Abstract
Walnut is one of the world’s four largest nuts. Currently, the bottleneck in walnut breeding is the production of resistant variants. Soil salinization is a global problem, and the use of salt-tolerant rootstocks is a basic strategy to overcome the challenge of sustained walnut production. Providing a scientific basis for the selection of walnut salt-tolerant rootstocks is possible by studying the physiological and biochemical response characteristics and salt tolerance variations of different walnut genotypes under salt stress. In the present study, seedlings of four genotypes of walnut rootstocks, including J1 (Juglans hindsii), J2 (J. mandshurica), J3 (J. regia × J. mandshurica), and J4 (J. regia × J. hindsii), were employed as test materials to conduct a 28-day pot experiment under NaCl stress with five NaCl concentrations (0, 50, 100, 200, and 300 mmol/L). Under different NaCl treatment concentrations, seedling morphology, growth indices, chlorophyll content, photosynthetic parameters, relative electrical conductivity (REC), malondialdehyde (MDA), proline (Pro), soluble sugar (SS), and the activity of superoxide dismutase (SOD) and peroxidase (POD) in the leaves were examined. Salt stress altered the morphological characteristics and growth indices of seedlings from four genotypes to varying degrees. In addition, according to the analysis of physiological and biochemical data, salt stress had a considerable impact on both the physiological and biochemical processes of seedlings. Salt stress decreased the chlorophyll content and photosynthetic parameters of four genotypes, the REC, MDA content, Pro content, and SS content of each genotype increased by different degrees, and the enzymatic activities showed different trends. The salt tolerance of rootstocks was evaluated thoroughly using principal component analysis and membership function analysis based on the 16 parameters. The results of a comprehensive evaluation of salt tolerance showed that the order of salt tolerance of the four genotypes was J4 > J1 > J3 > J2, which corresponded to the order of the morphological symptoms of salt injury. In conclusion, J4 has strong salt tolerance and is an important germplasm resource for walnut salt-tolerant rootstock breeding.
1. Research Background
Soil salinization has become a global resource and environmental crisis, and it is one of the main factors restricting the development of agriculture and forestry [1]. According to statistics, the total area of all types of saline-alkali land in China is approximately 9.9 × 107 hm2, accounting for nearly 10.3% of the total land area in China, and involves mainly the northeast, northwest, inland, and coastal regions of North China [2]. In China, saline-alkali soil is an important land reserve resource. Renovating and rationally utilizing saline-alkali soil is critical for agricultural productivity and sustainable development of the ecological environment [3].
Sodium chloride (NaCl) is one of the most common and widely distributed salts in the soil; Cl− and Na+ in soil salt are lethal to plants and cause single salt toxicity [4,5]. Salt stress causes plant damage through a complex physiological and biochemical process. It is believed that salt stress causes damage to plants in three aspects: osmotic stress, ion toxicity, and nutritional imbalance [6]. Initially, plants are susceptible to osmotic stress, which inhibits water absorption by the roots, and then affects the transpiration rate and photosynthetic rate of plants, thereby, inhibiting plant growth and development. Under salt stress, the accumulation of Na+ and Cl− in plants can affect the osmotic pressure and acid-base balance of plant cells, destroy cell membrane structure, and lead to metabolic disorders, and the competition between salt ions and nutrient elements leads to a nutritional imbalance in plants, inhibiting normal plant growth and development [7,8,9]. Scholars, both at home and abroad, have conducted numerous studies on the response of plants to salt stress and screened salt-tolerant resources by determining the external morphological characteristics of plants, the activities of antioxidant enzymes, and the changes in osmotic substances under salt stress [4,10,11,12,13,14,15,16]. An economical and effective method for screening salt-tolerant or salt-resistant plant materials is studying the physiological and biochemical indices of plants under salt stress [17,18].
Walnut (Juglans regia L.) is a perennial deciduous angiosperm that has gained increasing popularity in recent years owing to its various values [19,20]. It is an important nut and woody oil tree species, and the walnut industry has grown to be a pillar industry in many parts of China, playing an essential role in poverty alleviation projects. It has exceptional edible value and medicinal properties. It is also widely used as an excellent economical timber species with broad development prospects [20,21]. Grafting is a popular method of walnut breeding. Rootstock is the core source of resistance to various stresses required to sustain the growth and development of the whole plant [18]. The foundation of high-quality and high-yield walnut increased rootstock, and research has shown that walnut rootstock has an important impact on the growth and development, stress resistance, fruit yield, and quality of its grafted varieties [22,23,24].
Walnut stress resistance encompasses both biotic and abiotic stress resistance. Currently, research on walnut abiotic stress resistance has focused specifically on cold resistance [25,26] and drought resistance [20,27]. Plain areas are more suitable for walnut planting; however, to avoid occupying the agricultural land, expand the walnut planting area moderately, and obtain more economic benefits, many difficult sites with better conditions, such as saline-alkali land, have also begun to develop the walnut industry by changing the land to suit trees or changing trees to suit the land. Therefore, studying and screening rootstock varieties with strong salt tolerance is critical for adjusting the agricultural industrial structure, promoting agricultural efficiency, increasing farmers’ income, and rural revitalization. However, only a few studies have explored the salt tolerance mechanism of walnut rootstocks. Therefore, the present study explored the growth, physiological, and biochemical reactions of four walnut genotypes under salt stress to determine their tolerance to salt stress, clarify the physiological and biochemical mechanisms of salt tolerance, and screen walnut rootstock resources with strong salt tolerance, providing a scientific basis for the cultivation and application of walnut in salinized areas.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
The experiment was conducted in the greenhouse of the Chinese Academy of Forestry, China (Latitude 40°0′10″ N, longitude 116°14′38″ E, altitude 61 m). Four walnut seed genotypes of J1 (J. hindsii), J2 (J. mandshurica), J3 (J. regia × J. mandshurica), and J4 (J. regia × J. hindsii) were tested. J1 (J. hindsii), a tree species introduced to China, is native to the United States and is an excellent rootstock for grafted seedlings of walnut and black walnut. J2 (J. mandshurica) is often used as a rootstock for walnut grafting in northern China. J3 (J. regia × J. mandshurica), a natural hybrid of walnut and J. mandshurica, demonstrates a strong growth potential, strong resistance to adversity, and beautiful texture of nuts; it is not only used as the breeding material for cold and disease resistance of walnut plants in northern China but also in the production of Wenwan Walnut. J4 (J. regia × J. hindsii), a hybrid of walnut and J. hindsii, possesses the characteristics of barren resistance, strong stress resistance, fast growth rate, and high grafting affinity. On 2 January 2021, seeds with uniform size and no pests and diseases were selected and placed in 60-L buckets, to which clean water was added (the water level was kept higher than the level of seeds in the bucket). The seeds were soaked for 7 days, and the water was changed once a day. After the water was fully absorbed, the seeds were stored in the sand and stratified to accelerate the germination process. On 9 April 2021, the seeds that had been stored in the sand for 3 months were sown in plastic pots (18-cm diameter, 25-cm height, each containing one seed). The seeds were cultured in the greenhouse at an average temperature of 25 °C and not higher than 30 °C during the day and not lower than 14 °C at night, with the transmittance being 50–60% and the average relative humidity being 55–85%. The substrates used for culturing were peat soil, vermiculite, and perlite (3:1:1 v/v). On 22 April, the seeds began to emerge, and the seedlings were maintained and managed normally during the seedling growth period.
In late June, the seedlings were cultivated for approximately 2 months. The average plant height of each seedling was 45 cm, the average ground diameter was 6.4 mm, and the average number of compound leaves was 6. Healthy seedlings with consistent growth were selected for 28 days (1–28 July) under NaCl stress. A total of 5 salt gradients (0, 50, 100, 200, and 300 mmol/L) were set. Each gradient was divided into 3 groups of 6 plants each, and a total of 90 plants were selected for each genotype. The NaCl solution of each gradient was applied to all walnut genotypes during the morning (8:00–10:00 AM) on the same day. Plants were irrigated once a week with 300 mL applied per pot for a total of four times. To prevent leakage of the salt solution, a suitable-sized tray was placed at the bottom of the pot and the salt solution was returned from the tray to the pot. Routine management of the seedlings was performed during the experiment. After NaCl treatment, some seedlings were randomly selected from each group for biomass determination, and the 1–2 pairs of functional leaves at the middle and upper compound leaves of the other part of the seedlings were collected to measure the relevant physiological and biochemical indices.
2.2. Determination of Growth Indicators
Before salt stress treatment, 3 seedlings showing consistent growth were randomly selected from each group and their seedling height H0 (measured with a tape measure, cm) and ground diameter D0 (measured with a vernier caliper, mm) were recorded. After 28 days of salt stress, the seedling height H1 and ground diameter D1 were measured again, and the increment in seedling height H∆ = H1 − H0 and ground diameter D∆ = D1 − D0 were calculated. After the treatment, the seedlings were removed from each treatment group pot and rinsed with tap water; surface water was wiped with an absorbent paper, and plant fresh weight (PFW) was measured on an electronic balance. The plants were then divided into aboveground and underground portions and placed in cowhide bags and then into an oven at 105 °C for 30 min; the oven temperature was set to 75 °C for drying to a constant weight, removed, and weighed again to determine the dry weight, followed by the use of the below calculation formula: plant dry weight (PDW) = dry weight of shoot (DWS) + dry weight of root (DWR). Additionally, morphological changes in the leaves were recorded at the end of the salt stress period.
2.3. Determination of Physiological and Biochemical Indicators
2.3.1. Determination of the Relative Electrical Conductivity
Relative electrical conductivity (REC) was measured using a DDS-11C conductivity meter and calculated using the method described by Ghalati et al. [28]. Briefly, approximately 0.1 g of fresh leaf sample was incubated in a 100 mL of water bath (40 °C, 30 min), and the electrical conductivity of the samples was measured (R1). After heating the samples in boiling water for 15 min, the electrical conductivity of the samples (R2) was measured again. The REC was used to represent the cell membrane permeability. The calculation formula used was as follows:
REC (%) = R1/R2 × 100
2.3.2. Determination of the Chlorophyll Content
The pigments of leaves were extracted with 95% (v/v) ethanol and assessed according to the method of Zhu et al. [18]. The chlorophyll a (Chl a) and chlorophyll b (Chl b) contents in with the extracts were determined at the maximum absorption peaks of 665 nm and 649 nm, respectively. The contents of Chl a, Chl b, and TChl were calculated as follows:
Chl a (mg/L) = 13.95A665 − 6.88A649
Chl b (mg/L) = 24.96 A649 − 7.32 A665
TChl (mg/L) = Chl a + Chl b
2.3.3. Determination of Photosynthetic Parameters
The first and second pairs of functional leaves of the middle and upper compound leaves were selected, and the photosynthetic indices such as net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci) were measured using the Li-6400 photosynthetic apparatus. The measurement time a was 09:00–11:00 AM on a sunny day, the CO2 concentration was 400 μmol·mol−1, and the measured light intensity was 1200 μmol·m−2·s−1. A standard blade chamber and open-air path were adopted, and the flow rate was set to 500 μmol·s−1.
2.3.4. Determination of the Malondialdehyde Content
The malondialdehyde (MDA) content was determined according to the thiobarbituric acid (TBA)-based colorimetric method [29]. Briefly, fresh leaves were collected and extracted in 5 mL of 5% (w/v) trichloroacetic acid (TCA). The supernatant was collected by centrifugation at 10,000 rpm at 4 °C for 10 min, then add 2 mL TBA to the supernatant and incubated at 100 °C for 30 min. The tubes were incubated in an ice bath for 10 min to stop the reaction, and the absorbance of the supernatant was read at 450, 532, and 600 nm. The MDA content (μmol/g FW) was calculated as follows:
where A532, A600, and A450 represent the absorbance of the supernatant at 532, 600, and 450 nm, respectively; V represents the volume of extraction (mL), V1 represents the volume of extracted liquid reacting with TBA, V2 represents the total volume of extract and the TBA reaction solution, and m represents the fresh weight of the samples (g).
