Sodium N-Lauroylsarcosinate (SNLS) as a Selective Collector for Calcareous Phosphate Beneficiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Techniques
2.2.1. Chemical Composition and X-ray Diffraction
2.2.2. Zeta-Potential Measurements
2.2.3. FT-IR Measurements
2.2.4. Flotation Experiments
3. Results and Discussion
3.1. Characterization of Pure Minerals and Natural Ore
3.2. Flotation of Single Minerals with NSLS or NaOL
3.2.1. Effect of pH
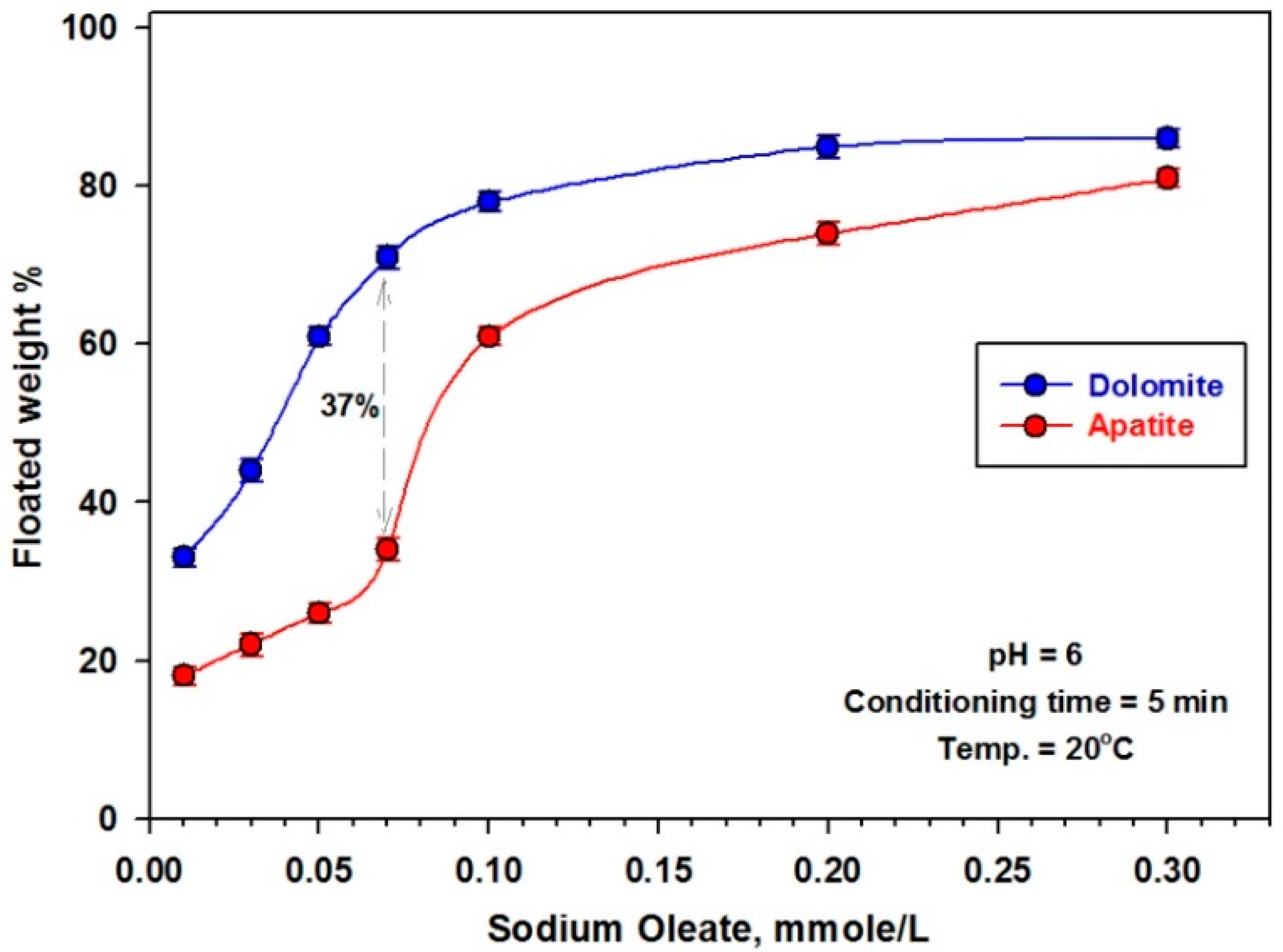
3.2.2. Effect of Collector Dose
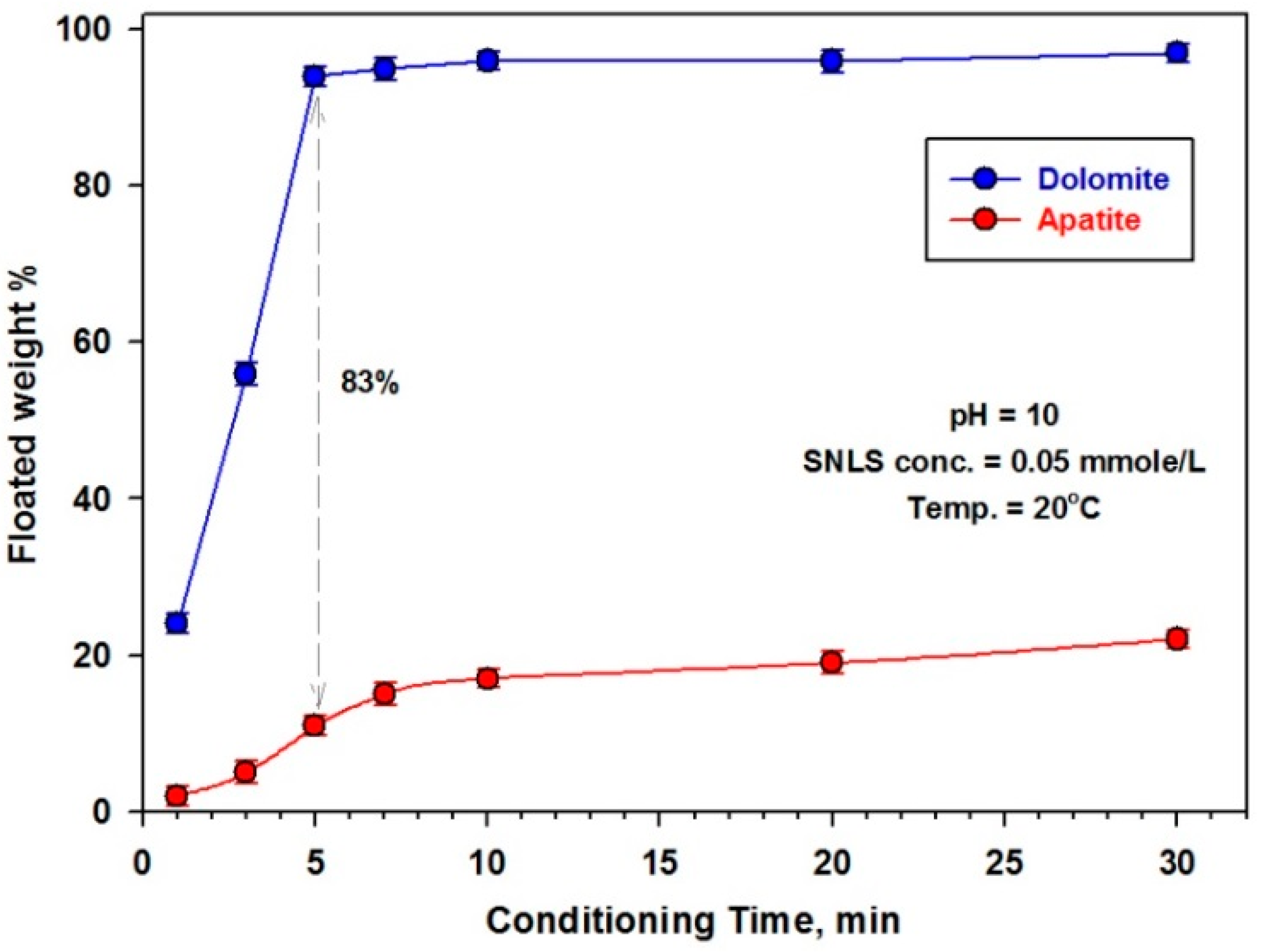
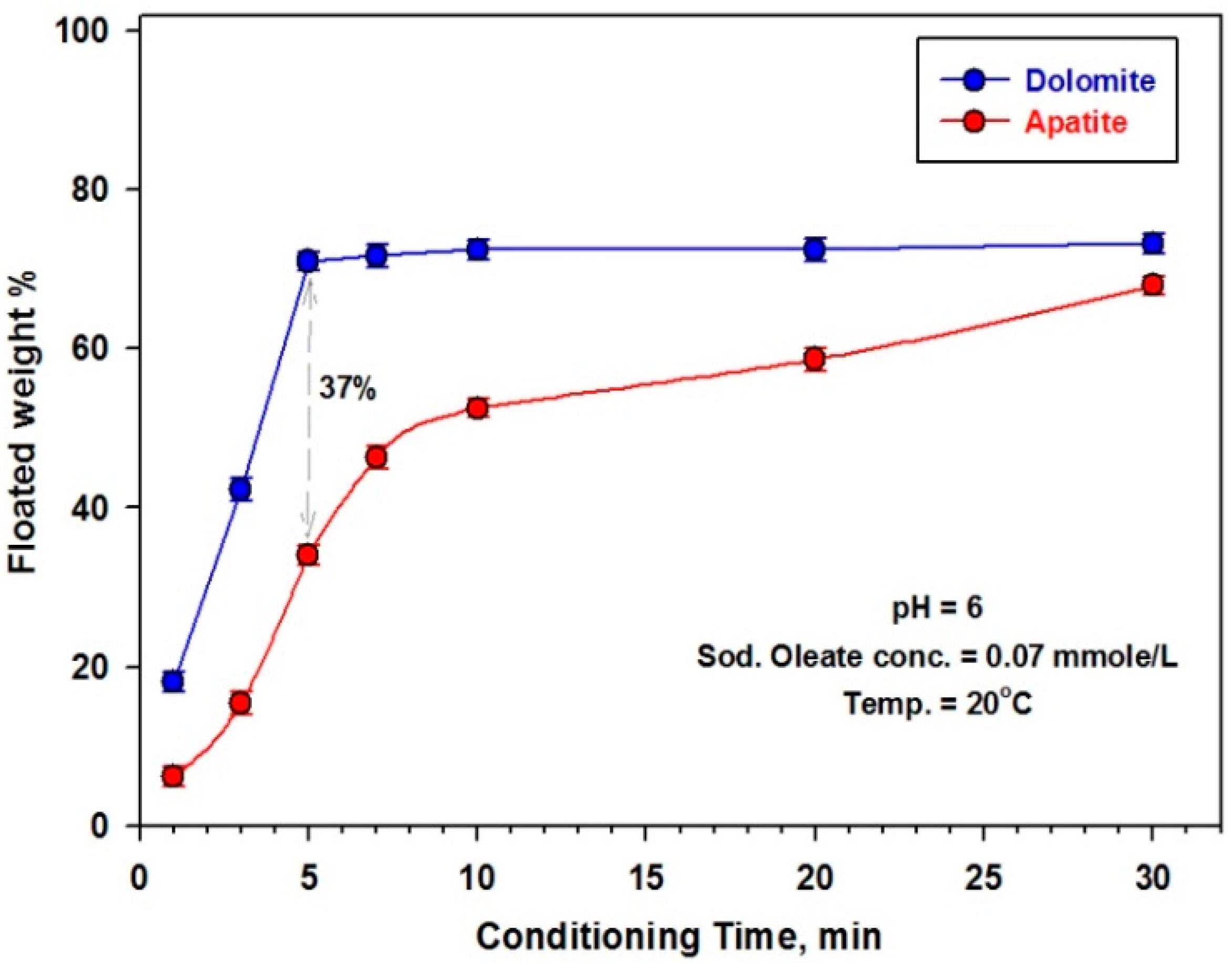
3.2.3. Effect of Conditioning Time
3.3. Flotation of Different Binary Mixed Minerals
