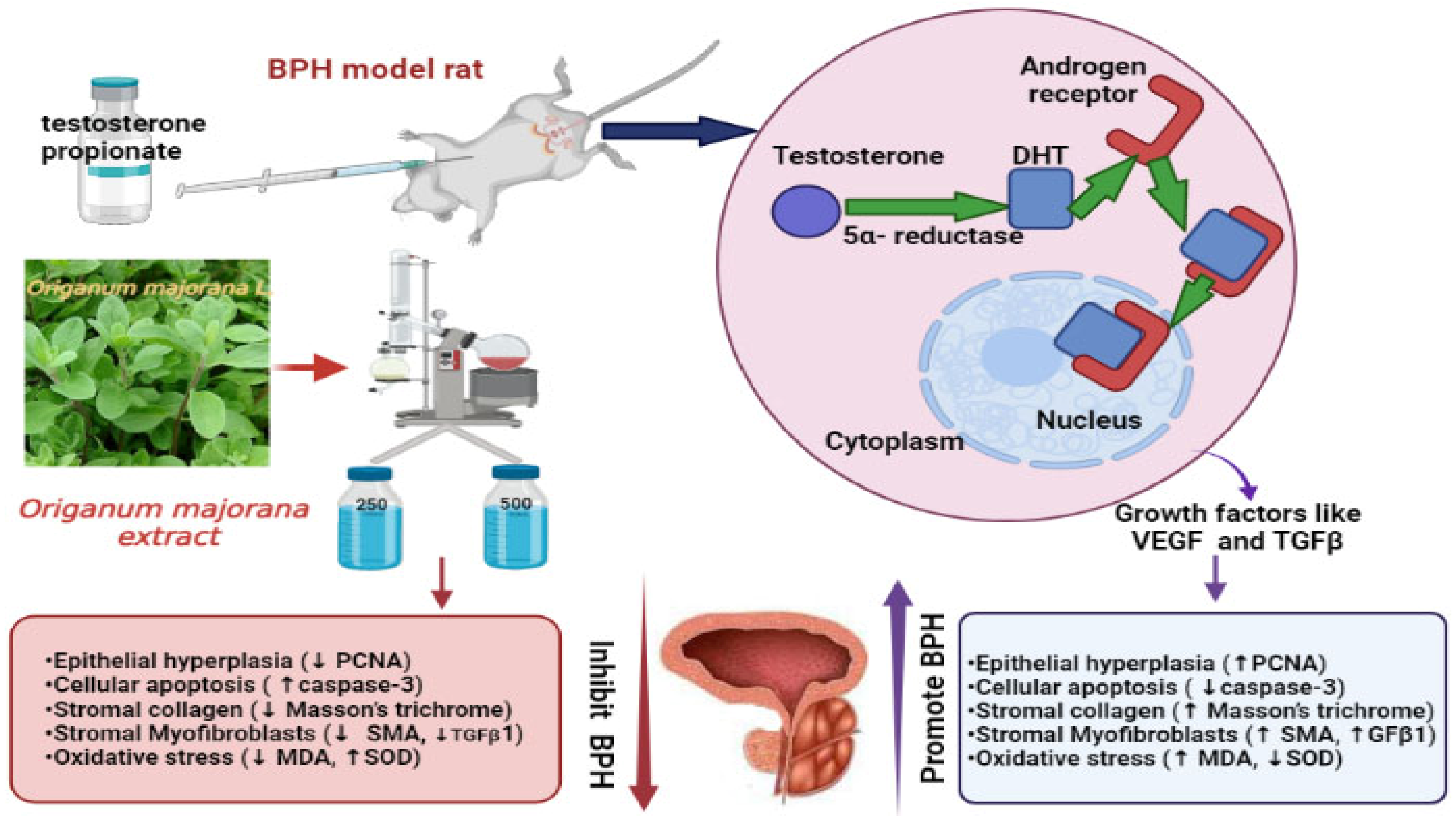
Origanum majorana L. Extract Attenuated Benign Prostatic Hyperplasia in Rat Model: Effect on Oxidative Stress, Apoptosis, and Proliferation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Animals
2.2. Methods
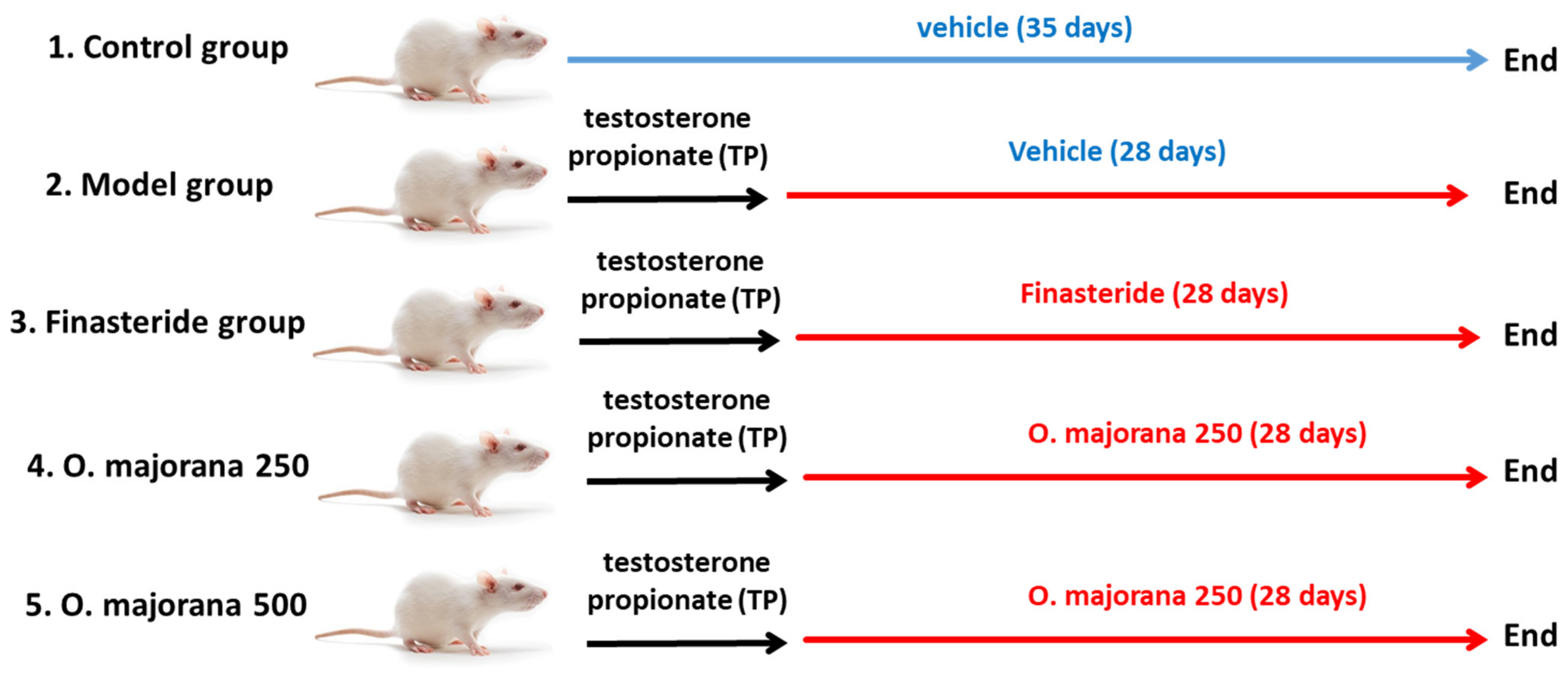
2.2.1. Testosterone Propionate-Induced Rat Model of BPH