[6.45 × (A532 − A600) − 0.56 × A450] × V2 × V/(m × V1 × 1000)
2.3.5. Determination of the Osmotic Adjustment Substances
Determination of the Proline Content
The content of proline (Pro) was measured using the ninhydrin reaction method [30]. Briefly, 0.1 g of fresh leaves were homogenized in 2.5 mL of 3% sulfosalicylic acid solution, and the homogenate was centrifuged at 10,000 rpm for 5 min. The extracted solution (2 mL) was treated with 2 mL of acid ninhydrin and 2 mL of glacial acetic acid and heated for 1 h at 100 °C. Then, 4 mL of methylbenzene was added to the solution to extract the mixture. The absorbance of the chromophore containing toluene was recorded at 520 nm.
Determination of the Soluble Sugar Content
The soluble sugar (SS) content was determined using the anthrone-sulfuric acid method as described by Liu et al. [6] and Kong et al. [31], with slight modifications. Frozen leaf material was extracted with 5 mL of distilled water at 100 °C for 30 min, after which the supernatant was collected. This step was repeated twice, and the supernatant was collected. Then, 0.5 mL of the extract was mixed with 1.5 mL of distilled water, 0.5 mL of anthrone reagent (1 g anthrone and 50 mL ethylacetate), and 5 mL of concentrated sulfuric acid; the mix was immediately placed in a boiling water bath for 1 min. After cooling, the SS content was analyzed through UV spectrophotometry at 630 nm.
2.3.6. Determination of the Activity of Antioxidant Enzymes
The extraction of antioxidant enzymes was assayed by the method of Khalid et al. [32] with some modifications. Briefly, 0.3 g of each sample was taken and homogenized in 3 mL of sodium phosphate buffer (pH 7.8) with a chilled mortar and pestle, followed by centrifugation at 10,000 rpm at 4 °C for 10 min to obtain the supernatant as a crude enzyme extract. The supernatant was collected and used to determine the superoxide dismutase (SOD) and peroxidase (POD) activities.
SOD activity was determined according to Liu et al. [6] and Khalid et al. [32], with slight modifications. The activity of SOD was determined by monitoring the decrease in absorbance (560 nm) of the nitrobluetetrazolium (NBT) by the enzyme. The SOD activity was calculated as U/g FW (unit of enzyme activity per gram of fresh weight). The reaction mixture contained 50 mM phosphate buffer (pH 7.8), 13 mM methionine, 75 μM NBT, 10 μM ethylene diaminetetraacetic acid (EDTA)-Na2, 2 μM riboflavin, and distilled water (15:3:3:3:3:2.5 v/v). Approximately 3 mL of the reaction solution was taken in test tubes in duplicate, to one tube, 0.05 mL of the enzyme extract was added, and to the other tube, the enzyme extract was replaced with the same amount of phosphate buffer, serving as the blank control group. The tubes were placed under 4000 Lx fluorescent lamps for 30 min. At the end of the reaction, the lamps were turned off and the tubes were incubated in the dark for analyses.
POD activity was determined using the guaiacol method [15,33]. Briefly, 28 μL guaiacol was added to 50 mL PBS (0.2 M, pH 6.0), and the mixture was heated and stirred until complete dissolution. After cooling, 19 μL (30%) of H2O2 was added to the mixture. The mixture was then stored in a refrigerator for further use. Then, 3 mL of the solution was taken and mixed with 40 μL of the enzyme solution. PBS served as the blank, and the absorbance of the solutions was measured at 470 nm. One unit of POD activity was expressed as the change of absorbance per min and was calculated as U·g−1·min−1 FW.
2.4. Statistical Analysis
The original data were analyzed by Microsoft Excel 2010, and the data were imported into SPSS 23.0 software for ANOVA and Duncan multiple comparisons. Two-way analyses of variance (ANOVA) were performed to detect the effects of genotype and salinity, and their interactions. All statistical effects were considered significant at p < 0.05, and the index change map was analyzed and plotted by Origin 2018.
The measured indices were analyzed and screened by principal component analysis, and the data were comprehensively analyzed using the fuzzy mathematical membership function method to evaluate the salt tolerance of the four walnut genotypes.
The calculation method of membership function value was as follows: R(Xi) = (Xi − Xmin)/(Xmax − Xmin), and inverse membership function value: R(Xi) = 1 − (Xi − Xmin)/(Xmax − Xmin), where Xi is the measured value of the index, and Xmin and Xmax are the minimum and maximum values of a certain index of all tested materials, respectively. Salt tolerance was determined by comparing the average values of the membership function values of all the measured indices of each genotype; the higher the average value, the stronger is the salt tolerance.
3. Results
3.1. Effects of Salt Stress on External Morphological Characteristics of Seedlings
The damage symptoms of four walnut genotypes appeared gradually with increasing salt concentration, however, significant differences were noted across genotypes (Figure 1, Table 1). At the NaCl concentration of 50 mmol/L, symptoms such as yellowing of the plants’ lower leaves and withered and curled leaf margins of the upper leaves emerged, and the yellowing, withering and curling of J2 was more severe. The symptoms of yellowing, withering, and curling of leaves of all genotypes were exacerbated after treatment with 100 mmol/L NaCl, with the symptoms of J2 being most severe. At the NaCl concentration of 200 mmol/L, the leaves of J2 and J3 were severely withered and curled, and deciduous leaves appeared; the lower leaves of J1 and J4 were yellowed, the middle and upper leaves were withered and curled, and a small number of leaves fallen off. When the NaCl treatment concentration reached 300 mmol/L, a large number of J2 leaves withered, curled, and fell off, whereas the number of withered and curled leaves in the middle and upper parts of J4 increased, and a small number of leaves fell off. During the period of NaCl stress, the salt stress symptoms of J4 appeared the latest compared with the other three genotypes. Therefore, the salt tolerance of the four genotypes can be preliminarily evaluated from the phenotypic symptoms as follows: J4 > J1 > J3 > J2.

Figure 1.
Comparison of the morphological performance of walnut rootstocks under saline irrigation treatment. Four walnut genotypes were grown under different NaCl concentrations (0, 50, 100, 200, and 300 mmol/L) for 28 days. Salt tolerance capacity was estimated after 28 days. Scale bar: 15 cm.

Table 1.
Morphological characteristics of 4 different walnut genotypes under NaCl stress.
3.2. Effect of Salt Stress on Plant Growth
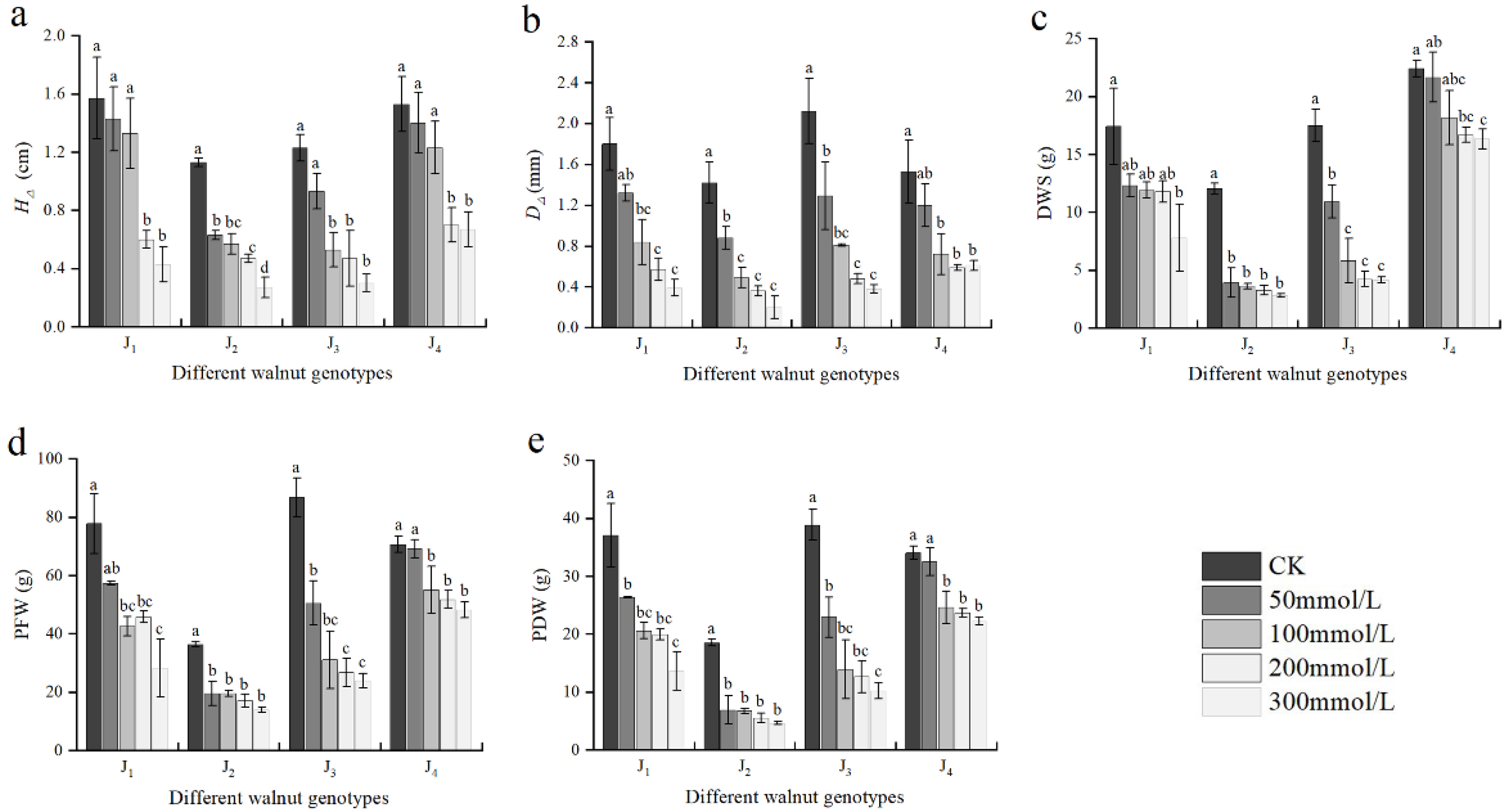
As shown in Figure 2, the growth indices differed remarkably between the genotypes under salinity stress conditions. With the increase in NaCl concentration, the H∆, D∆, DWS, PFW, and PDW of the four genotypes exhibited a declining trend. At 50 mmol/L, the aforementioned growth indices of J2 significantly decreased compared with those of the control, but did not differ significantly from those of the control in J4. At -100 mmol/L, the aforementioned indices of were significantly different from those of the control, and the D∆, PFW, and PDW were significantly different from those of the control in J1 and J4. The growth indices fell to a larger extent as the NaCl concentration increased. When the NaCl concentration was 300 mmol/L, the H∆, D∆, DWS, PFW, and PDW of J1 decreased by 72.61%, 78.33%, 55.31%, 63.73%, and 63.26%, respectively, compared with those of the control. These growth indices of J2 decreased by 76.11%, 85.92%, 76.53%, 61.51%, and 74.99%, respectively. Compared with those of the control, the H∆, D∆ DWS, PFW, and PDW of J3 decreased by 75.61%, 82.08%, 76.18%, 72.39%, and 73.85%, respectively, and the growth indices of J4 decreased by 56.21%, 60.13%, 27.13%, 31.84%, and 34.65% respectively. Under high salt stress, the seedling growth of four walnut genotypes was seriously inhibited, among which J2 and J3 were seriously inhibited. Furthermore, the PFW and PDW were significantly affected by the interaction of genotype × salinity (Table 2).