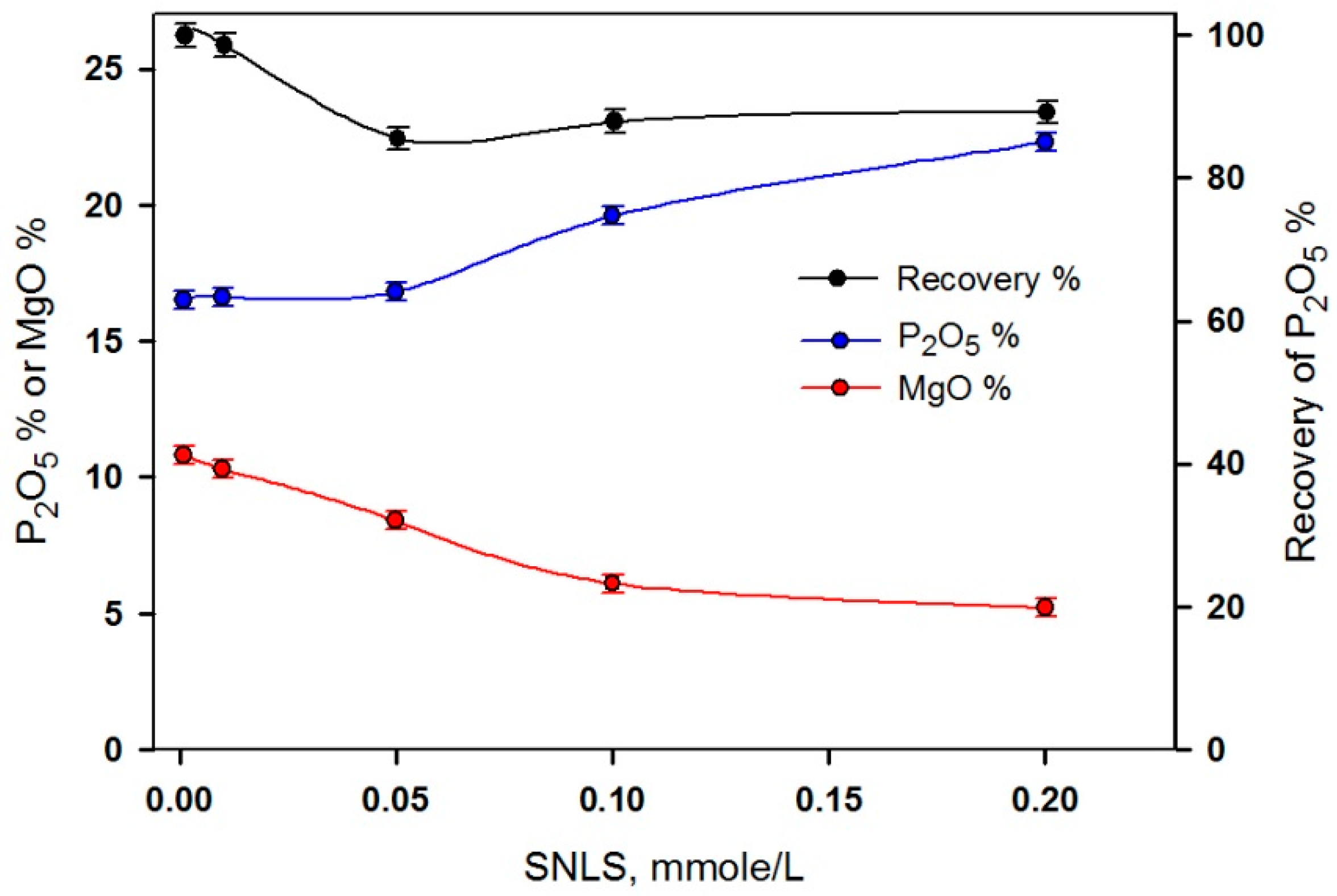
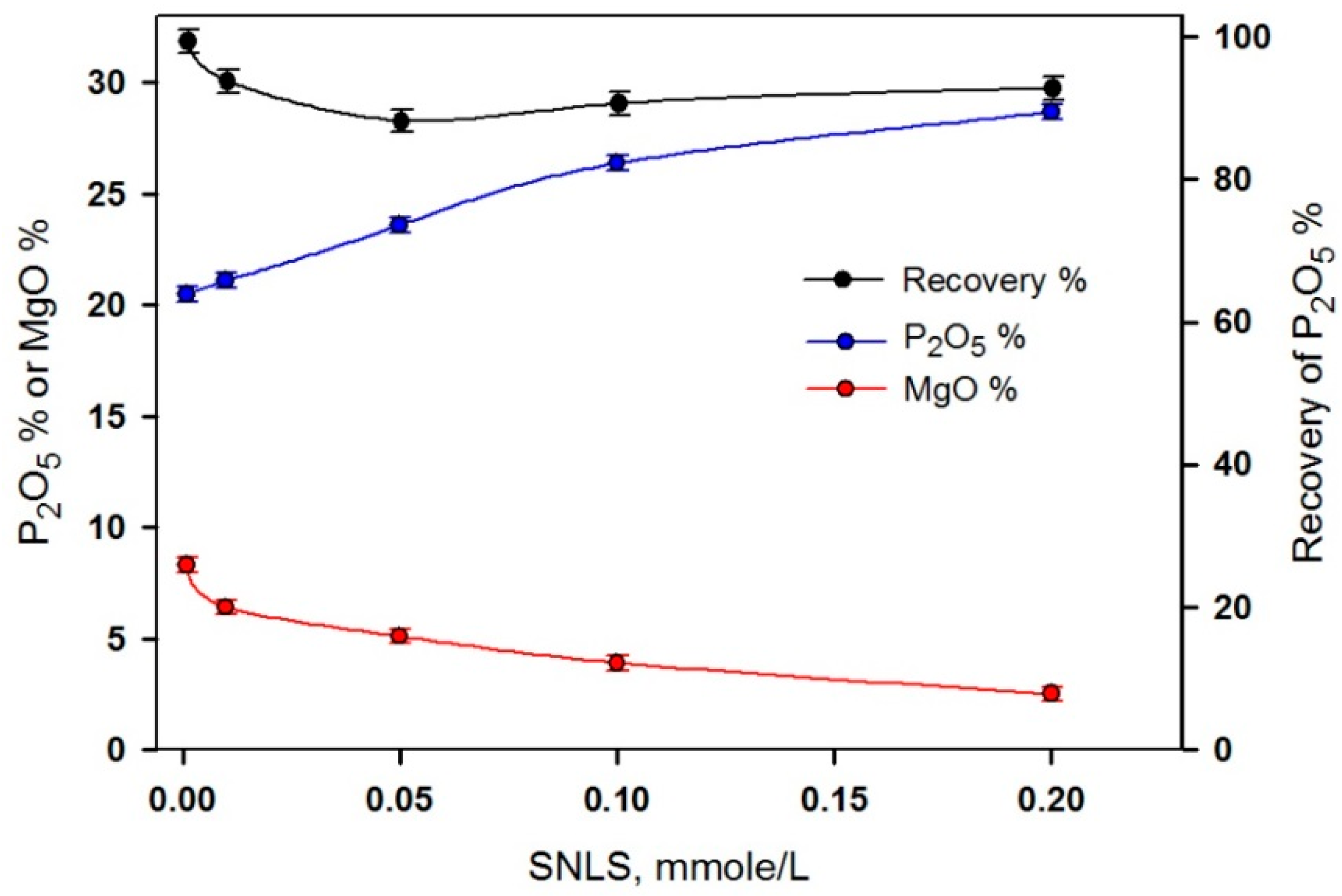
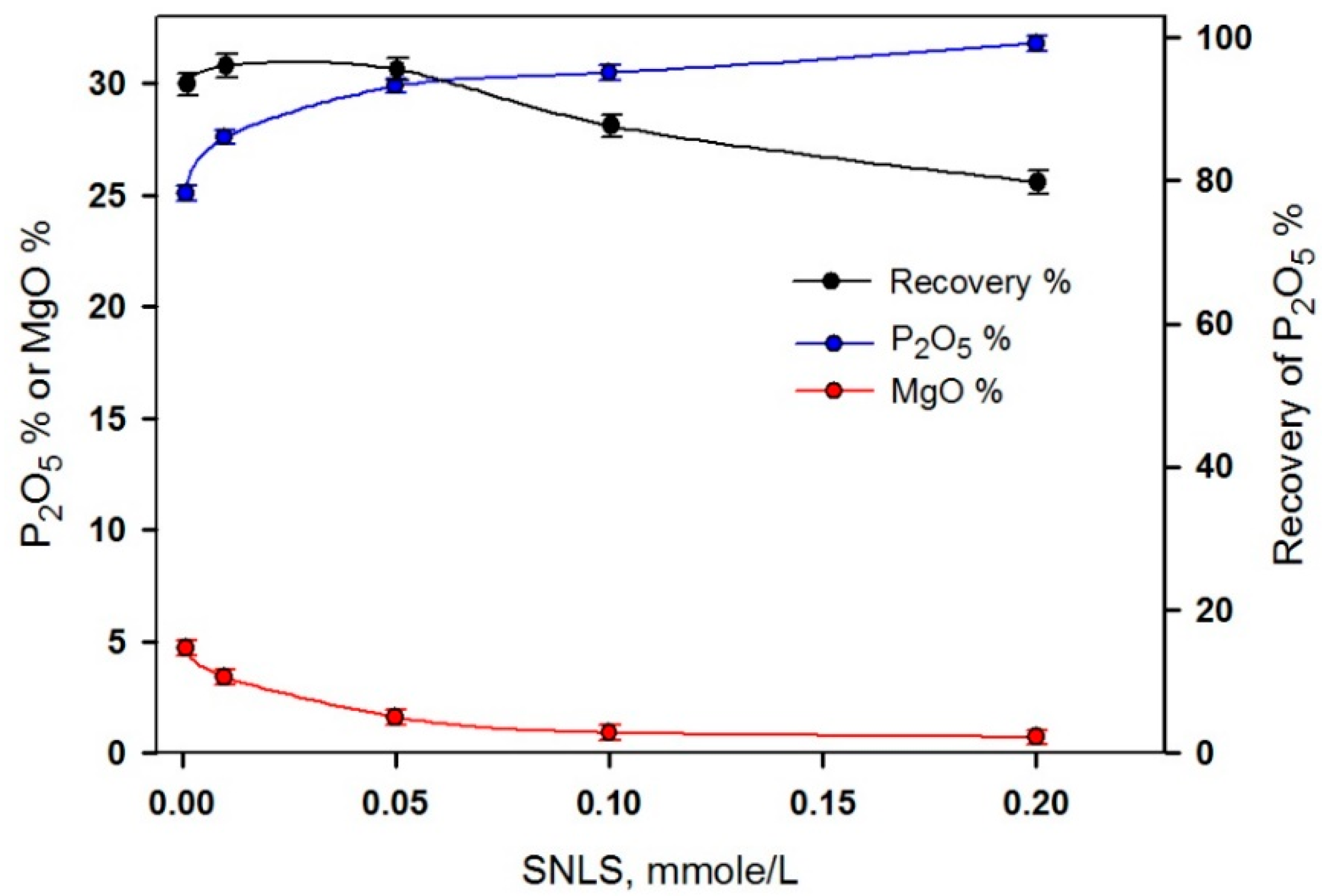
3.4. Application of the Natural Phosphate Ore
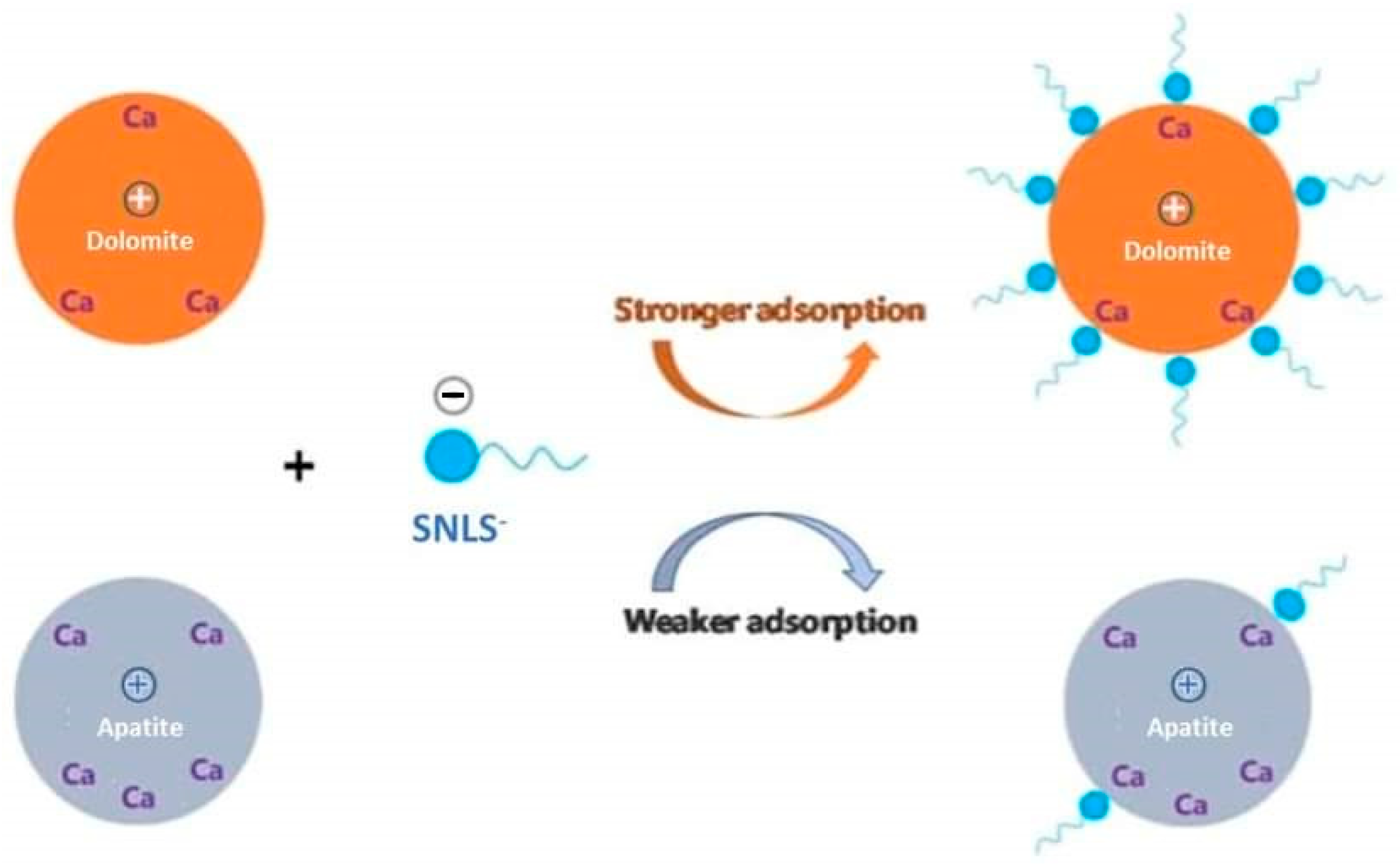
3.5. Surface Behavior of Treated Mineral
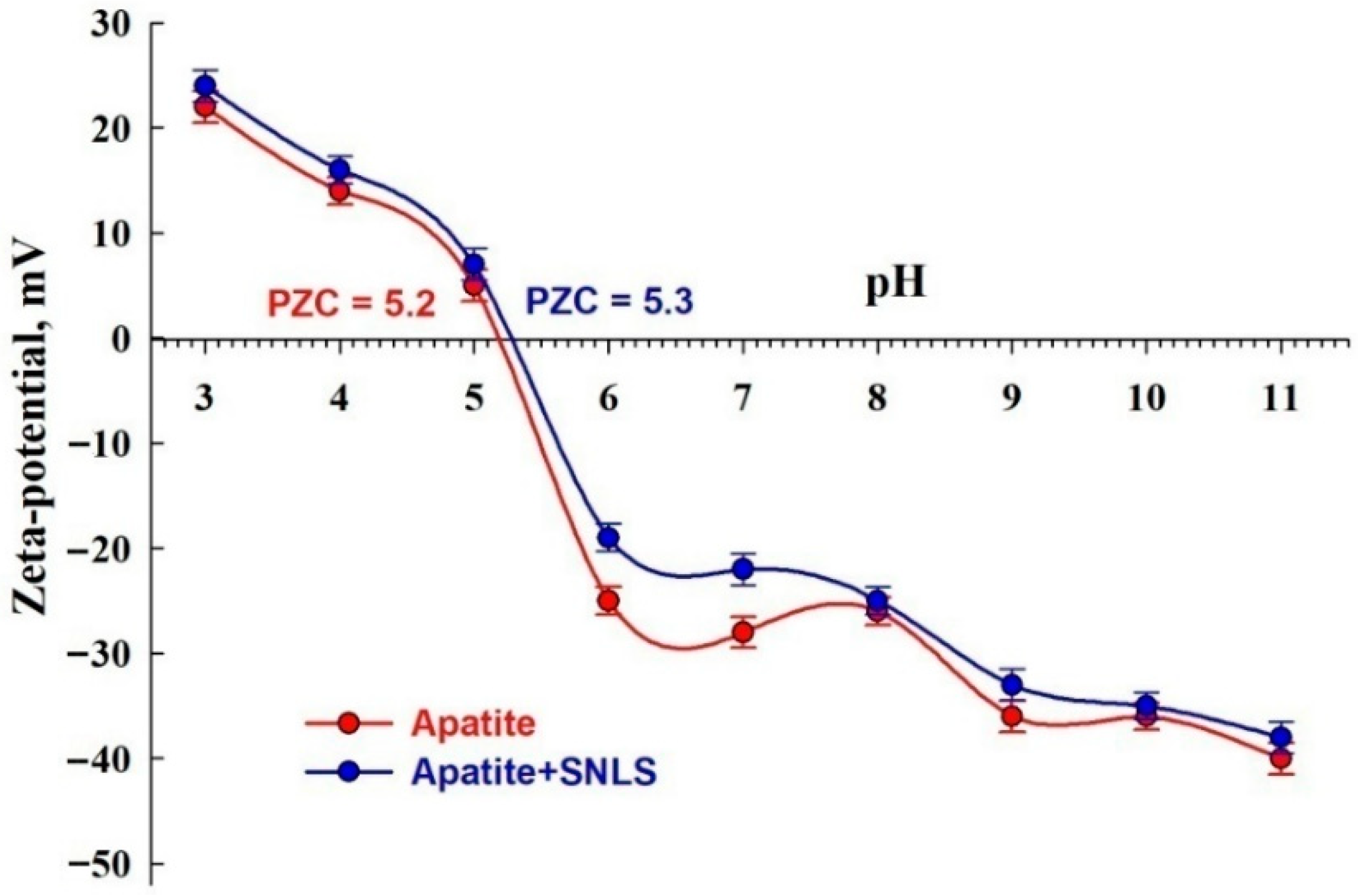
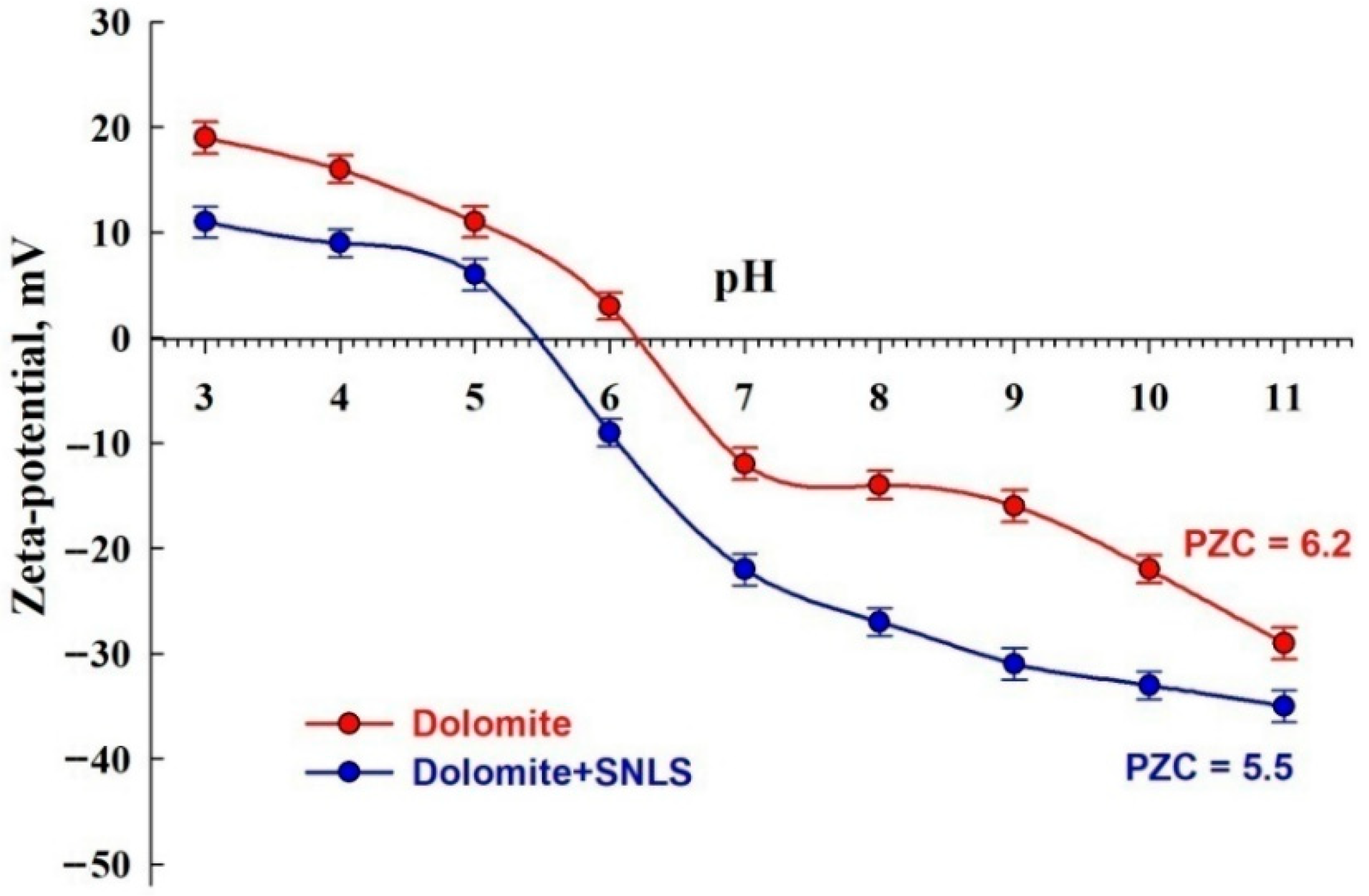
3.5.1. Zeta-potential Measurements