2.2.2. Body Weight and Prostate Weight
2.2.3. Hormonal Assessment
2.2.4. Measurement of Oxidant-Antioxidant Status in Prostate Tissues
2.2.5. Assessment of Nrf2 and HO-1 in Prostate Tissues by ELISA
2.2.6. Assessment of Caspase-3 and TGF-β1 in Prostate Tissues by ELISA
2.2.7. Quantitative Real-Time PCR Analysis for Caspase-3 and TGF-β1 in Prostate Tissues
- (a)
- Isolation of total RNA and complementary DNA (cDNA) Synthesis
- (b)
- The real-time PCR analysis process
2.2.8. Histopathological Examination
2.2.9. Immunohistochemical Staining Technique
2.2.10. Morphometric Analysis
- (1)
- In H&E-stained sections, the prostatic epithelial height (μm) was measured by manually drawing a line through the acinar epithelia at a magnification of ×400. The acinar luminal area (μm2) was measured by drawing a line around the luminar perimeter and calculating the acinar area at a magnification of ×100
- (2)
- Mean area % of collagen fiber was determined in the Masson’s trichrome-stained sections at a magnification of ×100
- (3)
- Mean area % of positive immunoreactivity for (α-SMA) and (TGF-B1) was measured at a magnification of ×400.
2.2.11. Statistical Analysis
3. Results
3.1. Effect of O. majorana on the Rat’s Body Weight Gain and Prostate Weight
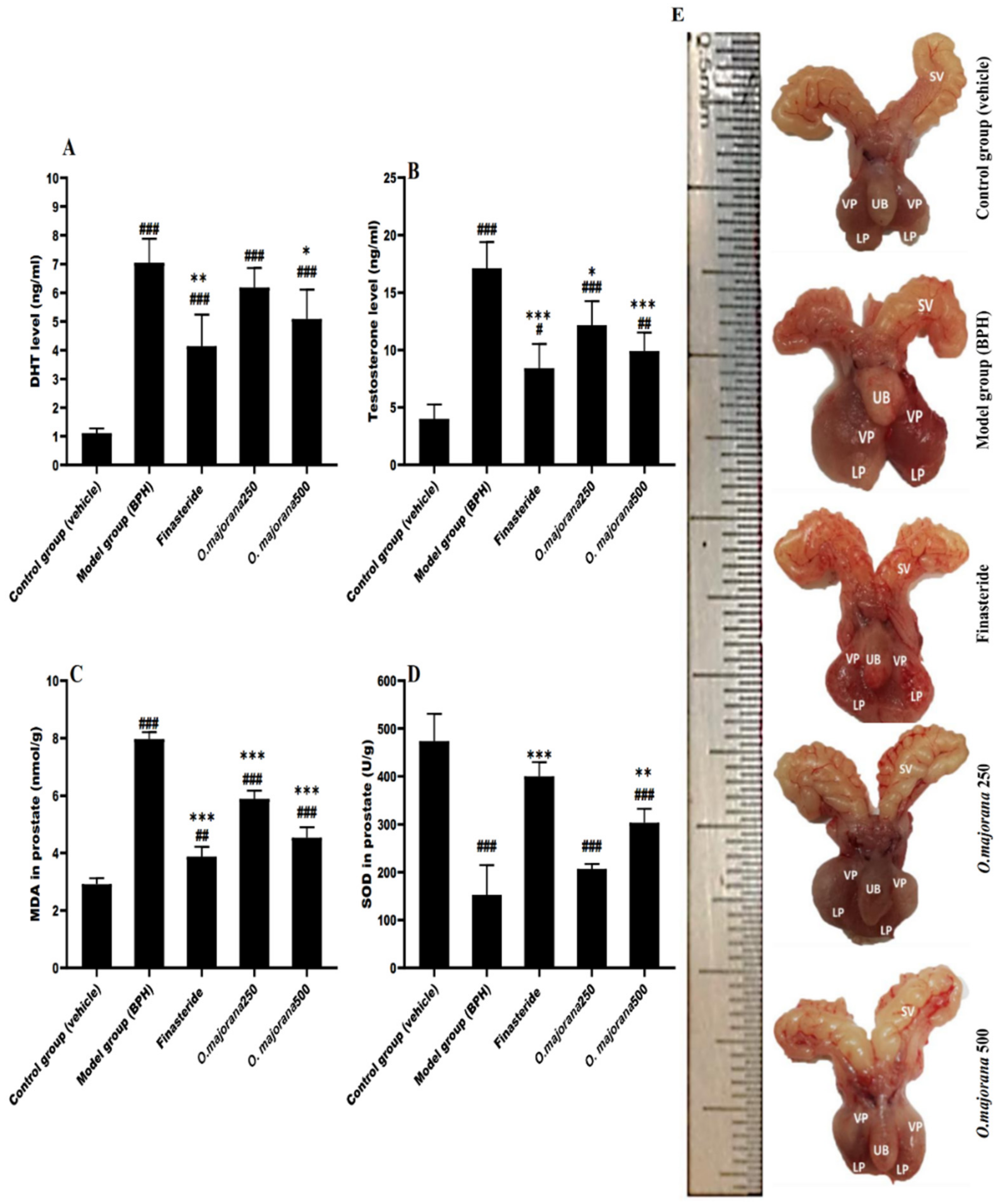