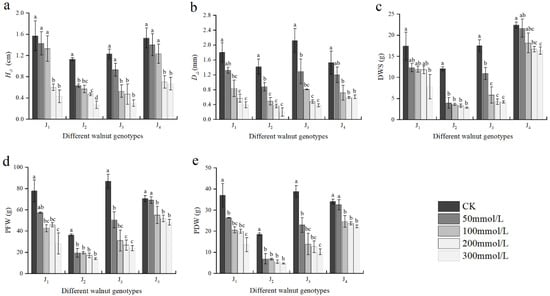
Figure 2.
Effect of salt stress on the growth of walnut. (a) Seedling height increment (H∆), (b) ground diameter increment (D∆), (c) dry weight of shoot (DWS), (d) plant fresh weight (PFW), and (e) plant dry weight (PDW). Four walnut genotypes were grown under different NaCl concentrations (0, 50, 100, 200, and 300 mmol/L) for 28 days. Each value indicates the mean ± standard error (SE) of three biological replicates. Bars of each parameter labeled with different letters are significantly different (p < 0.05) by Duncan’s test.

Table 2.
Main and interactive effects of genotype and salinity on growth, and physiological and biochemical parameters of walnut.
3.3. Effect of Salt Stress on Chlorophyll Content
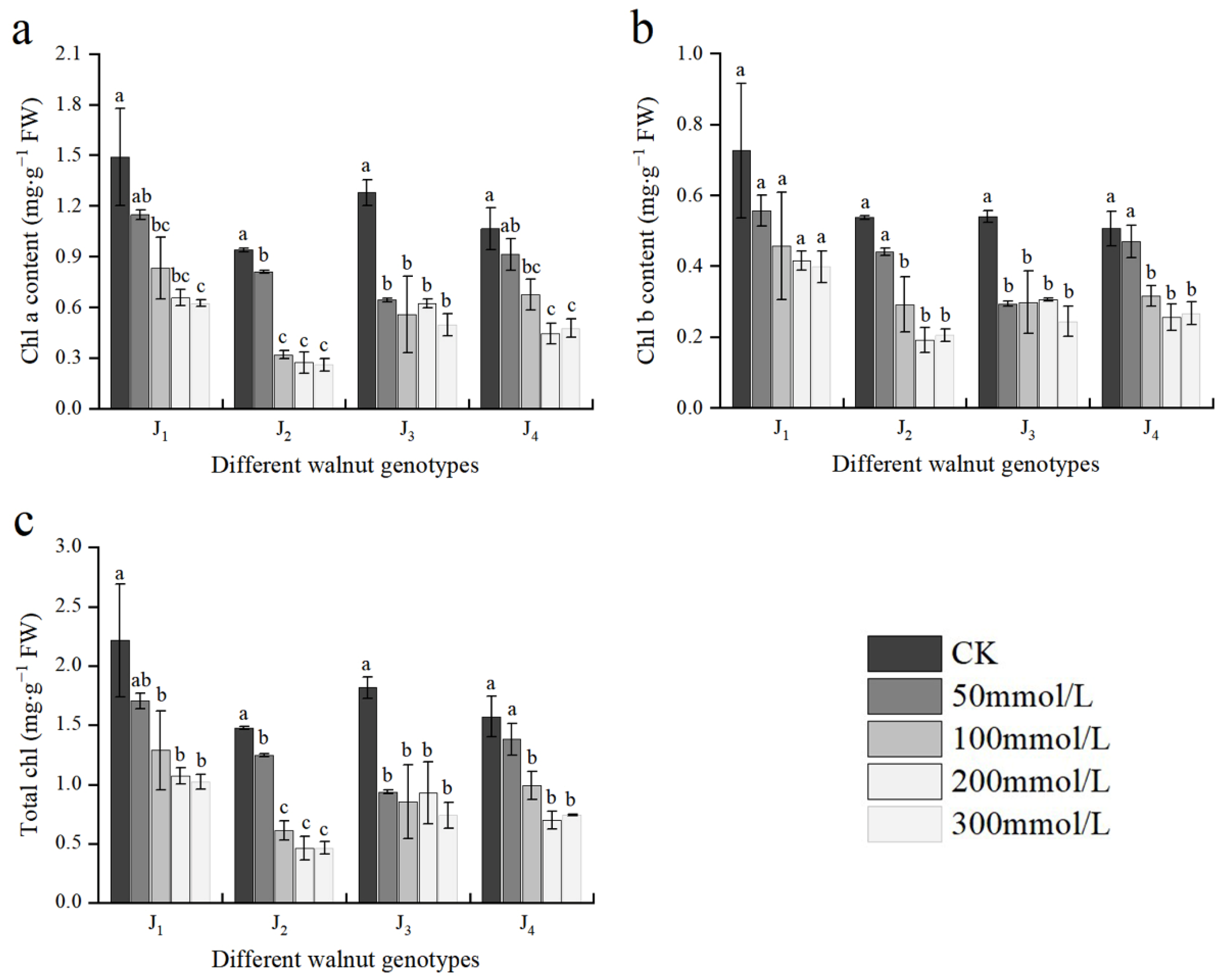
As shown in Figure 3, Chl a, Chl b, and TChl contents of all four genotypes declined with increasing salt concentration. The Chl a, Chl b, and TChl contents in J1, J2, and J4 did not differ significantly from those of the control at 50 mmol/L concentration; however, at the 100–300 mmol/L concentration range, the aforementioned indices of each treatment were different from those of the control. At 300 mmol/L, compared with the control, the contents of Chl a, Chl b, and TChl in J1 decreased by 58.12%, 45.18%, and 53.81%, respectively; the contents of Chl a, Chl b, and TChl in J2 decreased by 72.42%, 61.82%, and 68.43%, respectively; the contents of the corresponding indices of J3 decreased by 61.19%, 54.81%, and 59.30%, respectively; and the corresponding indices of J4 decreased by 55.40%, 47.23%, and 52.71%, respectively. The decrease in each index in J2 and J3 was significantly higher than that in the other two genotypes.
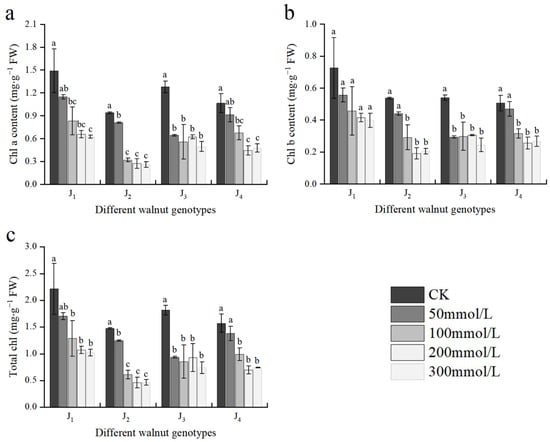
Figure 3.
Effect of salt stress on the chlorophyll content of walnut. (a) Chlorophyll a content (Chl a), (b) chlorophyll b content (Chl b), and (c) total chlorophyll content (TChl) in the leaves. Four walnut genotypes were grown under different NaCl concentrations (0, 50, 100, 200, and 300 mmol/L) for 28 days. Each value indicates the mean ± standard error (SE) of three biological replicates. Bars of each parameter labeled with different letters are significantly different (p < 0.05) by Duncan’s test.
3.4. Effects of Salt Stress on Photosynthetic Gas Exchange Parameters
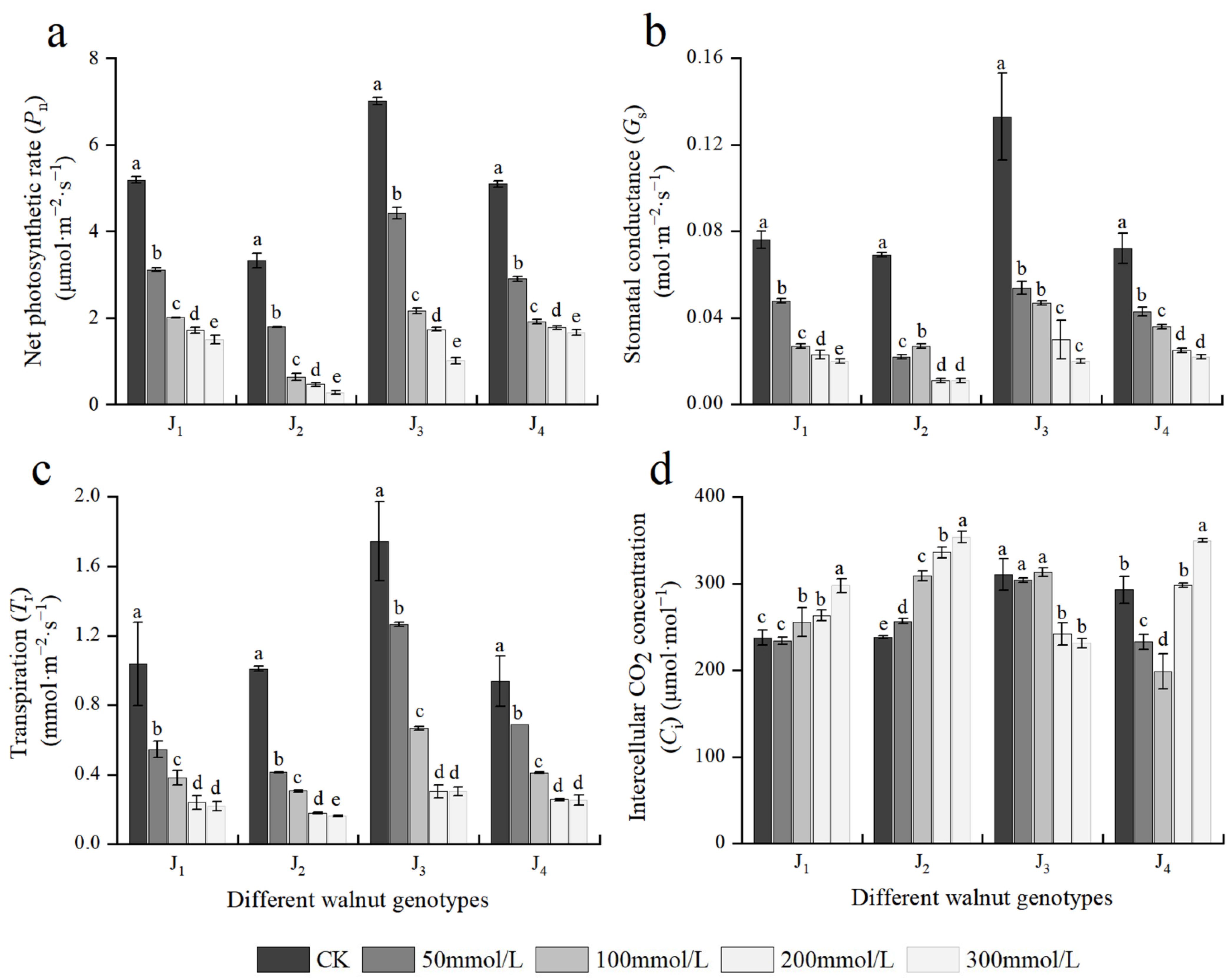
Photosynthesis is a metabolic process in plants that serve as the foundation for plant growth and development and a source of material and energy for plant growth. Figure 4 depicts the effects of different concentrations of salt stress on the photosynthetic parameters of the four walnut genotypes. The Pn, Gs, and Tr of the four genotype seedlings showed a decreasing trend with increasing NaCl concentration, and NaCl treatment with different concentrations showed significant differences. When the NaCl concentration was 50 mmol/L, the aforementioned indices of the four genotypes decreased rapidly, with J2 exhibiting the highest reduction, and Pn, Gs, and Tr decreased by 46.05%, 68.12%, and 58.95%, respectively, compared with the control. At 300 mmol/L, the aforementioned indices of the four genotypes decreased to the lowest; compared with the those of the control, the Pn, Gs, and Tr of J1 decreased by 71.07%, 73.68%, and 78.88%, respectively; the Pn, Gs, and Tr of J2 decreased by 91.70%, 84.06%, and 83.98%, respectively; the corresponding indices of J3 decreased by 85.57%, 84.96%, and 82.62%, respectively; and the corresponding indices of J4 decreased by 67.43%, 69.44%, and 72.95%, respectively. The decline in the aforementioned indices of J2 and J3 was higher than that in the other two genotypes. Under salt stress, when the NaCl mass concentration was 100–300 mmol/L, the Pn of J1 and J4 changed slowly at a lower level compared with J2 and J3.