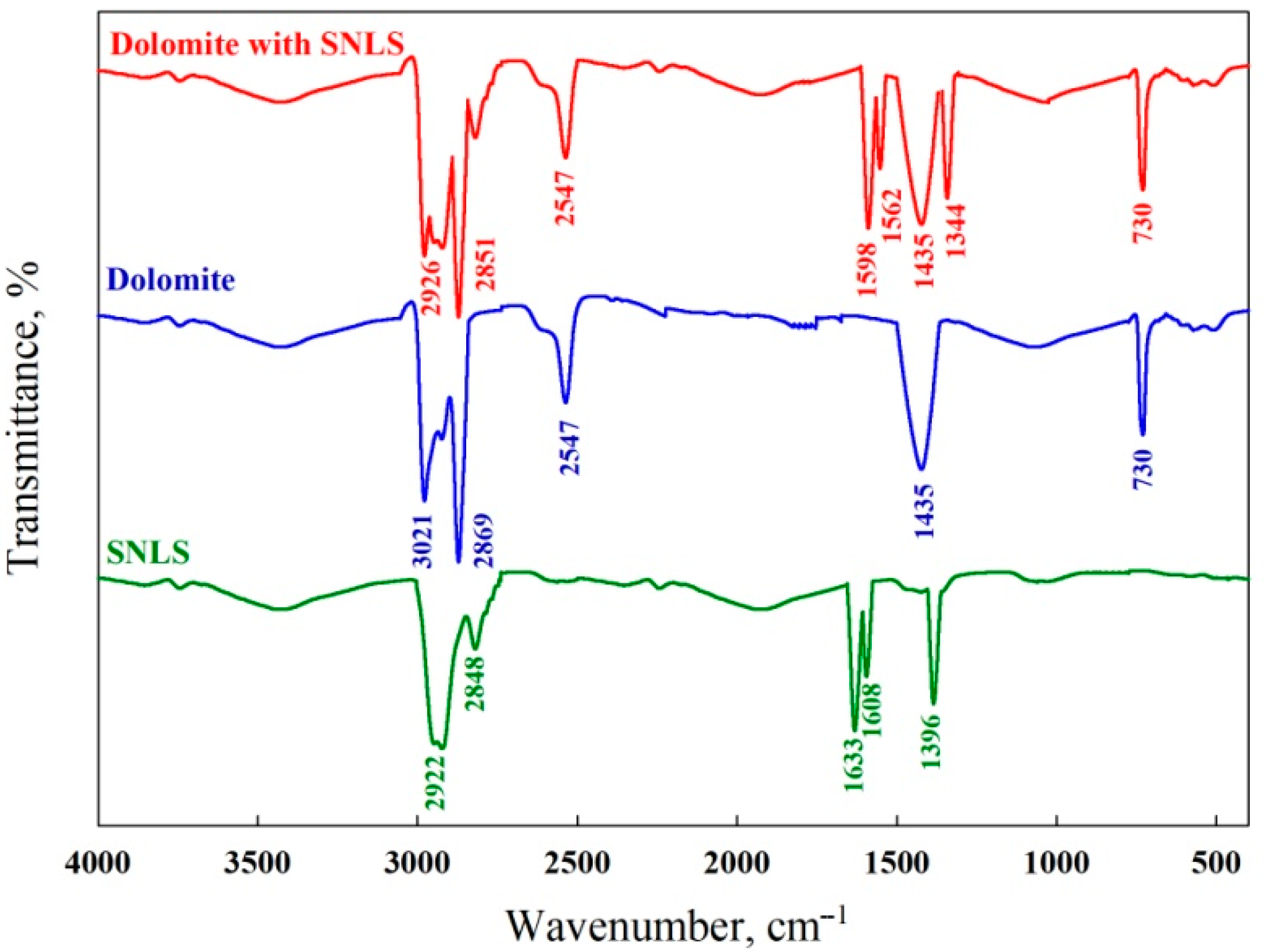
3.5.2. FT-IR Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kawatra, S.K.; Carlson, J.T. Beneficiation of Phosphate Ore; Society for Mining, Metallurgy & Exploration: Englewood, CO, USA, 2013; ISBN 978-0-87335-391-5. [Google Scholar]
- Lisiansky, L.; Baker, M.; Larmour-Ship, K.; Elyash, O. A Tailor Made Approach for the Beneficiation of Phosphate Rock. In ECI Symposium Series, Proceedings of the Beneficiation of Phosphates VII, Melbourne, Australia, 29 Mar–3 April 2015; Zhang, P., Miller, J., Leal, L., Kossir, A., Wingate, E., Eds.; Elsevier Inc.: New York, NY, USA, 2015; Available online: https://dc.engconfintl.org/phosphates_vii/24/ (accessed on 1 March 2022).