3.2. Effect of O. majorana on DHT and Testosterone Serum Levels
3.3. Effect of O. majorana on Prostatic MDA and SOD Levels
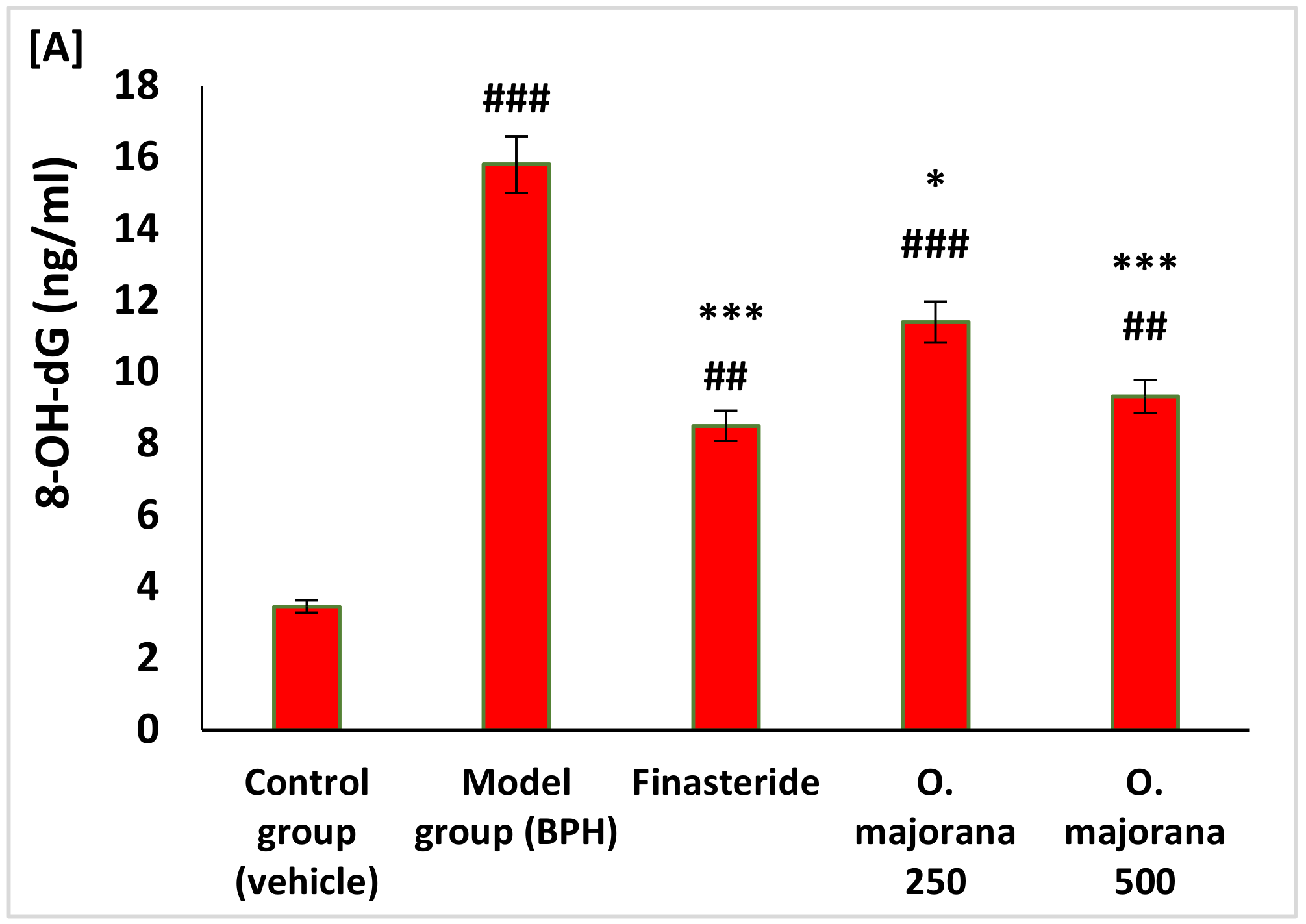
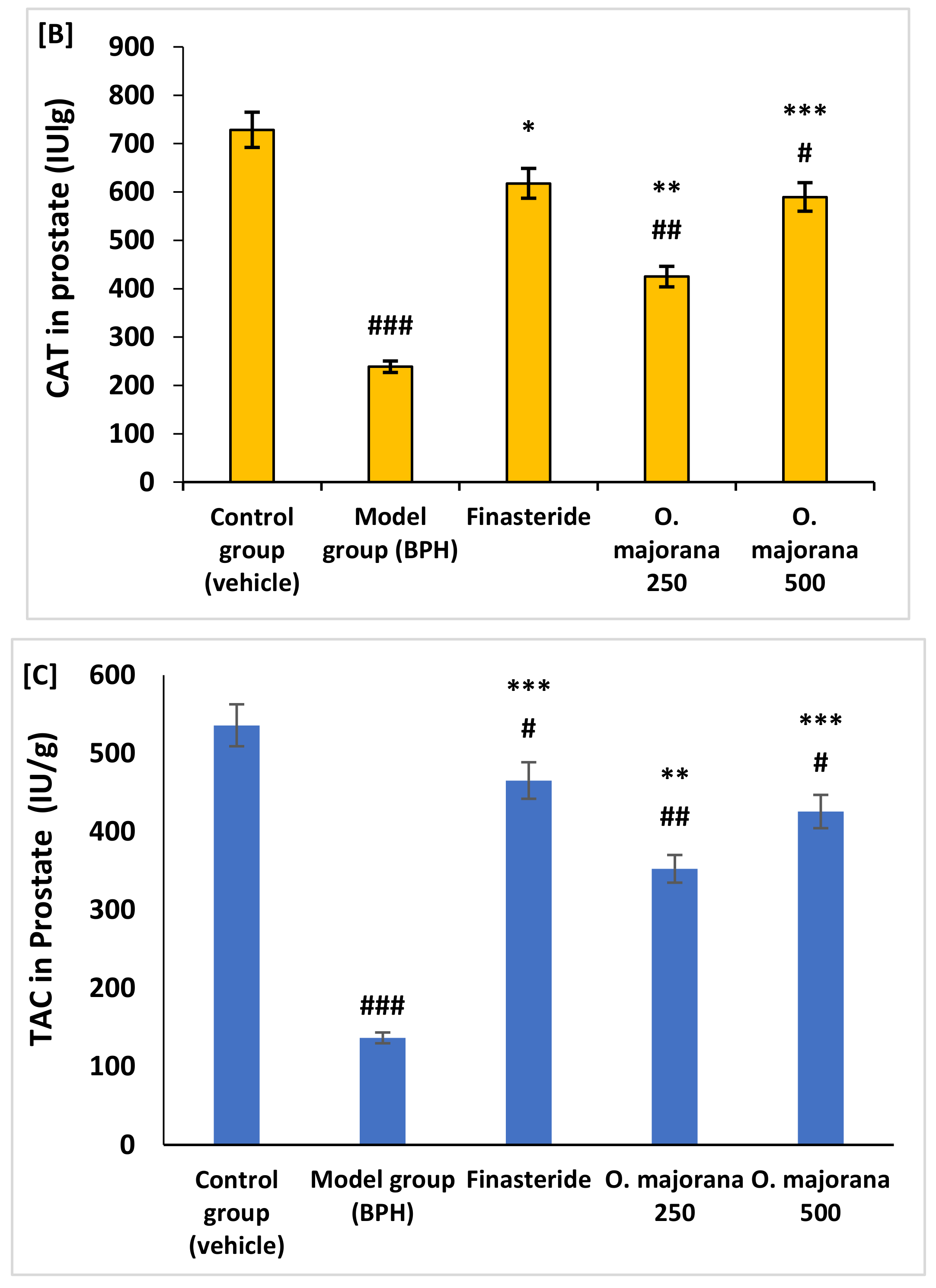
3.4. Effect of O. majorana on Prostate DNA Damage, CAT, and TAC
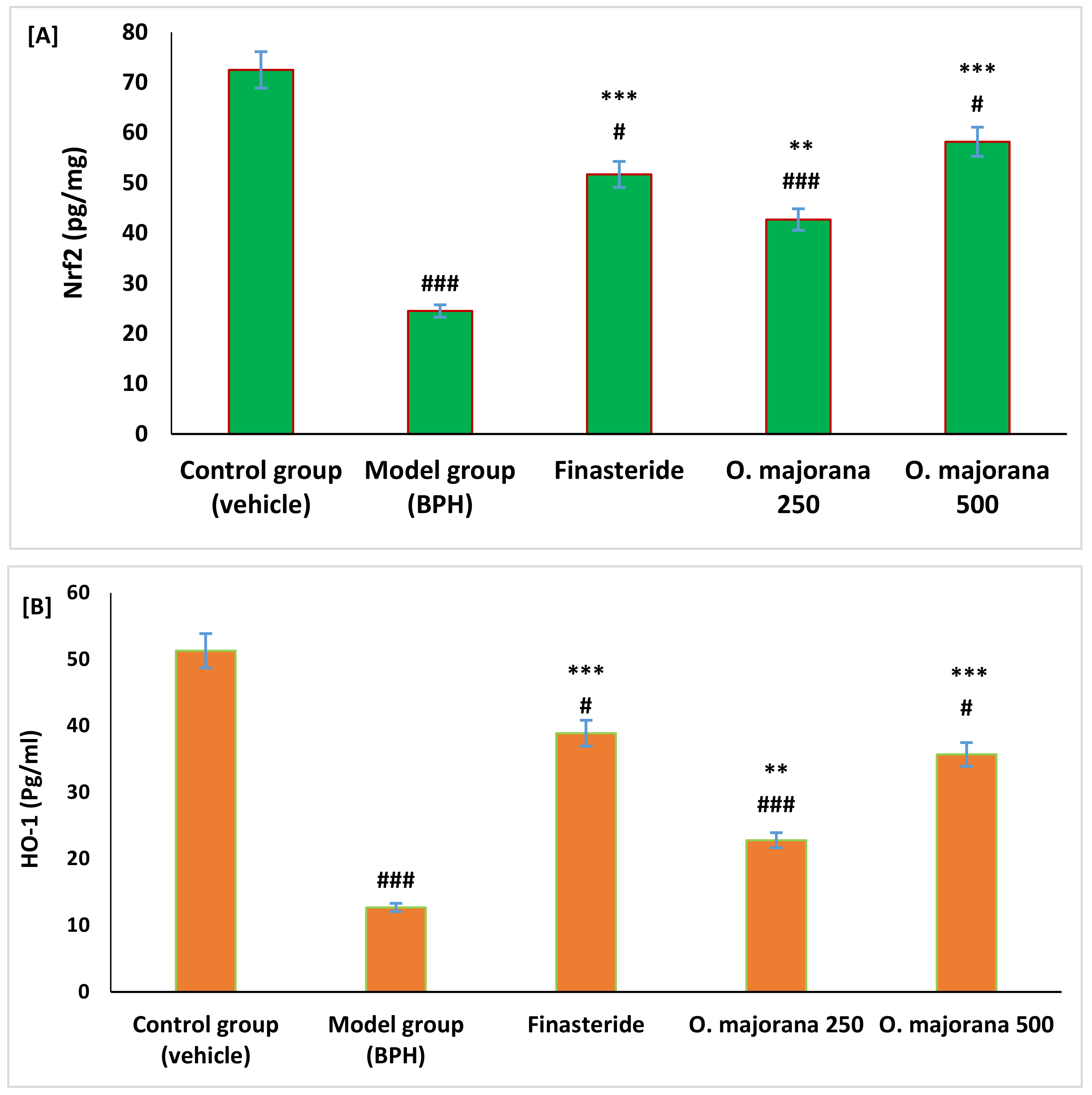