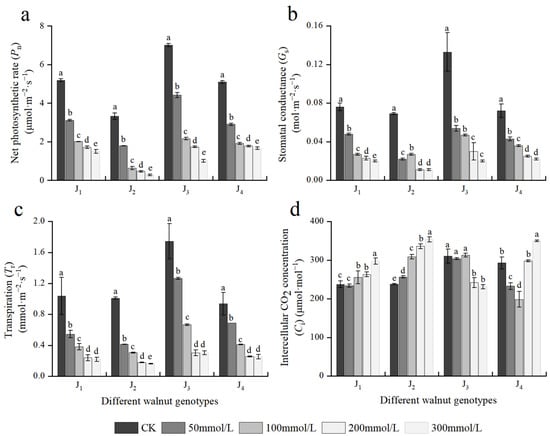
Figure 4.
Effect of salt stress on the photosynthetic parameters of walnut. (a) Net photosynthetic rate (Pn), (b) stomatal conductance (Gs), (c) transpiration (Tr), and (d) intercellular CO2 concentration (Ci) in the leaves. Four walnut genotypes were grown under different NaCl concentrations (0, 50, 100, 200, and 300 mmol/L) for 28 days. Each value indicates the mean ± standard error (SE) of three biological replicates. Bars of each parameter labeled with different letters are significantly different (p < 0.05) by Duncan’s test.
The Ci of four walnut genotypes showed different trends with increasing salt concentration. With the increase in salt concentration, the Ci of J1 and J2 increased, while that of J3 declined, and the Ci of J4 first decreased and then increased. The results showed that the main limiting factors for the decreased Pn caused by salt stress varied across four walnut genotypes. Furthermore, Pn, Gs, Tr, and Ci were significantly affected by the genotype × salinity interaction (Table 2).
3.5. Effects of Salt Stress on MDA Content and REC
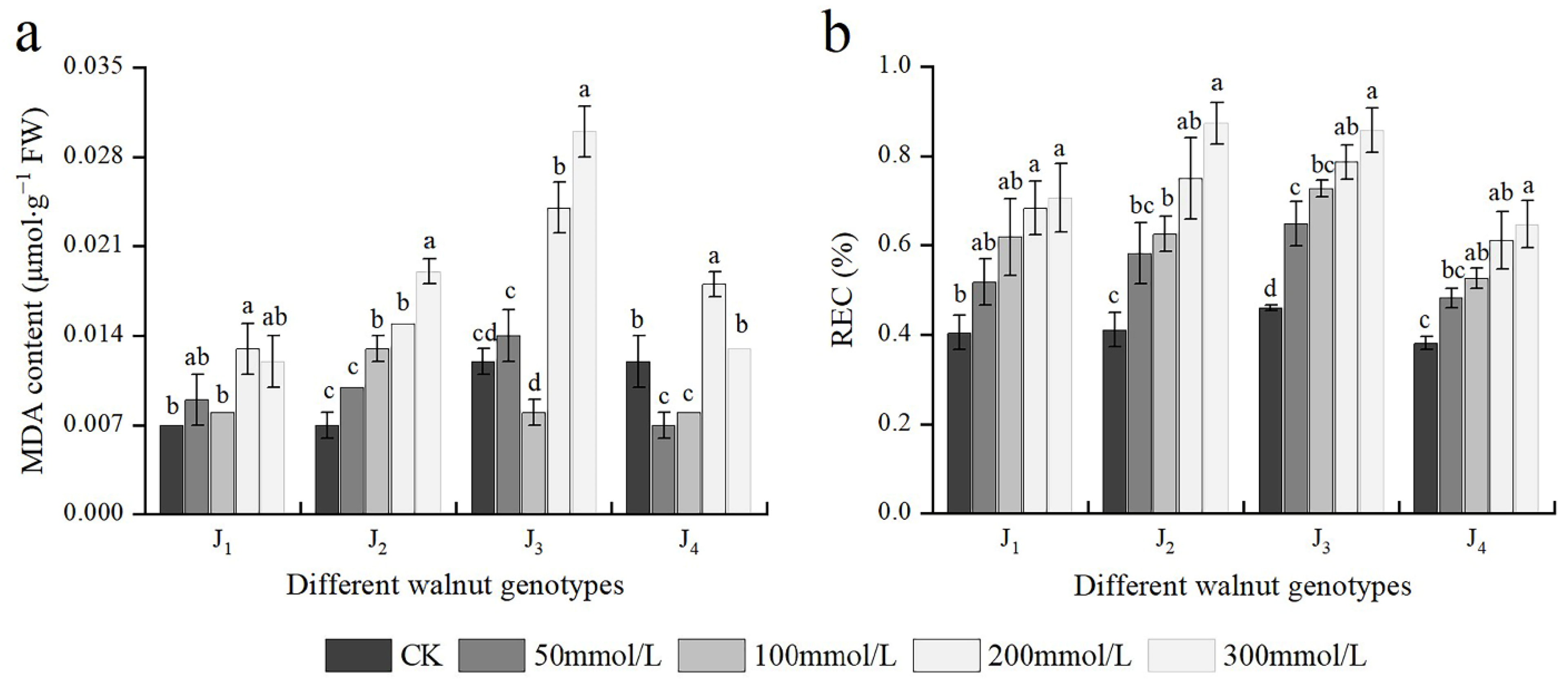
At 200 mmol/L, the MDA content of J1, J3, and J4 was significantly higher than that of the control; whereas at 100 mmol/L, the MDA content of J2 was considerably higher than that of the control. At 200–300 mmol/L, the MDA content of J2 and J3 was higher and increased (Figure 5a). In addition, the MDA content was significantly affected by the interaction of genotype × salinity (Table 2). With an increase in the NaCl concentration, REC of different genotypes also increased, with the increase of J2 and J3 being the maximum. Under 300 mmol/L NaCl stress, compared with the control, the REC of the four genotypes increased by 1.73, 2.12, 1.87, and 1.71 times, respectively (Figure 5b). The results showed that salt stress damaged the cell membrane of four walnut genotypes to a certain extent, with J1 and J4 showing reduced damage compared with the other two genotypes.
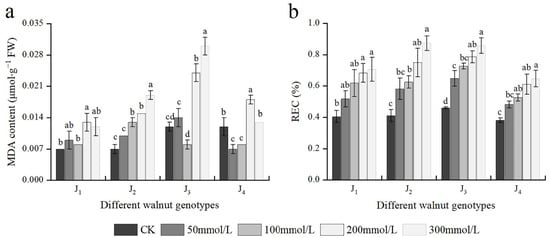
Figure 5.
Effect of salt stress on (a) the malondialdehyde (MDA) content, (b) relative electrical conductivity (REC) of walnut. Four walnut genotypes were grown under different NaCl concentrations (0, 50, 100, 200, and 300 mmol/L) for 28 d. Each value indicates the mean ± standard error (SE) of three biological replicates. Bars of each parameter labeled with different letters are significantly different (p < 0.05) by Duncan’s test.
3.6. Effects of Salt Stress on Osmotic Adjustment Substances
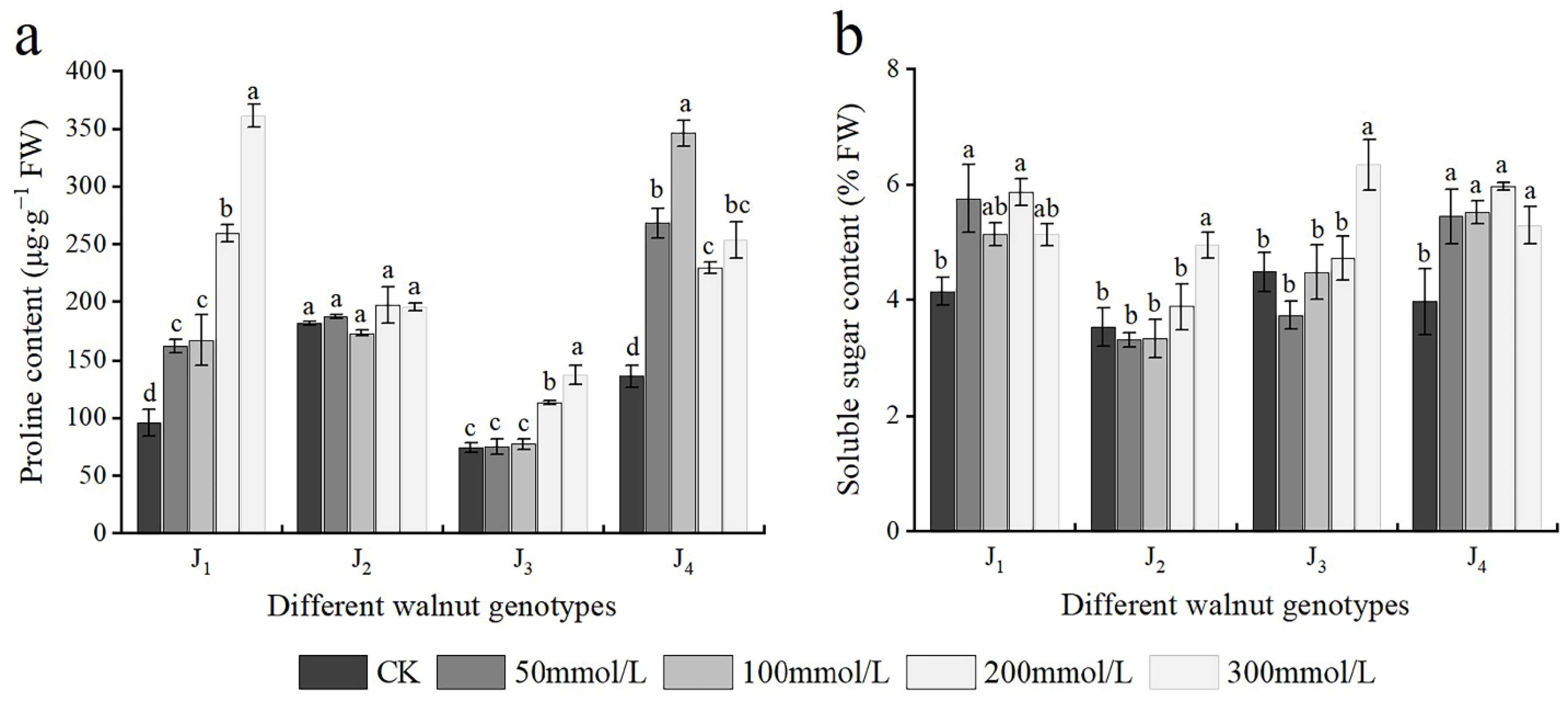
As shown in Figure 6a, the Pro content of J1 increased with increasing salt concentration, and significant differences in the Pro content were noted across treatments. The Pro content of J2 basically showed an upward trend; however, no significant difference was noted in the J2 Pro content across treatments. The Pro content of J3 also increased, and when the NaCl concentration reached 200 mmol/L, it differed considerably from that of the control. The Pro content of J4 first increased and subsequently declined, at the 0–100 mmol/L concentration range, the Pro content of J4 increased with the increase in salt concentration, whereas with further increase in the NaCl concentration, the Pro content decreased, and NaCl treatment with different concentrations showed significant differences.