- Cao, Q.; Cheng, J.; Wen, S.; Li, C.; Bai, S.; Liu, D. A mixed collector system for phosphate flotation. Miner. Eng. 2015, 78, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Abouzeid, A.Z.; Negm, A.T.; Elgillani, D.A. Upgrading of calcareous phosphate ores by flotation: Effect of ore characteristics. Int. J. Miner. Process. 2009, 90, 81–89. [Google Scholar] [CrossRef]
- Alouan, A. Phosphate Beneficiation Development for Customers Satisfaction in Sustainable Development Way OCP case Khouribga—JorfLasfar. Pro. Eng. 2016, 138, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Sajid, M.; Bary, G.; Asim, M.; Ahmad, R. Synoptic View on P Ore Beneficiation Techniques. Alex. Eng. J. 2021, 61, 3069–3092. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Zheng, H.; Huang, P.; Yang, P.; Chen, Q.; Weng, X.; He, D.; Song, S. Effect of geological origin of apatite on reverse flotation separation of phosphate ores using phosphoric acid as depressant. Miner. Eng. 2021, 172, 107182. [Google Scholar] [CrossRef]
- Guo, F.; Li, J. Selective Separation of Silica from a Siliceous-Calcareous Phosphate Rock. Min. Sci. Technol. 2011, 21, 135–139. [Google Scholar]
- Derhy, M.; Taha, Y.; Hakkou, R.; Benzaazoua, M. Review of the Main Factors Affecting the Flotation of Phosphate Ores. Minerals 2020, 10, 1109. [Google Scholar] [CrossRef]
- Ruan, Y.; He, D.; Chi, R. Review on Beneficiation Techniques and Reagents Used for Phosphate Ores. Minerals 2019, 9, 253. [Google Scholar] [CrossRef] [Green Version]
- Filippova, I.V.; Filippov, L.O.; Lafhaj, Z.; Barres, O.; Fornasiero, D. Effect of Calcium Minerals Reactivity on Fatty Acids Adsorption and Flotation. Colloid. Surf. A 2018, 545, 157–166. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, Q.; Zhang, G.; Liu, D.; Liu, R. Effect of Sodium Pyrophosphate on the Reverse Flotation of Dolomite from Apatite. Minerals 2018, 8, 278. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Ge, Y.; Guo, X.; Guo, W. The Depression Effect and Mechanism of NSFC on Dolomite in the Flotation of Phosphate Ore. Sep. Purif. Technol. 2016, 161, 88–95. [Google Scholar] [CrossRef]
- Liu, S.; Mao, Y.; Luo, Y.; Weng, X.; Chen, Q.; Li, H.; Yi, H.; Song, S.; He, D. Selective flotation separation of bastnaesite from dolomite using β-naphthyl sulfonate formaldehyde condensate as depressant: Experimental and calculational studies. Colloid. Surf. A 2022, 639, 128380. [Google Scholar] [CrossRef]
