |
| Ferulic acid | - | - | - | - | - | - | + | + | + | 16.1 ± 3.9 |
| Chlorogenic acid | - | + | - | - | - | - | + | + | + | + |
| Chicoric acid | + | - | + | + | + | + | - | - | - | - |
| Rosmarinic acid | + | - | + | + | + | + | - | - | - | - |
| Roots | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variables | AOA (DPPH) 1 | TPC | TFC | Protocatechuic Acid | Hesperetin | Epicatechin | Apigenin Derivative | Catechin |
| AOA (DPPH) | 1 | 0.909 ** | −0.334 ns | 0.859 ** | 0.883 ** | 0.876 ** | 0.754 * | 0.893 ** |
| TPC | 1 | −0.189 ns | 0.797 ** | 0.837 ** | 0.870 ** | 0.800 ** | 0.753 * | |
| TFC | 1 | −0.470 ns | −0.418 ns | −0.454 ns | −0.403 ns | −0.406 ns | ||
| Protocatechuic acid | 1 | 0.987 ** | 0.936 ** | 0.776 ** | 0.935 ** | |||
| Hesperetin | 1 | 0.909 ** | 0.757 * | 0.954 ** | ||||
| Epicatechin | 1 | 0.851 ** | 0.818 ** | |||||
| Apigenin derivative | 1 | 0.663 * | ||||||
| Catechin | 1 | |||||||
| Shoots | ||||||||
| AOA (DPPH) | 1 | 0.492 ns | 0.050 ns | −0.452 ns | 0.216 ns | 0.015 ns | 0.097 ns | - |
| TPC | 1 | 0.552 ns | −0.846 ** | 0.720 * | −0.873 ns | 0.669 * | - | |
| TFC | 1 | −0.450 ns | 0.672 * | −0.996 ns | 0.594 ns | - | ||
| Protocatechuic acid | 1 | −0.768 ** | 0.978 ns | −0.768 ** | - | |||
| Hesperetin | 1 | −0.997 ns | 0.914 ** | - | ||||
| Epicatechin | 1 | −0.996 ns | - | |||||
| Apigenin derivative | 1 | - | ||||||
| Inflorescence | ||||||||
| AOA (DPPH) | 1 | 0.054 ns | 0.288 ns | −0.558 ns | −0.702 * | - | −0.082 ns | - |
| TPC | 1 | 0.064 ns | −0.557 ns | −0.346 ns | - | 0.208 ns | - | |
| TFC | 1 | 0.281 ns | 0.099 ns | - | −0.302 ns | - | ||
| Protocatechuic acid | 1 | 0.778 * | - | −0.517 ns | - | |||
| Hesperetin | 1 | - | −0.122 ns | - | ||||
| Epicatechin | 1 | - | - | |||||
| Apigenin derivative | 1 | - |
| Roots | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | AOA (DPPH) 1 | TPC | TFC | THA | Isoquercitrin | Quercetin Derivative | Kaempferol Derivative | Astra Galin | Proto Catechuic Acid | p-Coumaric Acid |
| AOA (DPPH) | 1 | 0.389 ns | 0.504 ns | 0.237 ns | −0.389 ns | 0.025 ns | 0.310 ns | - | −0.146 ns | - |
| TPC | 1 | −0.127 ns | 0.419 ns | −0.231 ns | −0.237 ns | 0.397 ns | - | −0.474 ns | - | |
| TFC | 1 | −0.255 ns | −0.622 ns | 0.415 ns | −0.350 ns | - | −0.099 ns | - | ||
| THA | 1 | −0.396 ns | −0.889 ** | 0.791 ** | - | −0.604 ns | - | |||
| Isoquercitrin | 1 | 0.289 ns | −0.152 ns | - | 0.812 ** | - | ||||
| Quercetin derivative | 1 | −0.853 ** | - | 0.536 ns | - | |||||
| Kaempferol derivative | 1 | - | −0.348 ns | - | ||||||
| Protocatechuic acid | - | 1 | - | |||||||
| Shoots | ||||||||||
| AOA (DPPH) | 1 | 0.700 * | 0.199 ns | 0.691 * | −0.521 ns | −0.263 ns | −0.681 * | −0.714 * | −0.678 * | −0.185 ns |
| TPC | 1 | −0.201 ns | 0.486 ns | −0.871 ** | −0.607 ns | −0.934 ** | −0.842 ** | −0.676 * | 0.303 ns | |
| TFC | 1 | 0.577 ns | 0.585 ns | 0.784 ** | 0.271 ns | 0.105 ns | −0.270 ns | −0.516 ns | ||
| Isoquercitrin | 1 | 0.904 ** | 0.903 ** | 0.839 ** | 0.495 ns | −0.498 ns | ||||
| Quercetin derivative | 1 | 0.668 * | 0.657 * | 0.136 ns | −0.607 ns | |||||
| Kaempferol derivative | 1 | 0.861 ** | 0.702 * | −0.391 ns | ||||||
| Astragalin | 1 | 0.768 ** | −0.286 ns | |||||||
| Protocatechuic acid | 1 | 0.092 ns | ||||||||
| p-Coumaric acid | 1 |
| Art | Plant Parts | Soil Salinity | Diameter of Zone of Inhibition, mm | |||||
|---|---|---|---|---|---|---|---|---|
| E. coli | ||||||||
| Kanamycin | 10% DMSO | Extract Concentration, mg Disk−1 | ||||||
| 50 µg Disk−1 | 20 µL Disk−1 | 0.25 | 0.50 | 1.00 | 2.00 | |||