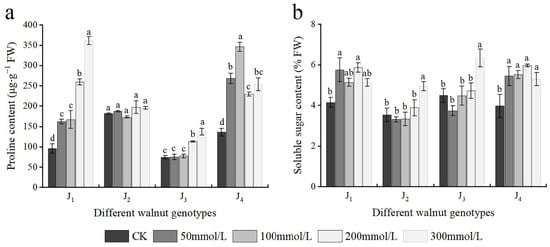
Figure 6.
Effect of salt stress on the osmotic adjustment substances of walnut. (a) Proline (Pro) content, (b) soluble sugar (SS) content in the leaves. Four walnut genotypes were grown under different NaCl concentrations (0, 50, 100, 200, and 300 mmol/L) for 28 days. Each value indicates the mean ± standard error (SE) of three biological replicates. Bars of each parameter labeled by different letters are significantly different (p < 0.05) by Duncan’s test.
The SS content of J1 and J4 first increased and subsequently decreased with increasing salt concentration. The SS content of J1 and J4 increased significantly at 50 mmol/L, and with further increase in the NaCl concentration, the SS content continued increasing; however, at 300 mmol/L concentration, the SS content decreased. Overall, the SS content of J2 and J3 exhibited an increasing trend. At the 0–200 mmol/L concentration range, the SS content of J2 and J3 increased slowly with the increase of salt concentration, whereas at 300 mmol/L, the SS content reached the maximum and was significantly higher than that of the control treatment; the SS content of J2 and J3 increased by 40.00% and 41.50%, respectively, compared with that of the control (Figure 6b). In addition, Pro and SS were significantly affected by the interaction of genotype × salinity (Table 2).
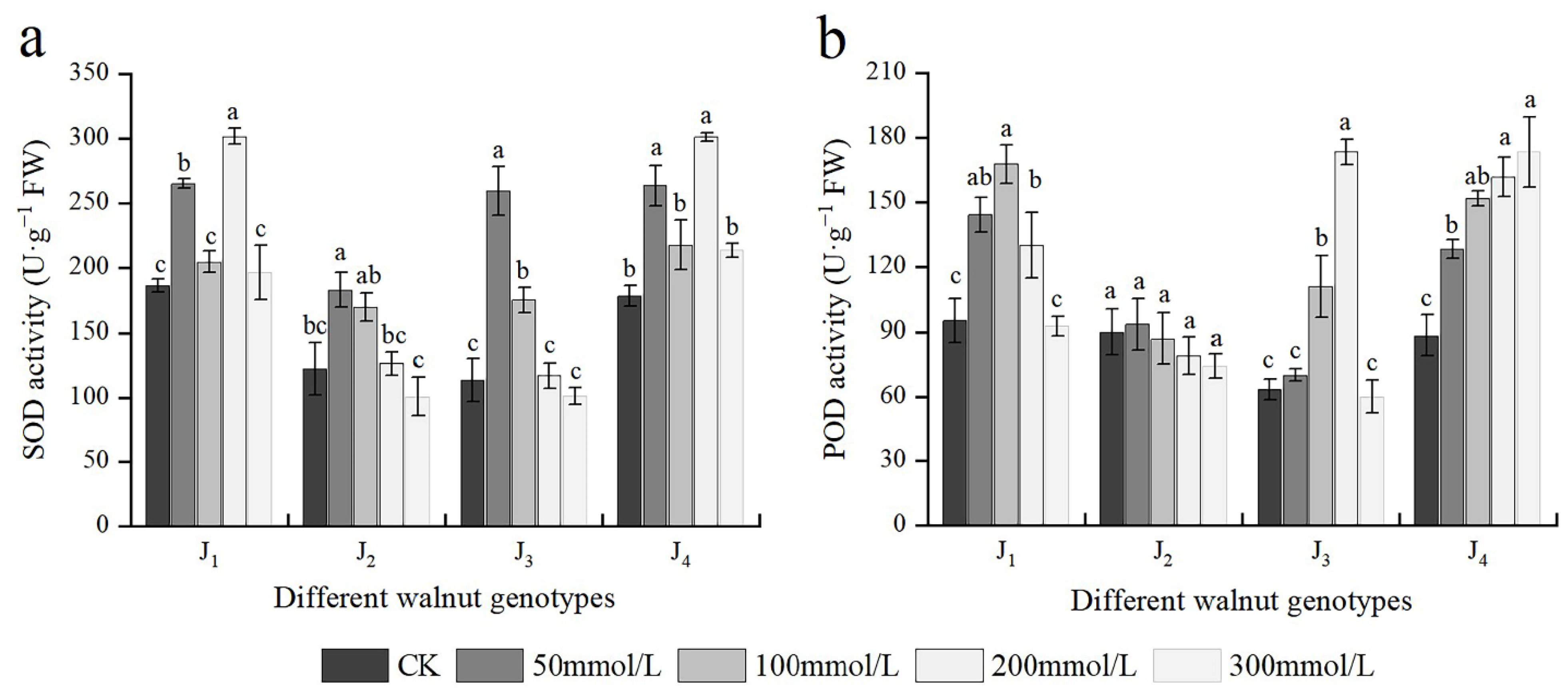
3.7. Effects of Salt Stress on Antioxidant Enzyme Activities
The SOD activity of four walnut genotypes basically first increased and then decreased under salt stress with different concentrations of salt; however, the extent of increase or decrease varied across different treatments and genotypes. The SOD activity in J1 and J4 increased progressively in response to increasing NaCl concentrations from 0 to 200 mmol/L, with plants exposed to 200 mmol/L showing the highest increase, and the SOD activity of J1 and J4 increased by 62.18% and 69.35%, respectively, compared with the control. J2 and J3 exhibited the maximum SOD activity at 50 mmol/L, which was significantly higher than the control. The SOD activity of J2 and J3 decreased again with continuous increasing salt concentration, and at 300 mmol/L, the SOD activity was lower than the control, although the difference was not significant. Under varying salt concentrations, the SOD activity of J1 and J4 was higher than that of the other two genotypes (Figure 7a). The four genotypes’ POD activity changed to different extents under different salt treatments. The POD activity of J4 increased with increasing salt concentration, peaking at 300 mmol/L, which was significantly higher than the control. J1, J2, and J3 increased initially and then declined with increasing salt concentration, reaching a maximum at 100, 50, and 200 mmol/L, respectively; However, at 300 mmol/L, the POD activity decreased to the control level, and the difference was not significant compared with the control. The fluctuation in POD activity of J2 was minimal among all treatments, and there was no significant difference between treatments, and the POD activity of J2 at each concentration was lower than that of the other three genotypes (Figure 7b). Furthermore, POD and SOD were significantly affected by the genotype × salinity interaction (Table 2).
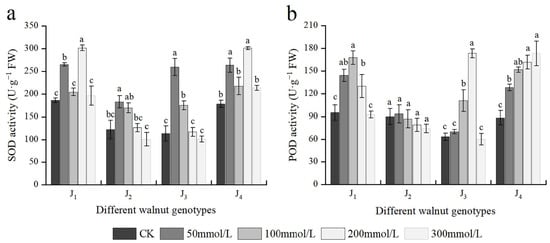
Figure 7.
Effect of salt stress on the activities of antioxidant enzymes of walnut. (a) Superoxide dismutase (SOD) activity, and (b) peroxidase (POD) activity of the leaves. Four walnut genotypes were grown under different NaCl concentrations (0, 50, 100, 200, and 300 mmol/L) for 28 days. Each value indicates the mean ± standard error (SE) of three biological replicates. Bars of each parameter labeled with different letters are significantly different (p < 0.05) by Duncan’s test.
3.8. Comprehensive Evaluation of Salt Tolerance
3.8.1. Principal Component Analysis (PCA)
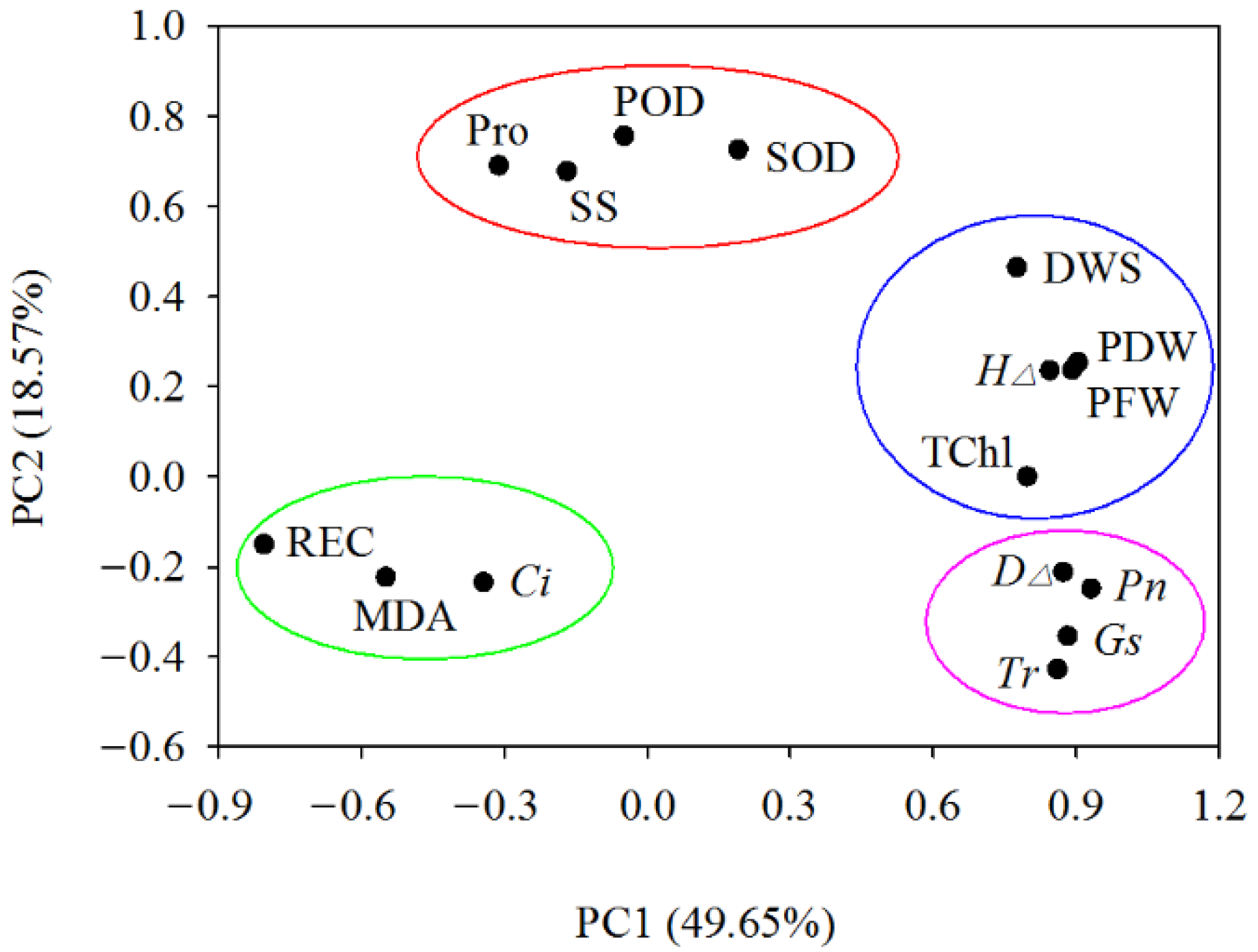
Through the principal component analysis of 16 indicators in four walnut genotypes grown in different salt treatments, four principal components with eigenvalues >1 were obtained, and the cumulative contribution rate reached 82.12%. On the PC1 (49.65%) and PC2 (18.57%) planes, four naturally segregating groups were formed. The first group is mainly characterized by photosynthetic parameters, namely net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), and ground diameter increment (D∆). The second group is mainly characterized by growth traits, which include the dry weight of shoot (DWS), plant fresh weight (PFW), plant dry weight (PDW), seedling height increment (H∆), and total chlorophyll content (TChl). The third group is mainly characterized by osmotic adjustment substances and antioxidant enzyme activities, including proline (Pro), soluble sugar (SS), and the activity of superoxide dismutase (SOD) and peroxidase (POD). The fourth group mainly represents characteristics of membrane system traits, including relative electrical conductivity (REC), malondialdehyde (MDA), and intercellular CO2 concentration (Ci) (Figure 8).