- Yang, B.; Cao, S.; Zhu, Z.; Yin, W.; Sheng, Q.; Sun, H.; Yao, J.; Chen, K. Selective Flotation Separation of Apatite from Dolomite Utilizing a Novel Eco-Friendly and Efficient Depressant for Sustainable Manufacturing of Phosphate Fertilizer. J. Clean Prod. 2020, 286, 124949. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, Y.; Weng, X.; Jin, Y.; Kasomo, R.M.; Ao, S. Flotation Separation of Dolomite from Fluorapatite Using Sodium Dodecyl Benzene Sulfonate as the Efficient Collector under Low Temperature. Minerals 2022, 12, 228. [Google Scholar] [CrossRef]
- Liu, X.; Ruan, Y.; Li, C.; Cheng, R. Effect and mechanism of phosphoric acid in the apatite/dolomite flotation system. Int. J. Miner. Process. 2017, 167, 95–102. [Google Scholar] [CrossRef]
- Elouear, Z.; Bouzid, J.; Boujelben, N.; Feki, M.; Jamoussi, F.; Montiel, A. Heavy Metal Removal from Aqueous Solutions by Activated Phosphate Rock. J. Hazard. Mater. 2008, 156, 412–420. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Janik, L.J.; Raupach, M. Diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy in soil studies. Aust. J. Soil Res. 1991, 29, 49–67. [Google Scholar] [CrossRef]
- Coulibaly, V.; Sei, J.; Koffi, L.; Oyetola, S.; Jdid, E.; Thomas, F. Mineralogical and Chemical Characteristics of Clays Consumed in the District of Abidjan (CÔte D’Ivoire). Mater. Sci. Appl. 2014, 5, 1048–1059. [Google Scholar]
- Soejoko, D.S.; Tjia, M.O. Infrared spectroscopy and X ray diffraction study on the morphological variations of carbonate and phosphate compounds in giant prawn (Macrobrachium rosenbergii) skeletons during its moulting period. J. Mater. Sci. 2003, 38, 2087–2093. [Google Scholar] [CrossRef]
- Solotchina, E.P.; Solotchin, P.A. Composition and structure of low-temperature natural carbonates of the calcite-dolomite series. J. Struct. Chem. 2014, 55, 779–785. [Google Scholar] [CrossRef]
- Al-Maliky, M.A.; Frentzen, M.; Meister, J. Laser-assisted prevention of enamel caries: A 10-year review of the literature. Lasers Med. Sci. 2020, 35, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Yusufoglu, Y.; Akinc, M. Deposition of Carbonated Hydroxyapatite (CO3HAp) on Poly (Methylmethacrylate) Surfaces by Decomposition of Calcium-EDTA Chelate. J. Am. Ceram. Soc. 2008, 91, 3147–3153. [Google Scholar] [CrossRef]
- Yang, B.; Zhu, Z.; Sun, H.; Yin, W.; Hong, J.; Cao, S.; Tang, Y.; Zhao, C.; Yao, J. Improving Flotation Separation of Apatite from Dolomite Using PAMS as a Novel Eco-Friendly Depressant. Miner. Eng. 2020, 156, 106492. [Google Scholar] [CrossRef]
- Lin, A.C.; Xie, F.; McCarthy, L.J.; Rodgers, D.R.; Hoff, K.G.; von Welczeck, M.R.; Zhai, S.; Saw, A.C.; Scott, G.E.; Zhang, S. Photonic Liquid Crystals of Graphene Oxide for Fast Membrane Nanofiltration. Carbon Trends. 2022, 7, 100150. [Google Scholar] [CrossRef]