| S. marina | Roots | Slight | 29.8 ± 0.2 a* | - | - | - | - | - |
| Strong | 26.9 ± 1.0 b | - | - | - | - | - | ||
| Shoots | Slight | 27.7 ± 2.9 a | - | - | - | - | - | |
| Strong | 29.0 ± 0.4 a | - | - | - | - | - | ||
| Inflorescence | Slight | 29.3 ± 0.7 a | - | - | - | - | - | |
| Strong | 28.7 ± 0.7 b | - | - | - | - | - | ||
| G. maritima | Roots | Slight | 30.4 ± 1.9 a | - | - | - | - | - |
| Strong | 29.2 ± 1.6 a | - | - | - | - | - | ||
| Shoots | Slight | 29.4 ± 0.9 a | - | - | - | - | - | |
| Strong | 29.9 ± 2.5 a | - | - | - | 7.9 ± 0.4 | 9.5 ± 0.4 | ||
| B. subtilis | ||||||||
| S. marina | Roots | Slight | 24.6 ± 1.7 a | - | - | - | - | - |
| Strong | 24.6 ± 0.3 a | - | - | - | - | - | ||
| Shoots | Slight | 26.5 ± 1.9 a | - | - | - | - | - | |
| Strong | 25.3 ± 0.2 a | - | - | - | - | - | ||
| Inflorescence | Slight | 26.7 ± 0.6 a | - | - | - | - | - | |
| Strong | 26.0 ± 0.6 b | - | - | - | - | - | ||
| G. maritima | Roots | Slight | 26.1 ± 1.4 a | - | - | - | - | 7.0 ± 0.2 |
| Strong | 26.0 ± 1.4 a | - | - | - | - | - | ||
| Shoots | Slight | 24.8 ± 0.6 b | - | - | - | - | - | |
| Strong | 27.6 ± 0.7 a | - | - | - | - | - | ||
| C. albicans | ||||||||
| S. marina | Roots | Slight | 27.8 ± 0.4 a | - | - | - | - | - |
| Strong | 27.7 ± 0.6 a | - | - | - | - | - | ||
| Shoots | Slight | 29.1 ± 2.0 a | - | - | - | - | - | |
| Strong | 29.3 ± 0.7 a | - | - | - | 6.4 ± 0.1 | 7.3 ± 0.8 | ||
| Inflorescence | Slight | 28.4 ± 0.3 a | - | - | - | - | - | |
| Strong | 28.4 ± 1.1 a | - | - | - | - | 6.4 ± 0.1 | ||
| G. maritima | Roots | Slight | 27.8 ± 0.8 a | - | - | - | 6.8 ± 0.1 a | 7.5 ± 0.3 a |
| Strong | 29.2 ± 3.2 a | - | - | - | 6.7 ± 0.1 a | 7.0 ± 0.1 b | ||
| Shoots | Slight | 28.3 ± 0.7 a | - | - | - | 6.5 ± 0.1 | 6.5 ± 0.1 a | |
| Strong | 29.8 ± 1.0 a | - | - | - | - | 6.5 ± 0.1 a | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pungin, A.; Lartseva, L.; Loskutnikova, V.; Shakhov, V.; Krol, O.; Popova, E.; Kolomiets, A.; Nikolaeva, N.; Volodina, A. The Content of Certain Groups of Phenolic Compounds and the Biological Activity of Extracts of Various Halophyte Parts of Spergularia marina (L.) Griseb. and Glaux maritima L. at Different Levels of Soil Salinization. Plants 2022, 11, 1738. https://doi.org/10.3390/plants11131738
Pungin A, Lartseva L, Loskutnikova V, Shakhov V, Krol O, Popova E, Kolomiets A, Nikolaeva N, Volodina A. The Content of Certain Groups of Phenolic Compounds and the Biological Activity of Extracts of Various Halophyte Parts of Spergularia marina (L.) Griseb. and Glaux maritima L. at Different Levels of Soil Salinization. Plants. 2022; 11(13):1738. https://doi.org/10.3390/plants11131738
Chicago/Turabian StylePungin, Artem, Lidia Lartseva, Violetta Loskutnikova, Vladislav Shakhov, Olesya Krol, Elena Popova, Andrey Kolomiets, Nadezhda Nikolaeva, and Aleksandra Volodina. 2022. "The Content of Certain Groups of Phenolic Compounds and the Biological Activity of Extracts of Various Halophyte Parts of Spergularia marina (L.) Griseb. and Glaux maritima L. at Different Levels of Soil Salinization" Plants 11, no. 13: 1738. https://doi.org/10.3390/plants11131738
APA StylePungin, A., Lartseva, L., Loskutnikova, V., Shakhov, V., Krol, O., Popova, E., Kolomiets, A., Nikolaeva, N., & Volodina, A. (2022). The Content of Certain Groups of Phenolic Compounds and the Biological Activity of Extracts of Various Halophyte Parts of Spergularia marina (L.) Griseb. and Glaux maritima L. at Different Levels of Soil Salinization. Plants, 11(13), 1738. https://doi.org/10.3390/plants11131738