3.5. Effect of O. majorana on Nrf2 and HO-1 Levels in Prostate Tissue
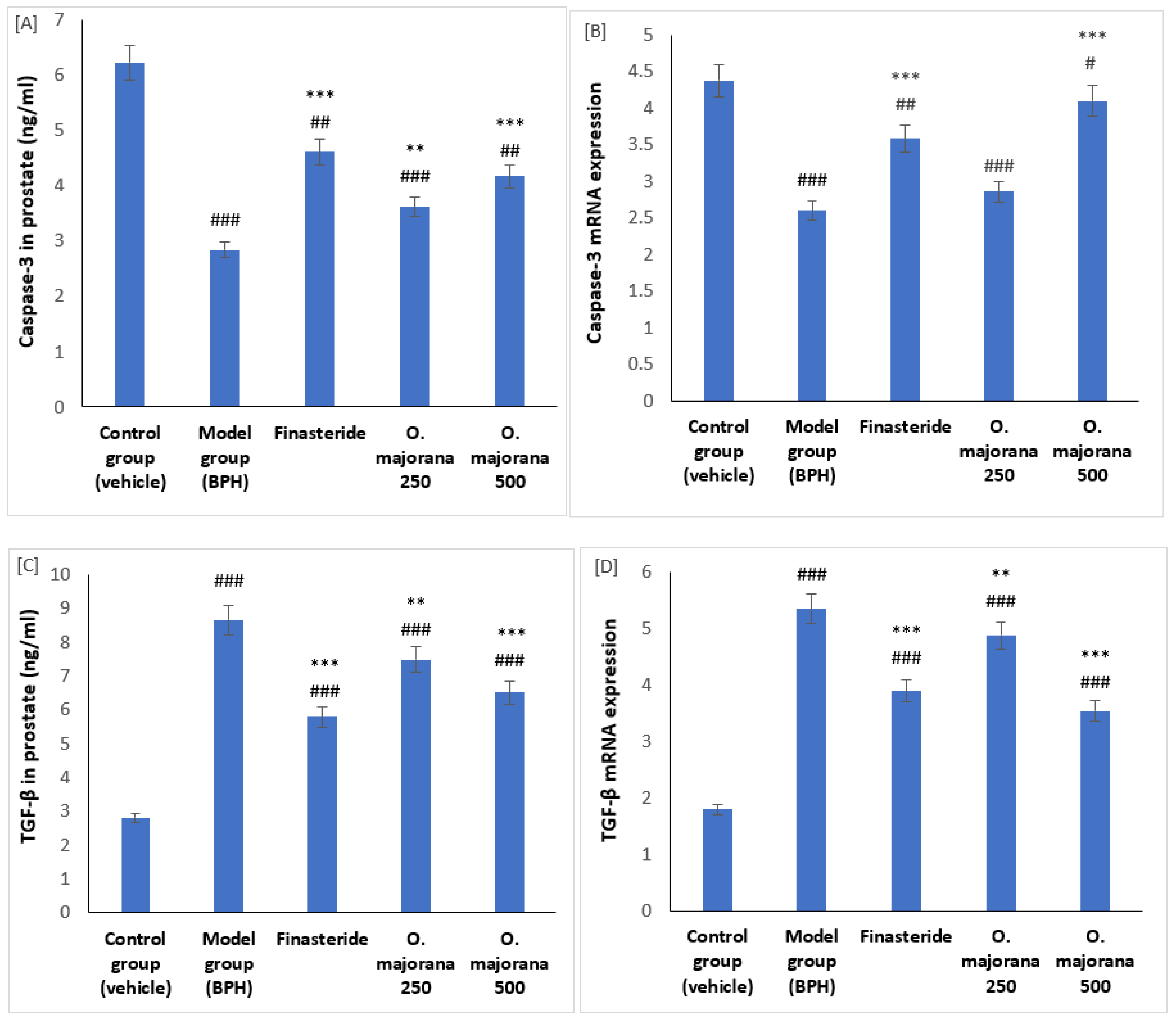
3.6. Effect of O. majorana on mRNAs and Proteins of Caspase-3 and TGF-β1 in Prostate Tissue
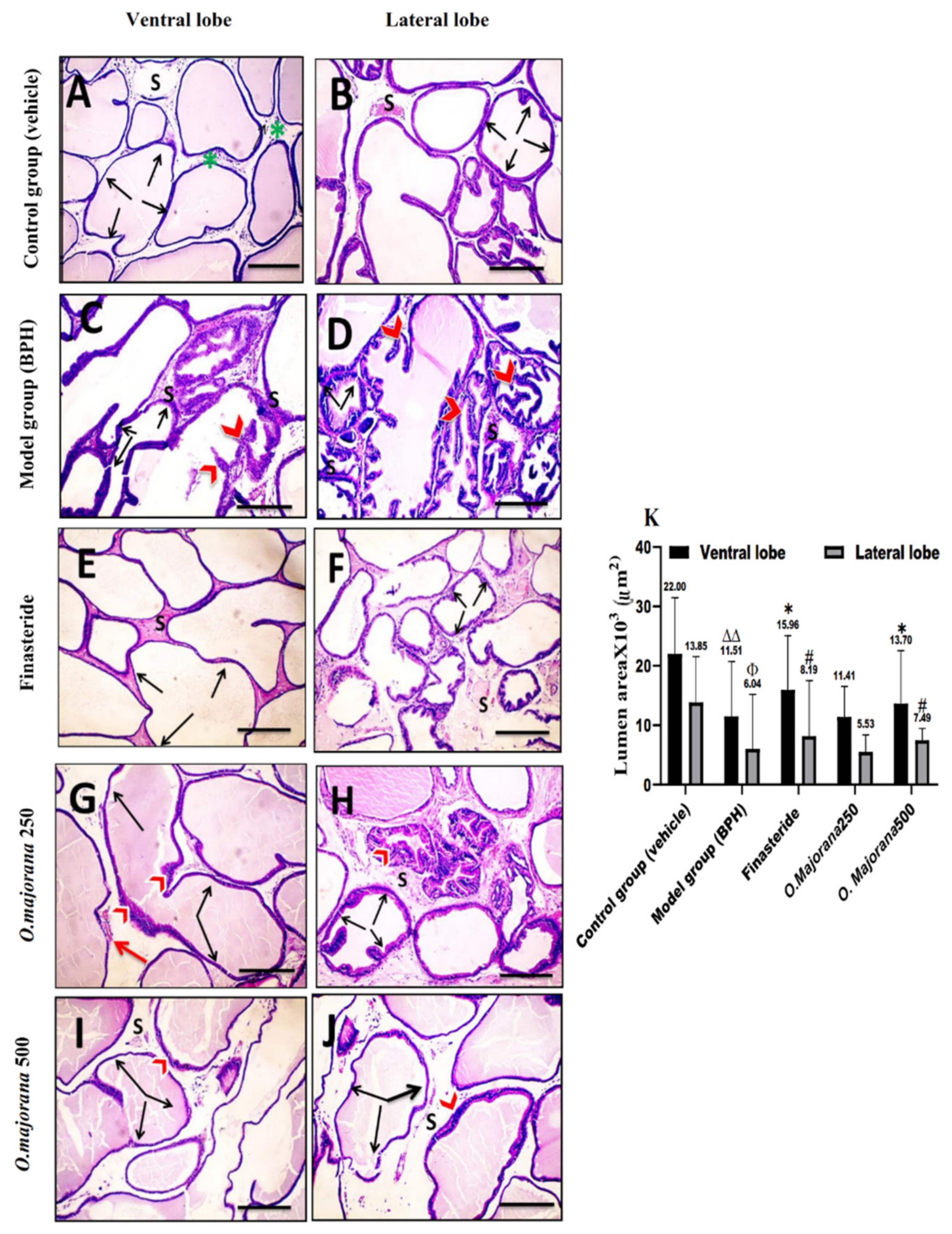
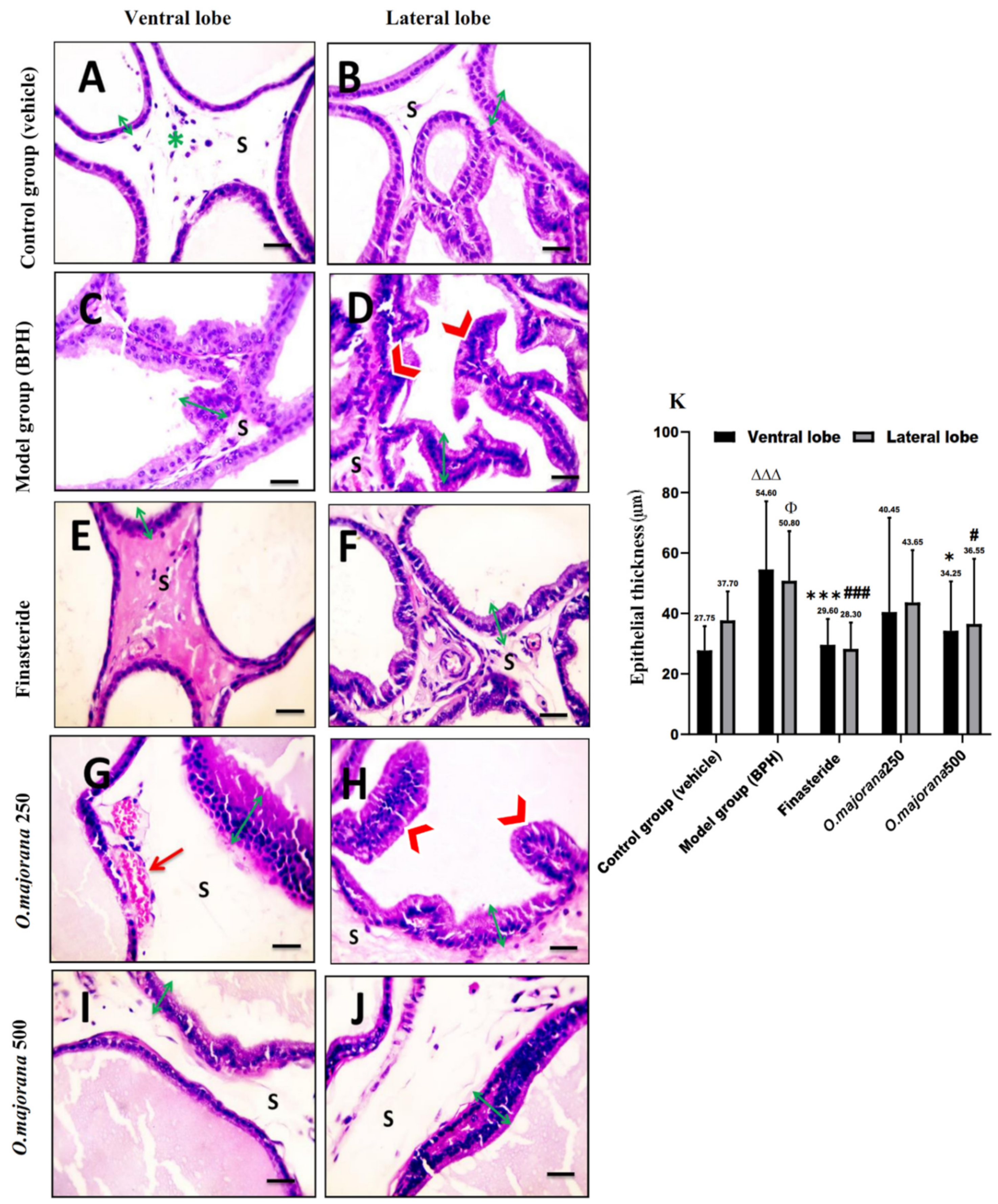
3.7. The Effect of O. majorana on Prostate Histological Structure