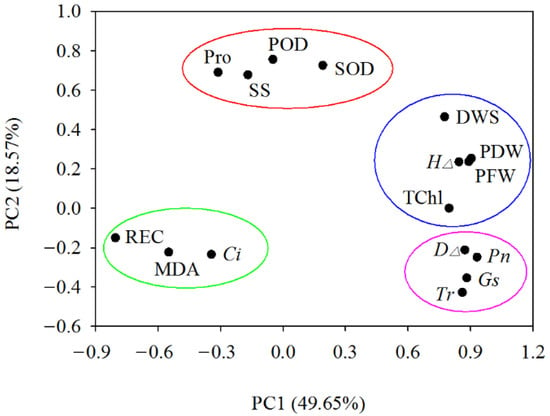
Figure 8.
Principal component analysis of walnut genotypes under different salt concentrations. Drawing a scatterplot with PC1 as the X-axis and PC2 as the Y-axis, four naturally segregating groups were formed. Abbreviations of the corresponding indicators are shown in Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7.
3.8.2. Membership Function Analysis
Membership function analysis assessed 16 indices from four walnut genotypes, and the total average value was calculated to rank the salt tolerance (Table 3). The membership function value of J4 was the largest (0.668), followed by that of J1 (0.595), whereas the membership function value of J2 and J3 were 0.236 and 0.452, respectively. The results showed that the comprehensive order of salt tolerance of the four walnut genotypes was J4 > J1 > J3 > J2, which corresponded to the morphological performance of each genotype under salt stress and demonstrated the viability of the assessment approach.

Table 3.
The average membership function values of the four walnut genotypes under salt stress.
4. Discussion
External morphological characteristics of plants can directly reflect the effects of salt stress on plants, and thus serve as an index of plant salt tolerance [10,34]. The changes in the plant growth indices and biomass are the comprehensive embodiment of plants to salt stress, and it is also the most intuitive index to judge the salt tolerance of plants [35]. By observing the external morphology of four genotypes after salt stress, it was found that the onset of stress symptoms occurred later in J4 than in the other three genotypes; after 28 days of stress, all genotypes showed symptoms of salt injury to varying degrees under different salt concentrations; however, the salt injury symptom of J2 was the most serious under all concentrations and that of J4 was less serious than that of other genotypes. Salt stress also inhibited the growth of four walnut genotypes, and H∆, D∆, DWS, PFW, and PDW of all genotypes decreased with increasing salt concentration. Low-concentration (50 mmol/L) salt stress had no significant effect on the growth of J1 and J4, but it did have an inhibitory role, with the effect on J2 growth being more significant. The four walnut genotypes’ growth was significantly inhibited by 300 mmol/L salt stress, with J4 having the smallest decline in growth indices under high salt stress. According to the phenotypic symptoms of each genotype, the order of the four walnut genotypes according to their salt tolerance was: J4 > J1 > J3 > J2.
Salt stress might inhibit and influence plant photosynthesis to some extent. First, chlorophyll is the most important pigment involved in photosynthesis in plants, and it is essential in organic compounds and light energy conversion [36]. Chlorophyll content can reflect the strength of photosynthesis under salt stress, which is one of the important physiological indices used to measure plant stress resistance, and its content has a direct effect on plants’ growth and development [30]. The contents of Chl a, Chl b, and TChl of four walnut genotypes decreased with the increasing salt concentration in this study. At the NaCl concentration of 50 mmol/L, Chl a, Chl b and TChl content of J1 and J4 exhibited no significant differences compared to the control group, indicating that low salt stress had a slight effect on the photosynthetic pigments of the aforementioned two walnut genotypes. Under high salt (300 mmol/L) stress, the photosynthetic pigment of four walnut genotypes was significantly lower than that of the control, with the above-mentioned indicators of J2 having a relatively large reduction. In general, a decrease of these pigments under salt stress is considered to be a result of slow synthesis or fast breakdown of the pigments in the cells [30]. Salt stress enhanced the synthesis of ROS, which caused lipid peroxidation and resulted in a large accumulation of MDA content. Hight concentration of MDA can promote the destruction of chloroplast membrane structure and accelerate the degradation of chlorophyll [29,37]. Chlorophyll synthesis was disrupted under NaCl stress, and the chlorophyll content decreased, which decreased the photosynthetic rate of plants, affecting plant growth and development and biomass accumulation. In J4 plants, the chlorophyll content decreased slightly under NaCl stress, the chlorophyll system loss was the least, and the salt tolerance was the best. Second, salt stress can affect plant photosynthetic gas exchange parameters. Pn can directly reflect plant assimilation capacity per unit leaf area and is an important index for measuring plant photosynthetic capacity. The results of this experiment showed that with increasing salt concentration, the Pn, Gs, and Tr of the four walnut genotypes showed a downward trend, which was significantly inhibited, and this phenomenon is consistent with the previous research results of 4 olive cultivars [38] and Prunus rootstocks [5] under salt stress. The decrease in Pn of J4 under high salt stress was less than that in the other three genotypes, indicating that J4 has a stronger ability to produce organic matter under high salt stress. According to studies, the decrease of Pn in plant leaves can be attributed to two categories: if the decrease in Pn is accompanied by the increase of Ci, the main limiting factor for photosynthesis is the nonstomatal factor; if both Ci and Gs decrease at the same time, the main limiting factor is stomatal [39,40]. In this experiment, with increasing salt concentration, the Pn of J1, and J2 decreased gradually, accompanied by an increase in Ci, indicating that the nonstomatal restriction was the primary cause of the decrease in Pn in the aforementioned two genotypes. It may be due to the temporary enhancement of cell respiration under high salt stress, as well as the accumulation of excess salt ions in the cells, which destroyed the chloroplast structure and leaf photosynthetic organs, resulting in a decrease in the chlorophyll content and photosynthetic activity of leaves. In J3, the gradual decrease in Pn was accompanied by a decrease in Ci, indicating that stomatal limitation was the main factor for the decrease in Pn. It may be inferred that osmotic stress caused by salt stress reduced Gs and increased the resistance of diffusion from the outside to the cell, resulting in photosynthetic inhibition. During the stress period, the Ci of J4 first decreased and subsequently increased, therefore, changing the photosynthesis limitation factor of J4 from stomatal to nonstomatal.
Studies have shown that under stress conditions such as salt stress, the membrane system of plants is destroyed, the selective permeability of cell membranes increases, and a large number of intracellular substances extravasate. The increased membrane permeability and electrolyte extravasation lead to increased REC in plants. Therefore, REC represents the degree of damage to the plant cell membrane under stress conditions [18,28]. MDA is a membrane lipid peroxidation and serves as a significant physiological marker indicating the degree of cell membrane damage and the strength of its response to adversity [41]. The contents of REC and MDA increased to varying degrees in this experiment, demonstrating that different concentrations of salt stress can cause lipid peroxidation of plant cell membranes and damage plant cell structures to varying degrees. J1 and J4 showed a lower degree of cell membrane lipid peroxidation and smaller increases in the REC and MDA contents. This finding indicated that under NaCl stress, J1 and J4 could better maintain the stability of the cell membrane structure and alleviate the oxidative damage to cells caused by high salt stress than J2 and J3.
Excessive salt ions in the soil limit soil water potential, making it difficult for plants to absorb water or water outflow, resulting in osmotic stress. Plants can regulate intracellular osmotic potential by synthesizing and accumulating organic osmolytes in excess under salt stress conditions to avoid osmotic stress damage, maintain osmotic balance, and ensure the normal physiological functioning of cells [42]. Pro as an osmotic adjustment substance in plants, can be used as a reference index for the degree of salt and alkali stress in plants. It can regulate osmotic potential, scavenge free radicals, and stabilize subcellular structures [29]. Some studies have shown a positive correlation between Proline accumulation and NaCl concentration in plants [4,30,43]. The contents of Pro in the four genotypes increased at different levels of salt stress in this experiment. The Pro content of J1 and J4 increased significantly under high salt concentration and the relative Pro content of these two genotypes was higher than that of the other two genotypes. This result showed that under high salt stress, J1 and J4 could deal with the damage induced by salt stress by accumulating more Pro, which enhances their adaptability to the NaCl stress environment. SS is an important osmotic regulatory substance in plants, regulating the change in osmotic potential in cells, and a carbon framework for intracellular macromolecules and an energy source in plants [44]. The SS content of four walnut genotypes increased in varying degrees with the increase in NaCl concentration in this experiment, which is consistent with the results of previous studies on the effect of salt stress on bean seedlings [45], Oriza sativa L. [46], and 4 kiwifruit genotypes [10]. Additionally, the SS content of J1 and J4 decreased under high salt stress, which might be because under high concentration salt stress, these two genotypes needed to consume more energy to maintain their resistance physiology.
When plants are stressed, the body produces reactive oxygen species (ROS) and other harmful substances, which cause plant senescence, oxidative stress, and other damages. When plants are subjected to salt stress, a substantial quantity of ROS accumulates, causing oxidative damage to plant cells. To limit the damage caused by ROS, plants produce a sophisticated and complex antioxidant system to eliminate excessive ROS, thus improving plant salt tolerance [44]. SOD and POD enzymes are components of the cellular defense system that maintain a metabolic balance [47]. SOD is an essential enzyme in plant antioxidant systems for scavenging oxygen free radicals, and it can decompose superoxide anion into singlet oxygen and H2O2 and resist ROS damage to the cell membrane. H2O2 retains strong oxidability, and POD enzyme is required for further decomposition into O2 and H2O [44,48]. The SOD enzyme activity of four walnut genotypes first increased and then decreased with increasing salt concentration, and the SOD enzyme activity of J1 and J4 was higher than that of the other two genotypes, which is consistent with the previous studies on 4 citrus rootstocks [15] and Phoenix dactylifera L. [49]. The SOD enzyme activities of four genotypes decreased under high salt stress, but the enzyme activities of J1 and J4 were higher than those of the control, suggesting that J1 and J4 could remove the damage caused by excessive ROS and alleviate the damage to cell membrane lipid peroxidation by maintaining a high SOD enzyme activity. The variation in the POD enzyme activity may reflect plant stress resistance [34,50]. In general, the higher the activity of the enzyme POD, the stronger the stress resistance of plants, and with the aggravation of stress, the POD activity steadily diminishes until inactivated [51]. The changes in the POD enzyme activity of four walnut genotypes showed different changing trends with increasing salt concentration. The POD activity of J4 increased with an increase in the salt concentration, and the differences across treatments were significant, with J4 demonstrating a strong salt tolerance. Some studies have shown that the higher the salt concentration, the greater the inhibition of plant growth and the higher the activity of antioxidant enzymes; however, when the salt concentration exceeds the tolerance range of plants, the antioxidant capacity of plants gradually diminishes [32,52,53]. The POD activity of J1 and J3 first increased and subsequently decreased; it increased significantly at 50 and 100 mmol/L concentrations, respectively, and remained at a high level. However, when the NaCl concentration increased to 300 mmol/L, the cells’ self-regulation ability was seriously limited, the cell membrane system was excessively damaged, and the POD activity of the two genotypes decreased to the control level. The POD enzyme activity of J2 also showed a trend of first increasing and then decreasing, at 50 mmol/L, the POD activity increased slightly, and there was no significant difference between the treatments and the control; and the POD activity of J2 under each salt concentration treatment was generally lower than that of the other three genotypes. Among the four genotypes, J4 exhibited higher antioxidant enzymes (SOD and POD) activities and showed strong salt tolerance.