- Cao, Q.; Zou, H.; Chen, X.; Wen, S. Flotation selectivity of N-hexadecanoylglycine in the fluorapatite–dolomite system. Miner. Eng. 2019, 131, 353–362. [Google Scholar] [CrossRef]
- Gao, Z.; Jiang, Z.; Sun, W.; Pooley, S.G.; Wang, J.; Liu, Y.; Xu, F.; Wang, Q.; Zeng, L.; Wu, Y. New Role of the Conventional Foamer Sodium N-Lauroylsarcosinate as a Selective Collector for the Separation of Calcium Minerals. J. Mol. Liq. 2020, 318, 114031. [Google Scholar] [CrossRef]

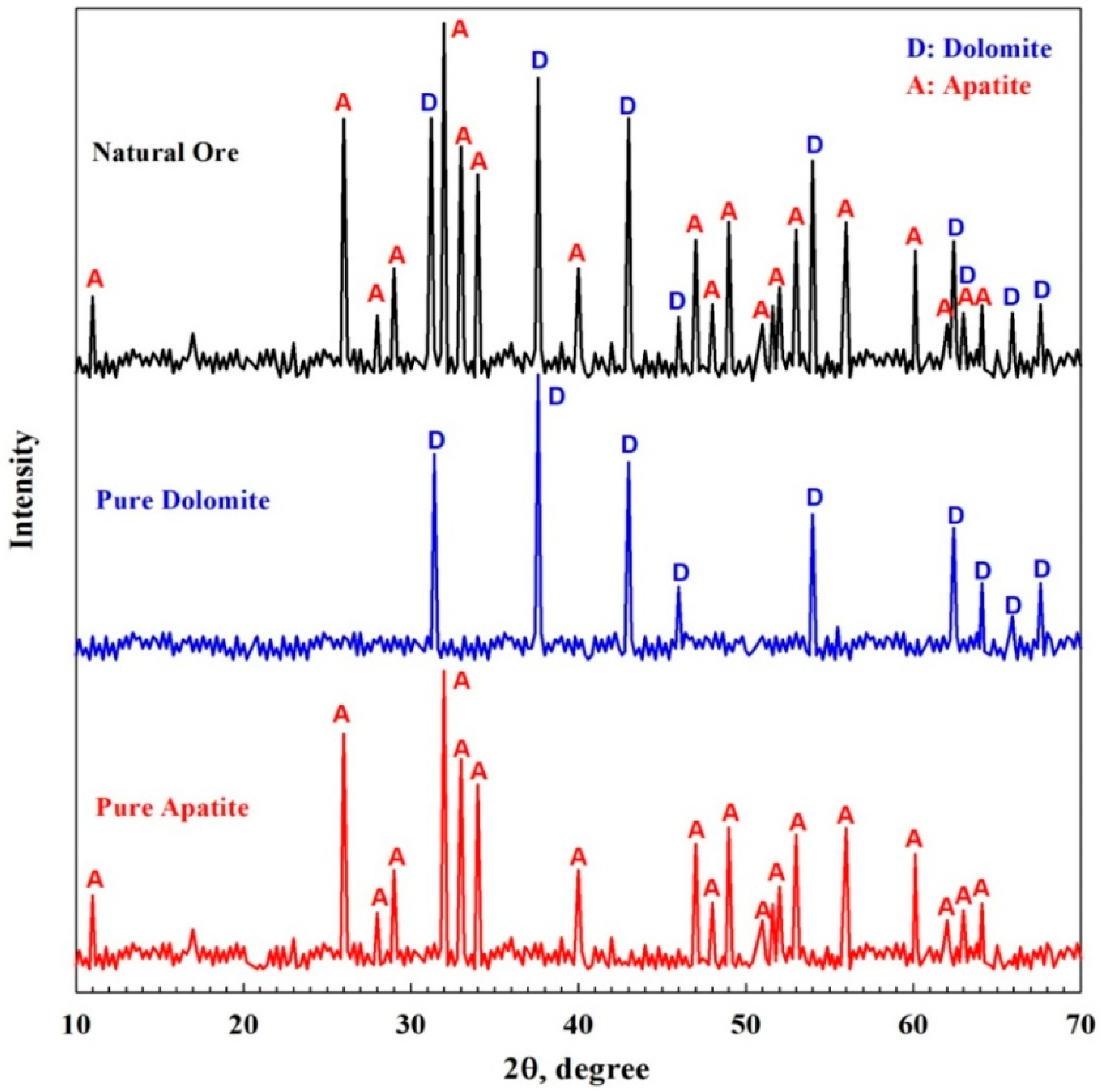
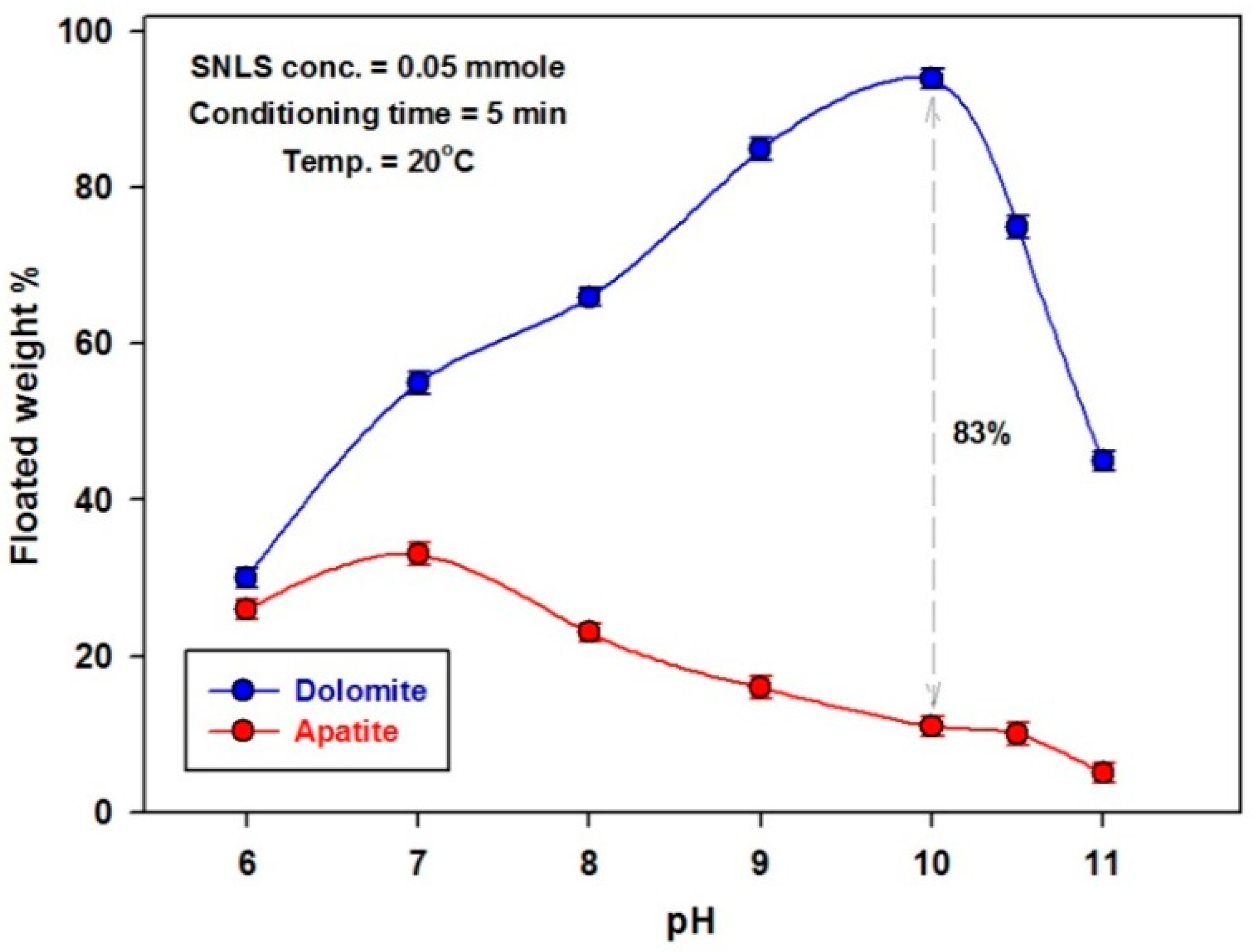
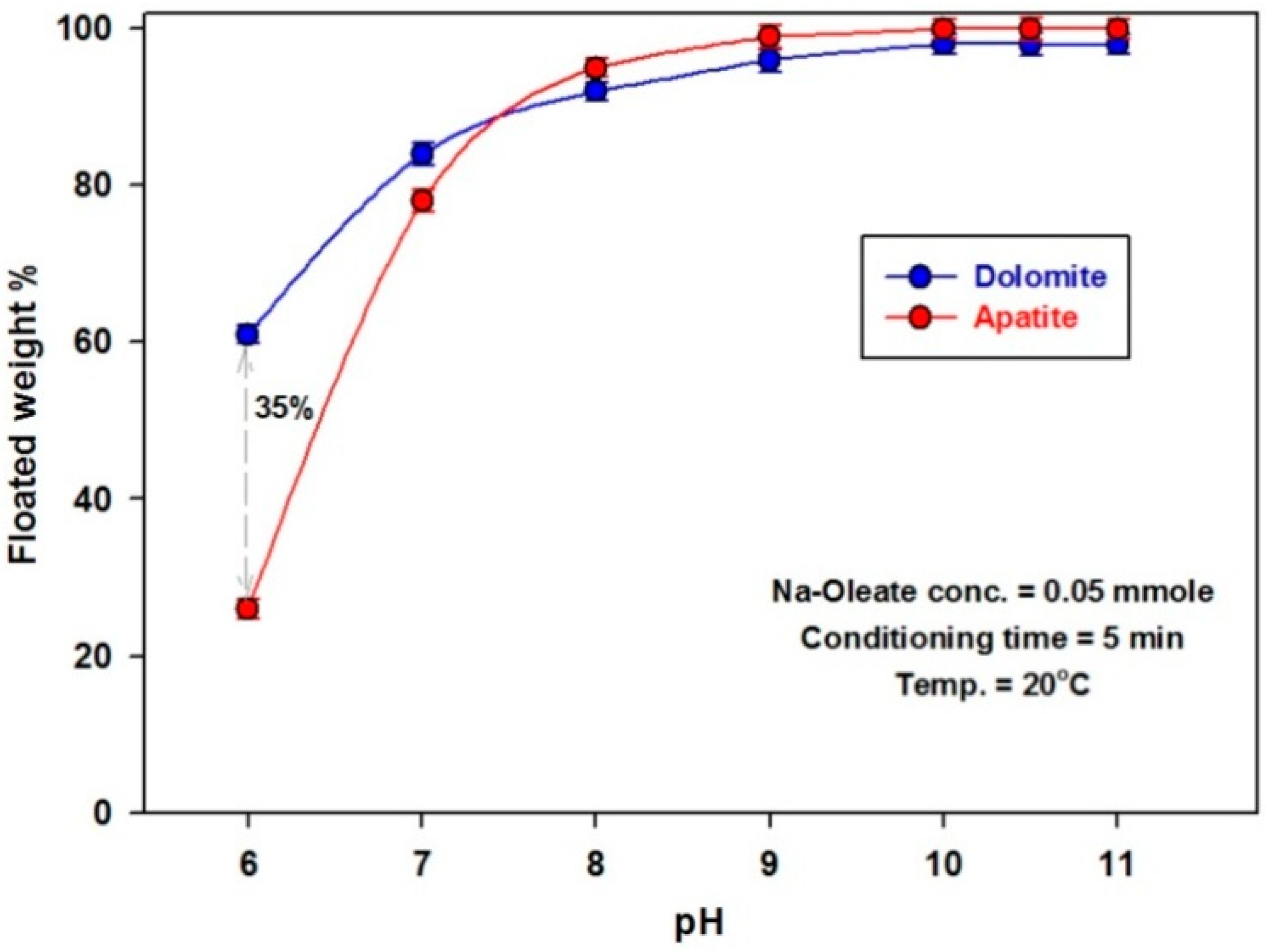
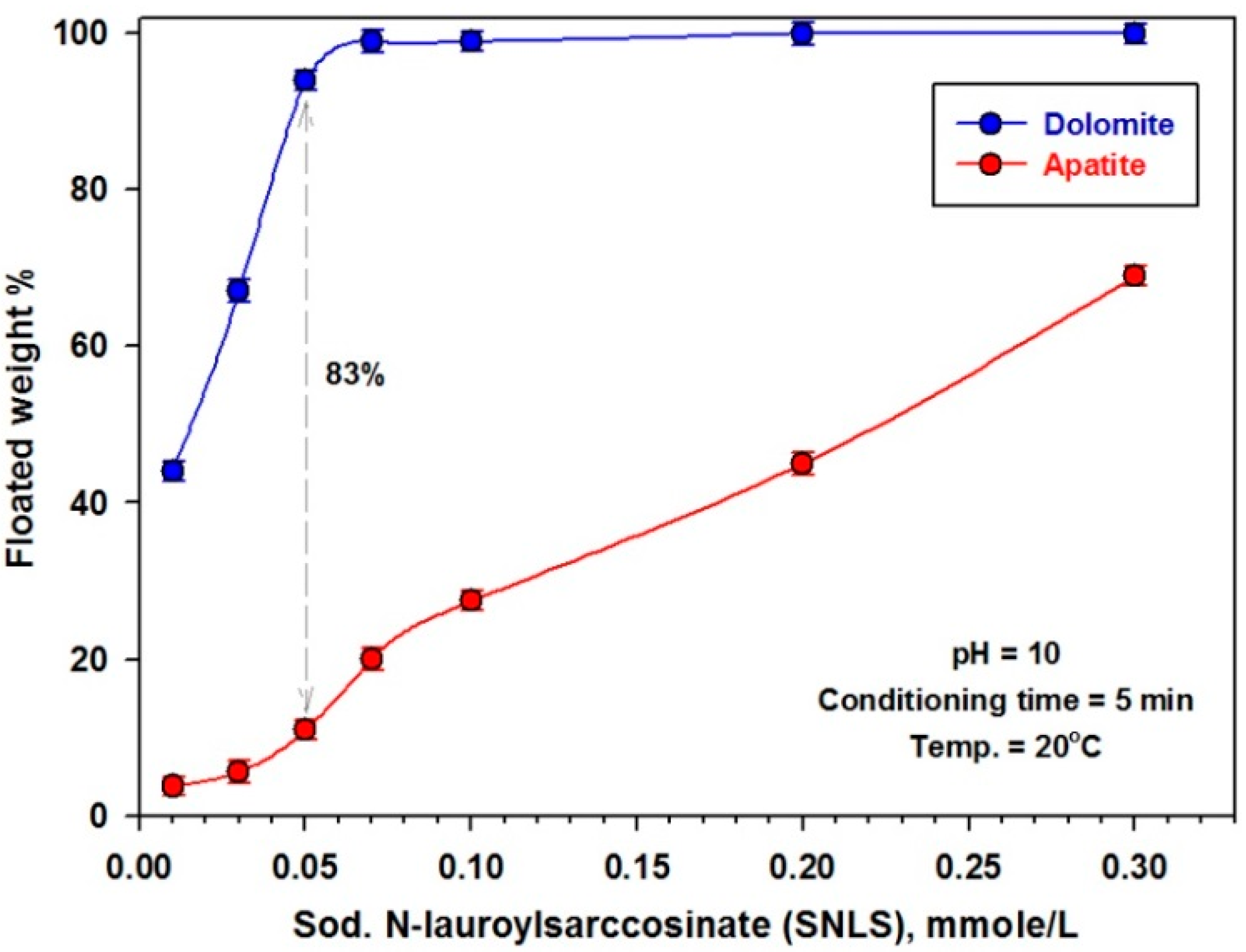

| Item | Weight % | ||
|---|---|---|---|
| Dolomite | Apatite | Natural Ore | |
| P2O5 | 0.002 | 34.58 | 25.41 |
| CaO | 30.37 | 56.97 | 50.36 |
| MgO | 21.69 | 0.005 | 5.48 |
| Fe2O3 | 0.016 | 0.001 | 0.013 |
| SiO2 | 0.012 | 0.003 | 0.378 |
| Al2O3 | 0.028 | 0.016 | 0.026 |
| K2O | 0.016 | 0.012 | 0.013 |
| Na2O | 0.015 | 0.018 | 0.021 |
| CO2 | 47.85 | 4.498 | 15.38 |
| F | 0.001 | 3.897 | 2.92 |
| Total | 100 | 100 | 100 |
| Mix | Feed (Mixture) | Concentrate | ||||||
|---|---|---|---|---|---|---|---|---|
| Weight (g) | % | Weight % | % | |||||
| Apatite | Dolomite | P2O5 | MgO | Float | Sink | Rec. | P2O5 | |
| 1 | 1.0 | 1.0 | 16.5 | 10.8 | 16 | 84 | 85.5 | 16.8 |
| 2 | 1.2 | 0.8 | 19.8 | 8.7 | 26 | 74 | 88.2 | 23.6 |
| 3 | 1.5 | 0.5 | 24.7 | 5.4 | 21 | 79 | 95.6 | 29.9 |
| SNLS (mmol/L) | Concentrate | |||
|---|---|---|---|---|
| Weight % | Rec. % | P2O5 % | MgO % | |
| 0.001 | 96 | 97.5 | 25.8 | 5.16 |
| 0.010 | 83 | 90.2 | 27.6 | 3.24 |
| 0.050 | 77 | 87.0 | 28.7 | 1.56 |
| 0.100 | 74 | 85.9 | 29.5 | 0.89 |
| 0.200 | 67 | 81.5 | 30.9 | 0.79 |
| Head | 100 | 100 | 25.4 | 5.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Halim, M.M.; Abdel Khalek, M.A.; Zheng, R.; Gao, Z. Sodium N-Lauroylsarcosinate (SNLS) as a Selective Collector for Calcareous Phosphate Beneficiation. Minerals 2022, 12, 829. https://doi.org/10.3390/min12070829
Abdel-Halim MM, Abdel Khalek MA, Zheng R, Gao Z. Sodium N-Lauroylsarcosinate (SNLS) as a Selective Collector for Calcareous Phosphate Beneficiation. Minerals. 2022; 12(7):829. https://doi.org/10.3390/min12070829
Chicago/Turabian StyleAbdel-Halim, Mohamed M., Mohamed A. Abdel Khalek, Renji Zheng, and Zhiyong Gao. 2022. "Sodium N-Lauroylsarcosinate (SNLS) as a Selective Collector for Calcareous Phosphate Beneficiation" Minerals 12, no. 7: 829. https://doi.org/10.3390/min12070829