At present, plant salt tolerance physiological research is mainly based on neutral salt NaCl stress, this study is also based on NaCl stress to explore the physiological and biochemical changes of four walnut genotypes. However, the large areas of saline-alkali land in China are complex saline-alkali land; and two major problems are soil alkalization caused by alkaline salts such as Na2CO3 and NaHCO3 and soil salinization caused by neutral salts such as NaCl and Na2SO4; and in actual saline-alkali land, the problems of soil salinization and alkalization often coexist. Furthermore, because plant salt tolerance is regulated by various trait genes, gene expression varies significantly under different ecological environment conditions and growth stages, and the salt tolerance performance may change. Thus, the salt tolerance of different walnut genotypes should be further studied in terms of physiological and ecological responses and gene network regulation mechanisms in the soil salt environment.
5. Conclusions
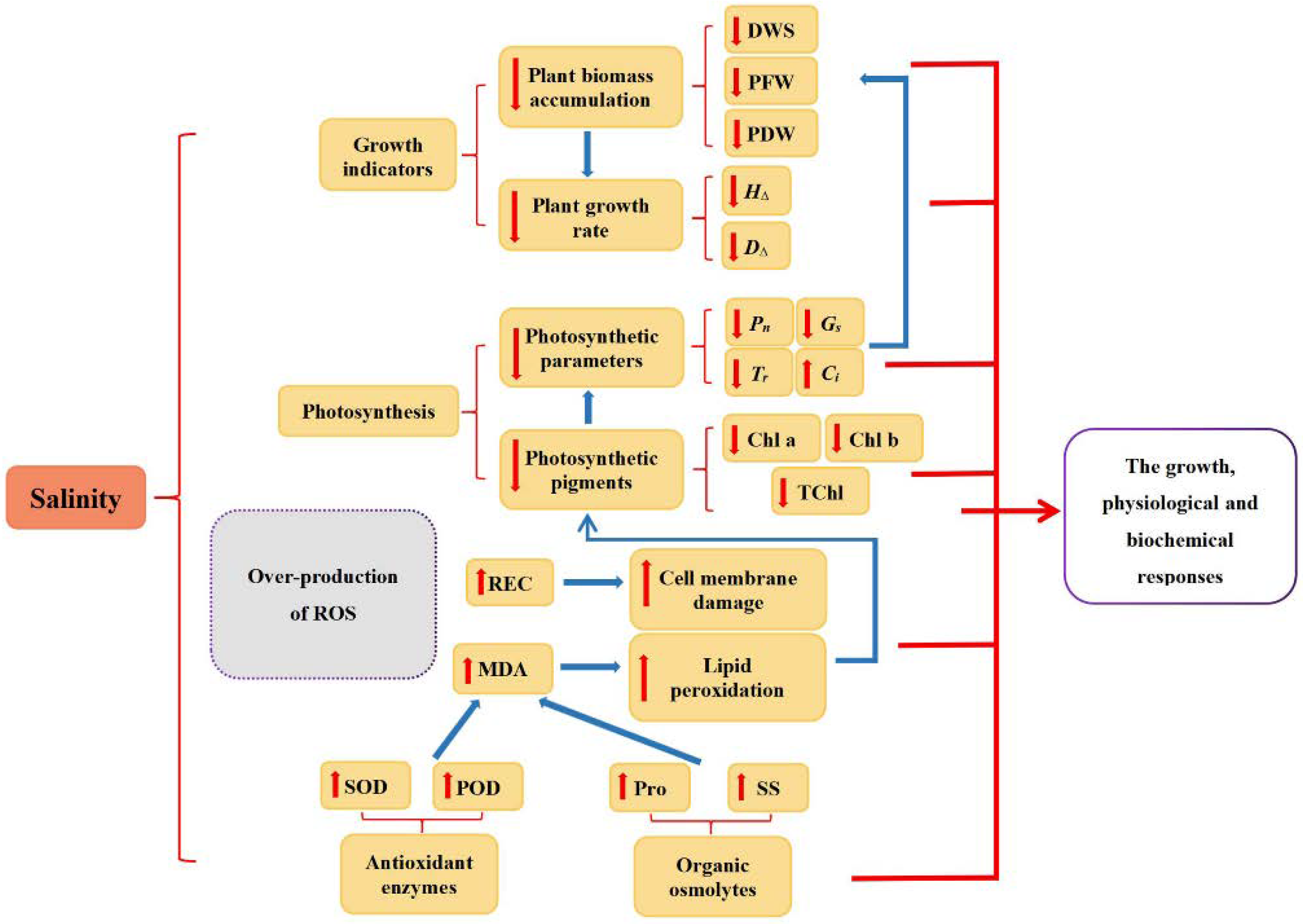
In the present study, we investigated the growth, physiological and biochemical responses of four different walnut genotypes against salt stress. According to the results, salt stress is reflected in obvious impairment in photosynthesis and inhibition of stomatal conductance, transpiration, and chlorophyll synthesis, with an increase in the concentration of MDA and REC highlighting the lipid peroxidation and oxidative damage of cell membranes. However, the adverse effects of salt stress could be mitigated via various internal mechanisms in the cell, such as the accumulation of antioxidant enzymes (SOD and POD) and osmolytes (Pro and SS) (Figure 9). The salt stress-induced diverse changes among all four walnut genotypes. Considering the results of principal component analysis and membership function, the comprehensive order of the four genotypes according to their salt tolerance was: J4 > J1 > J3 > J2. It was consistent with each genotype’s morphological performance under salt stress and demonstrated variances in growth characteristics, and physiological and biochemical responses of four walnut genotypes under different salt stress.
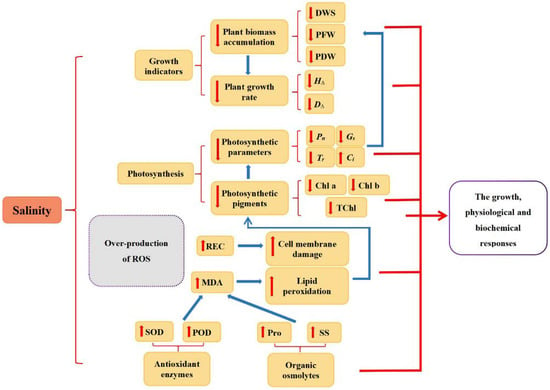
Figure 9.
Schematic representation of salinity-induced growth inhibition. H∆,seedling height increment; D∆,ground diameter increment; DWS, dry weight of shoot; PFW, plant fresh weight; PDW, plant dry weight; Chl a, chlorophyll a content; Chl b, chlorophyll b content; TChl, total chlorophyll content; Pn, net photosynthetic rate; Gs, stomatal conductance; Tr, transpiration; Ci, intercellular CO2 concentration; MDA, malondialdehyde; REC, relative electrical conductivity; Pro, proline; SS, soluble sugar; SOD, superoxide dismutase; POD, peroxidase.
Author Contributions
Conceptualization, J.Z. and D.P.; methodology, J.T.; formal analysis, W.F., B.L. and Y.B.; investigation, J.H.; writing—original draft preparation, X.J.; writing—review and editing, D.P. and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (Grant Number: 2019YFD1001603).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- He, X.; Yang, Y.; Wei, H.; Yuan, Z. Soil microbiome-mediated salinity tolerance in poplar plantlets is source-dependent. Chemosphere 2021, 272, 129600. [Google Scholar] [CrossRef] [PubMed]
- Ci, L.J.; Yang, X.H. Desertification and Its Control in China; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 9783642018695. [Google Scholar]
- Libutti, A.; Cammerino, A.; Monteleone, M. Risk assessment of soil salinization due to tomato cultivation in Mediterranean climate conditions. Water 2018, 10, 1503. [Google Scholar] [CrossRef] [Green Version]
- Kulczyk-Skrzeszewska, M.; Kieliszewska-Rokicka, B. Influence of drought and salt stress on the growth of young Populus nigra ‘Italica’ plants and associated mycorrhizal fungi and non-mycorrhizal fungal endophytes. New For. 2021, 1–16. [Google Scholar] [CrossRef]
- Toro, G.; Pimentel, P.; Salvatierra, A. Effective categorization of tolerance to salt stress through clustering Prunus rootstocks according to their physiological performances. Horticulturae 2021, 7, 542. [Google Scholar] [CrossRef]
- Liu, C.; Yan, M.; Huang, X.; Yuan, Z. Effects of salt stress on growth and physiological characteristics of pomegranate (Punica granatum L.) cuttings. Pak. J. Bot. 2018, 50, 457–464. [Google Scholar]
- Capula-Rodríguez, R.; Valdez-Aguilar, L.A.; Cartmill, D.L.; Cartmill, A.D.; Alia-Tejacal, I. Supplementary Calcium and Potassium Improve the Response of Tomato (Solanum lycopersicum L.) to Simultaneous Alkalinity, Salinity, and Boron Stress. Commun. Soil Sci. Plant Anal. 2016, 47, 505–511. [Google Scholar] [CrossRef]
- Chen, W.; Cui, P.; Sun, H.; Guo, W.; Yang, C.; Jin, H.; Fang, B.; Shi, D. Comparative effects of salt and alkali stresses on organic acid accumulation and ionic balance of seabuckthorn (Hippophae rhamnoides L.). Ind. Crops Prod. 2009, 30, 351–358. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Abid, M.; Zhang, Y.J.; Li, Z.; Bai, D.F.; Zhong, Y.P.; Fang, J.B. Effect of Salt stress on growth, physiological and biochemical characters of Four kiwifruit genotypes. Sci. Hortic. 2020, 271, 109473. [Google Scholar] [CrossRef]
- Zhong, Y.P.; Qi, X.J.; Chen, J.Y.; Li, Z.; Bai, D.F.; Wei, C.G.; Fang, J.B. Growth and physiological responses of four kiwifruit genotypes to salt stress and resistance evaluation. J. Integr. Agric. 2019, 18, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Fu, Y.; Hu, D.; Yu, J.; Liu, H. Effect of green, yellow and purple radiation on biomass, photosynthesis, morphology and soluble sugar content of leafy lettuce via spectral wavebands “knock out”. Sci. Hortic. 2018, 236, 10–17. [Google Scholar] [CrossRef]
- Li, Q.; Lv, L.R.; Teng, Y.J.; Si, L.B.; Ma, T.; Yang, Y.L. Apoplastic hydrogen peroxide and superoxide anion exhibited different regulatory functions in salt-induced oxidative stress in wheat leaves. Biol. Plant. 2018, 62, 750–762. [Google Scholar] [CrossRef]
- Liu, A.; Hu, Z.; Bi, A.; Fan, J.; Gitau, M.M.; Amombo, E.; Chen, L.; Fu, J. Photosynthesis, antioxidant system and gene expression of bermudagrass in response to low temperature and salt stress. Ecotoxicology 2016, 25, 1445–1457. [Google Scholar] [CrossRef]
- Shahid, M.A.; Balal, R.M.; Khan, N.; Simón-Grao, S.; Alfosea-Simón, M.; Cámara-Zapata, J.M.; Mattson, N.S.; Garcia-Sanchez, F. Rootstocks influence the salt tolerance of Kinnow mandarin trees by altering the antioxidant defense system, osmolyte concentration, and toxic ion accumulation. Sci. Hortic. 2019, 250, 1–11. [Google Scholar] [CrossRef]
- Cha-Um, S.; Kirdmanee, K. Effect of salt stress on proline accumulation, photosynthetic ability and growth characters in two maize cultivars. Pak. J. Bot. 2009, 41, 87–98. [Google Scholar]
- Zhang, W.; Zwiazek, J.J. Effects of root medium pH on root water transport and apoplastic pH in red-osier dogwood (Cornus sericea) and paper birch (Betula papyrifera) seedlings. Plant Biol. 2016, 18, 1001–1007. [Google Scholar] [CrossRef]
- Zhu, S.; Nong, J.; Luo, G.; Li, Q.; Wang, F.; Jiang, D.; Zhao, X. Varied tolerance and different responses of five citrus rootstocks to acid stress by principle component analysis and orthogonal analysis. Sci. Hortic. 2021, 278, 109853. [Google Scholar] [CrossRef]
- Karimi, S.; Karami, H.; Vahdati, K.; Mokhtassi-Bidgoli, A. Antioxidative responses to short-term salinity stress induce drought tolerance in walnut. Sci. Hortic. 2020, 267, 109322. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, J.; Pei, D.; Yu, L. Combined effects of water stress and salinity on growth, physiological, and biochemical traits in two walnut genotypes. Physiol. Plant. 2021, 172, 176–187. [Google Scholar] [CrossRef]
- Song, D.; Pan, K.; Zhang, A.; Wu, X.; Tariq, A.; Chen, W.; Li, Z.; Sun, F.; Sun, X.; Olatunji, O.A.; et al. Optimization of growth and production parameters of walnut (Juglans regia) saplings with response surface methodology. Sci. Rep. 2018, 8, 9992. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Mei, L.; Jiang, H.; Hu, G.; Wang, Y. Evaluation of Carya cathayensis resistance to Botryosphaeria trunk canker using grafting on pecan. Sci. Hortic. 2019, 248, 184–188. [Google Scholar] [CrossRef]
- Meng, B.N.; Zhang, J.P.; Pei, D.; Xu, H.Z.; Wang, S.M.; Guo, Z.M. Effects of different rootstocks on photosynthesis characteristics in walnut. Nonwood For. Res. 2013, 2. [Google Scholar]
- Hao, L.C.; Wang, Y.Y.; Qi, G.H.; Zhang, X.M.; Li, B.G. Effect of rootstocks on the quality of nut of ‘Lyuling’ walnut. North. Hortic. 2015, 10, 29–32. [Google Scholar]
- Ebrahimi, A.; Zarei, A.; McKenna, J.R.; Bujdoso, G.; Woeste, K.E. Genetic diversity of Persian walnut (Juglans regia) in the cold-temperate zone of the United States and Europe. Sci. Hortic. 2017, 220, 36–41. [Google Scholar] [CrossRef]
- Aslamarz, A.A.; Vahdati, K.; Hassani, D.; Rahemi, M.; Mohammadi, N.; Leslie, C.J. Cold hardiness and its relationship with proline content in Persian walnut. Eur. J. Hortic. Sci. 2011, 76, 84–90. [Google Scholar]
- Yang, G.; Zhang, W.; Liu, Z.; Yi-Maer, A.Y.; Zhai, M.; Xu, Z. Both JrWRKY2 and JrWRKY7 of Juglans regia mediate responses to abiotic stresses and abscisic acid through formation of homodimers and interaction. Plant Biol. 2017, 19, 268–278. [Google Scholar] [CrossRef]
- Esfandiari Ghalati, R.; Shamili, M.; Homaei, A. Effect of putrescine on biochemical and physiological characteristics of guava (Psidium guajava L.) seedlings under salt stress. Sci. Hortic. 2020, 261, 108961. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, Q.; Tao, J. The Physiological Response of Three Narcissus pseudonarcissus under NaCl Stress. Am. J. Plant Sci. 2019, 10, 447–461. [Google Scholar] [CrossRef] [Green Version]
- Rahneshan, Z.; Nasibi, F.; Moghadam, A.A. Effects of salinity stress on some growth, physiological, biochemical parameters and nutrients in two pistachio (Pistacia vera L.) rootstocks. J. Plant Interact. 2018, 13, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Pan, J.; Zhang, M.; Xing, X.; Zhou, Y.; Liu, Y.; Li, D.; Li, D. ZmMKK4, a novel group C mitogen-activated protein kinase kinase in maize (Zea mays), confers salt and cold tolerance in transgenic Arabidopsis. Plant Cell Environ. 2011, 34, 1291–1303. [Google Scholar] [CrossRef]
- Khalid, M.F.; Hussain, S.; Anjum, M.A.; Ahmad, S.; Ali, M.A.; Ejaz, S.; Morillon, R. Better salinity tolerance in tetraploid vs. diploid volkamer lemon seedlings is associated with robust antioxidant and osmotic adjustment mechanisms. J. Plant Physiol. 2020, 244, 153071. [Google Scholar] [CrossRef]
- Muñoz-Muñoz, J.L.; García-Molina, F.; García-Ruiz, P.A.; Arribas, E.; Tudela, J.; García-Cánovas, F.; Rodríguez-López, J.N. Enzymatic and chemical oxidation of trihydroxylated phenols. Food Chem. 2009, 113, 435–444. [Google Scholar] [CrossRef]
- Urbinati, G.; Nota, P.; Frattarelli, A.; Lucioli, S.; Forni, C.; Caboni, E. Morpho-physiological responses of sea buckthorn (Hippophae rhamnoides) to NaCl stress. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2019, 154, 827–834. [Google Scholar] [CrossRef]
- Mao, P.; Zhang, Y.; Cao, B.; Guo, L.; Shao, H.; Cao, Z.; Jiang, Q.; Wang, X. Effects of salt stress on eco-physiological characteristics in Robinia pseudoacacia based on salt-soil rhizosphere. Sci. Total Environ. 2016, 568, 118–123. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Diaz-Vivancos, P.; Alvarez, S.; Fernandez-Garcia, N.; Sanchez-Blanco, M.J.; Hernandez, J.A. NaCl-induced physiological and biochemical adaptative mechanisms in the ornamental Myrtus communis L. plants. J. Plant Physiol. 2015, 183, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.J.; Uddin, M.J.; Hossain, M.A.; Henry, R.; Begum, M.K.; Sohel, M.A.T.; Mou, M.A.; Ahn, J.; Cheong, E.J.; Lim, Y.S. Exogenous putrescine attenuates the negative impact of drought stress by modulating physio-biochemical traits and gene expression in sugar beet (Beta vulgaris L.). PLoS ONE 2022, 17, e0262099. [Google Scholar] [CrossRef]
- Regni, L.; Del Pino, A.M.; Mousavi, S.; Palmerini, C.A.; Baldoni, L.; Mariotti, R.; Mairech, H.; Gardi, T.; D’Amato, R.; Proietti, P. Behavior of four olive cultivars during salt stress. Front. Plant Sci. 2019, 10, 867. [Google Scholar] [CrossRef]
- Moya, J.L.; Tadeo, F.R.; Gómez-Cadenas, A.; Primo-Millo, E.; Talón, M. Transmissible salt tolerance traits identified through reciprocal grafts between sensitive Carrizo and tolerant Cleopatra citrus genotypes. J. Plant Physiol. 2002, 159, 991–998. [Google Scholar] [CrossRef]
- Khoshbakht, D.; Ramin, A.A.; Baninasab, B. Effects of sodium chloride stress on gas exchange, chlorophyll content and nutrient concentrations of nine citrus rootstocks. Photosynthetica 2015, 53, 241–249. [Google Scholar] [CrossRef]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Front. Plant Sci. 2015, 6, 868. [Google Scholar] [CrossRef] [Green Version]
- Yan, K.; Shao, H.; Shao, C.; Chen, P.; Zhao, S.; Brestic, M.; Chen, X. Physiological adaptive mechanisms of plants grown in saline soil and implications for sustainable saline agriculture in coastal zone. Acta Physiol. Plant. 2013, 35, 2867–2878. [Google Scholar] [CrossRef]
- Mazumdar, P.; Lau, S.E.; Singh, P.; Takhtgahi, H.M.; Harikrishna, J.A. Impact of sea-salt on morpho-physiological and biochemical responses in banana (Musa acuminata cv. Berangan). Physiol. Mol. Biol. Plants 2019, 25, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, J.; Yan, D.; Yuan, H.; Wang, Y.; He, Y.; Wang, X.; Li, Z.; Mei, J.; Hu, M.; et al. Exogenous spermidine improves salt tolerance of pecan-grafted seedlings via activating antioxidant system and inhibiting the enhancement of Na+/K+ ratio. Acta Physiol. Plant. 2020, 42, 83. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Torabian, S. Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol. Environ. Saf. 2017, 137, 64–70. [Google Scholar] [CrossRef]
- Abdallah, M.M.S.; Abdelgawad, Z.A.; El-Bassiouny, H.M.S. Alleviation of the adverse effects of salinity stress using trehalose in two rice varieties. S. Afr. J. Bot. 2016, 103, 275–282. [Google Scholar] [CrossRef]
- Hatami, E.; Shokouhian, A.A.; Ghanbari, A.R.; Naseri, L.A. Alleviating salt stress in almond rootstocks using of humic acid. Sci. Hortic. 2018, 237, 296–302. [Google Scholar] [CrossRef]
- Klein, A.; Hüsselmann, L.; Keyster, M.; Ludidi, N. Exogenous nitric oxide limits salt-induced oxidative damage in maize by altering superoxide dismutase activity. S. Afr. J. Bot. 2018, 115, 44–49. [Google Scholar] [CrossRef]
- Ait-El-Mokhtar, M.; Laouane, R.B.; Anli, M.; Boutasknit, A.; Wahbi, S.; Meddich, A. Use of mycorrhizal fungi in improving tolerance of the date palm (Phoenix dactylifera L.) seedlings to salt stress. Sci. Hortic. 2019, 253, 429–438. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, H.S.; Park, S.C.; Ji, C.Y.; Yang, J.W.; Lee, H.U.; Kwak, S.S. Downregulation of swpa4 peroxidase expression in transgenic sweetpotato plants decreases abiotic stress tolerance and reduces stress-related peroxidase expression. Plant Biotechnol. Rep. 2021, 15, 69–76. [Google Scholar] [CrossRef]
- Gökmen, E.; Ceyhan, E. Effects of drought stress on growth parameters, enzyme activities and proline content in chickpea genotypes. Bangladesh J. Bot. 2015, 44, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Anjum, S.A.; Xie, X.Y.; Wang, L.C.; Saleem, M.F.; Chen, M.; Lei; Wang, L. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar] [CrossRef]
- Oustric, J.; Morillon, R.; Ollitrault, P.; Herbette, S.; Luro, F.; Froelicher, Y.; Tur, I.; Dambier, D.; Giannettini, J.; Berti, L.; et al. Somatic hybridization between diploid Poncirus and Citrus improves natural chilling and light stress tolerances compared with equivalent doubled-diploid genotypes. Trees 2018, 32, 883–895. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).