3.7.1. H&E Stain
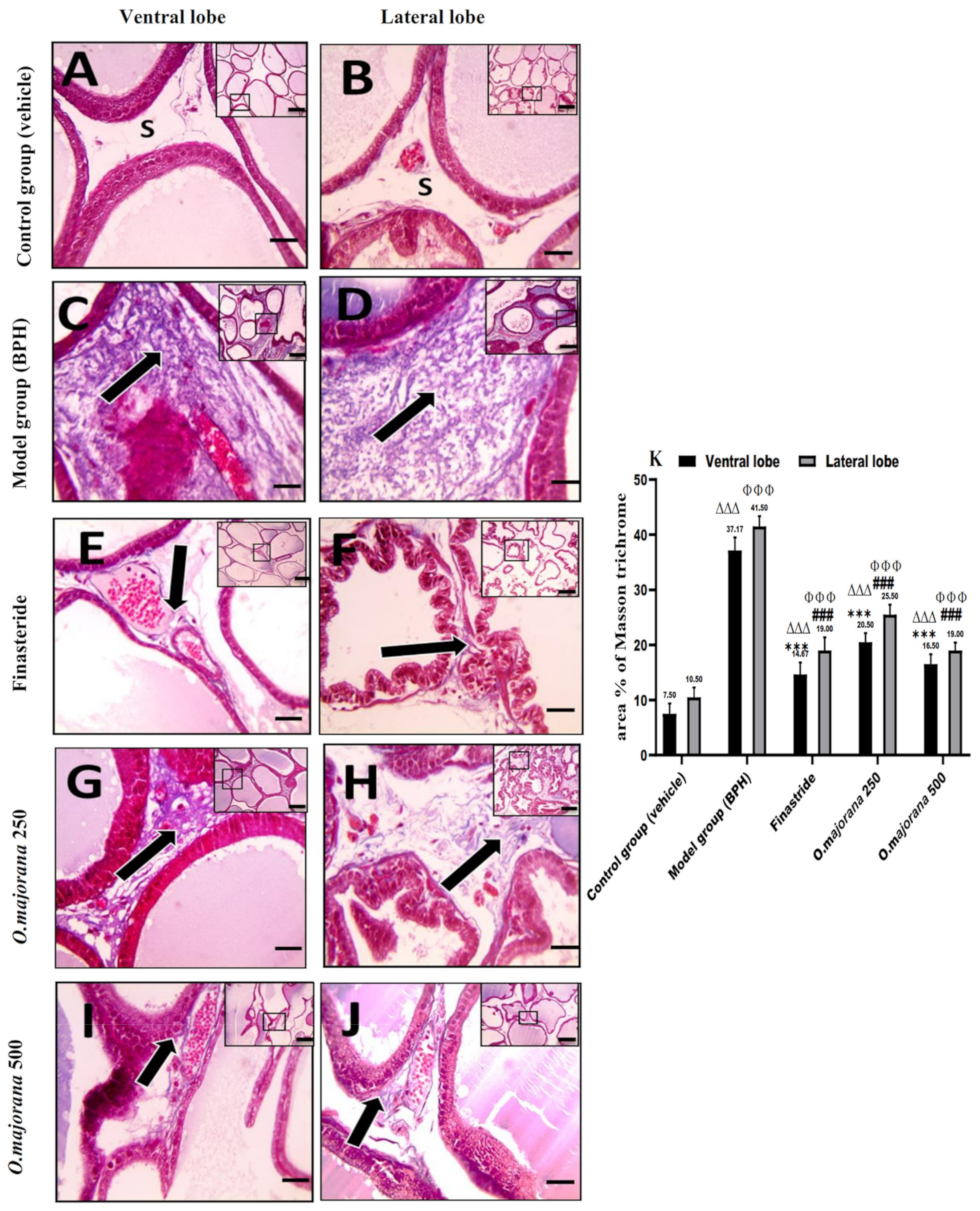
3.7.2. Masson’s Trichrome Stain
3.8. α-SMA and TGF-B1 Expression in Prostate Lobes
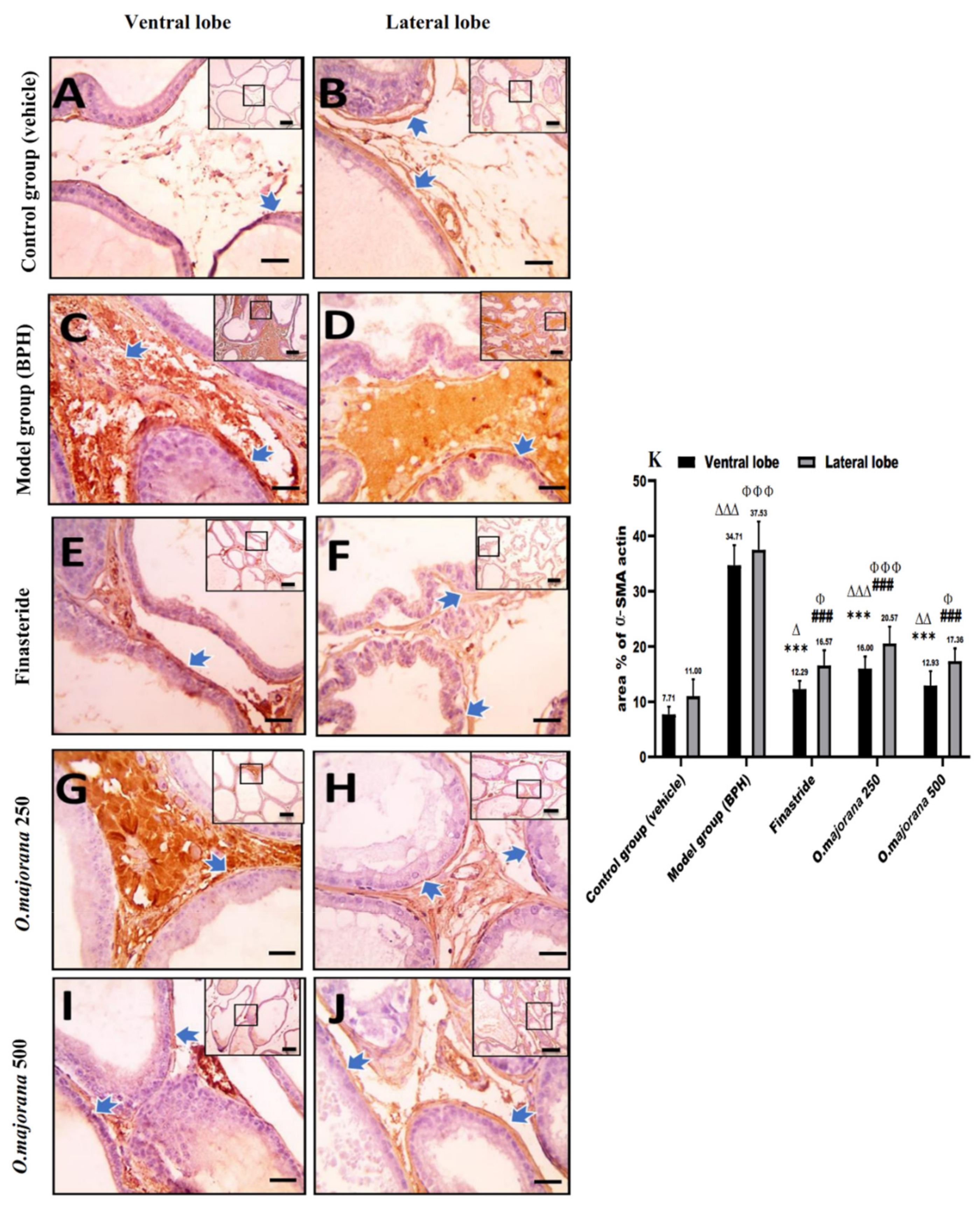
3.8.1. α-SMA Immunostaining
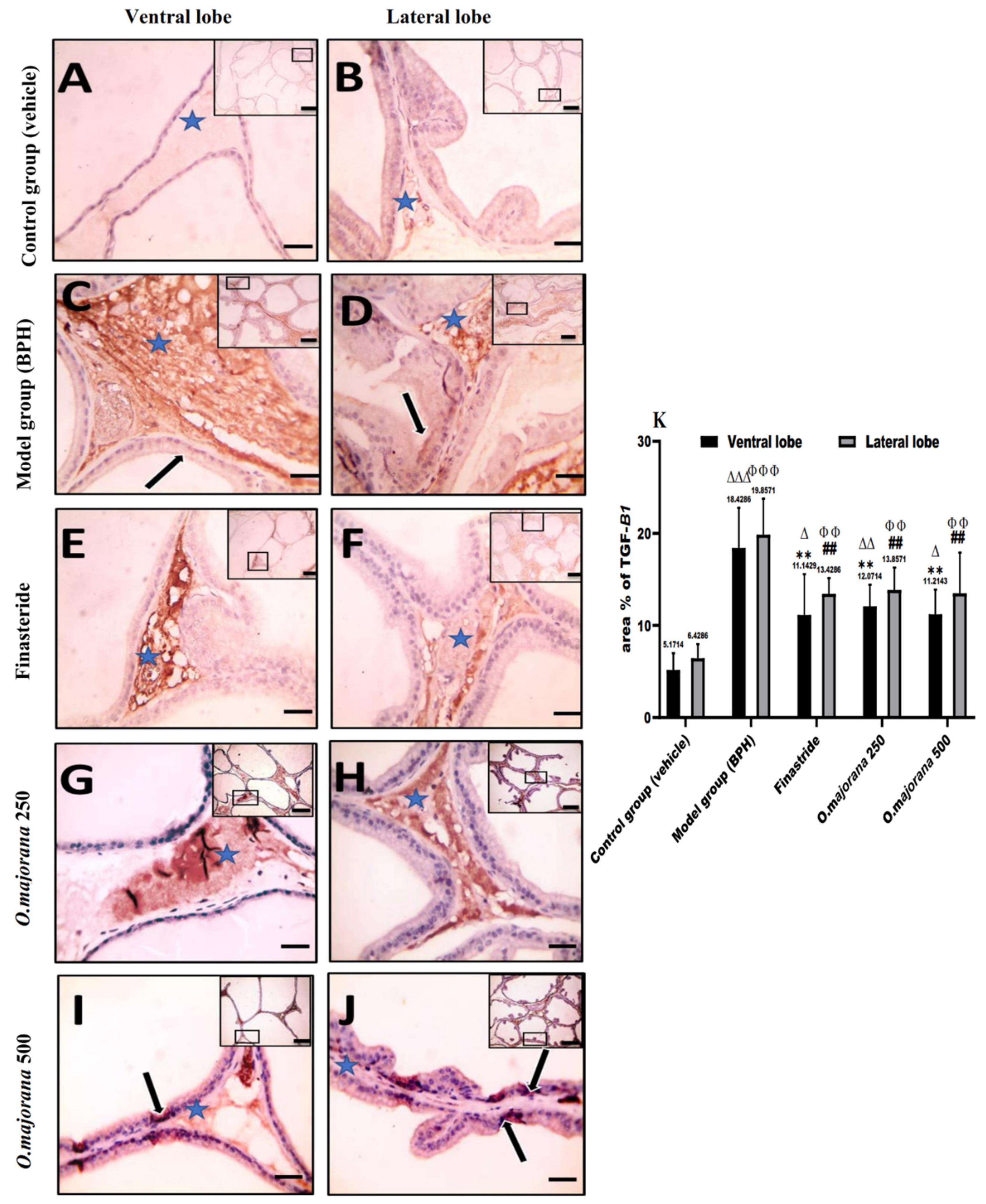
3.8.2. TGF-B1 Immunostaining
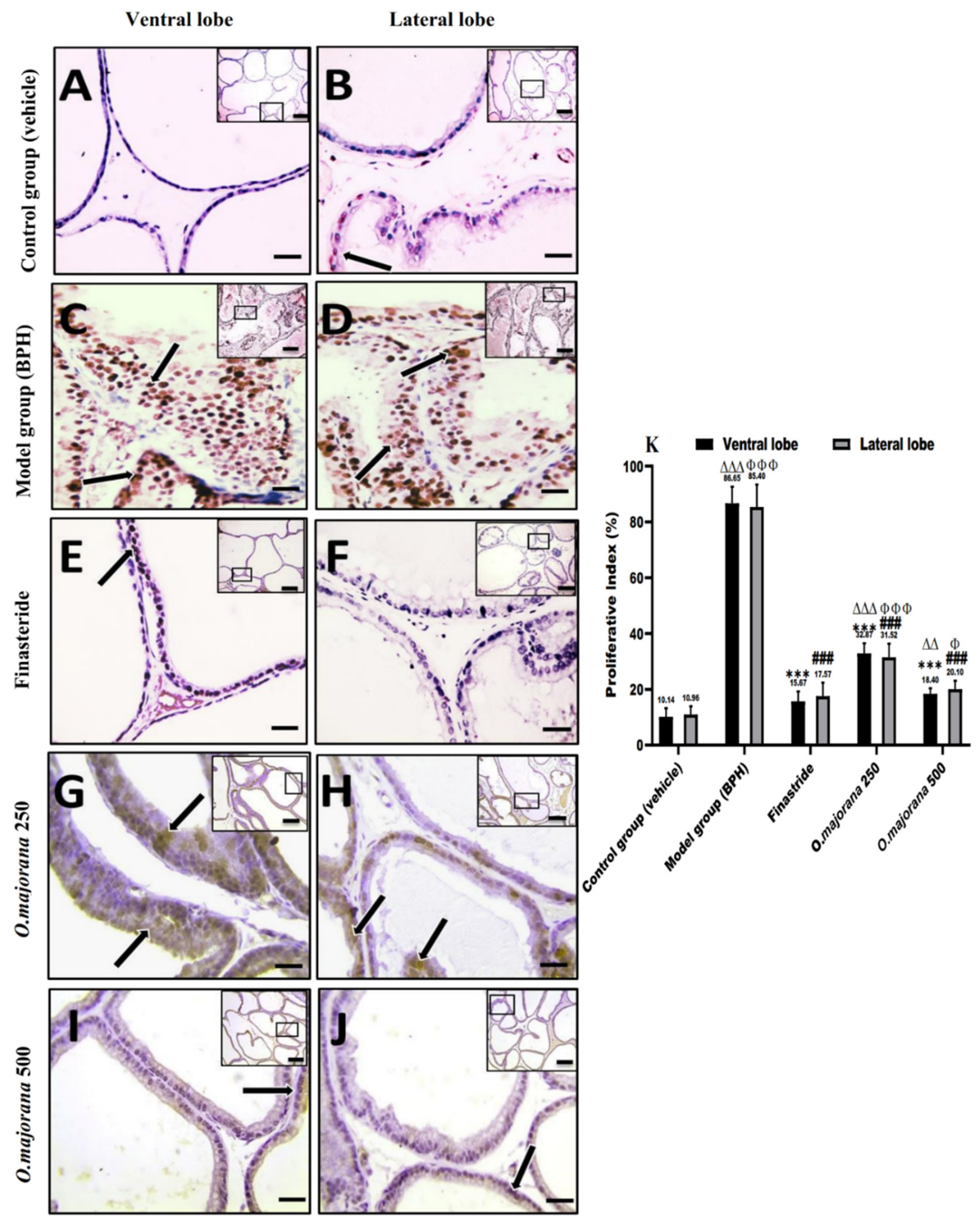
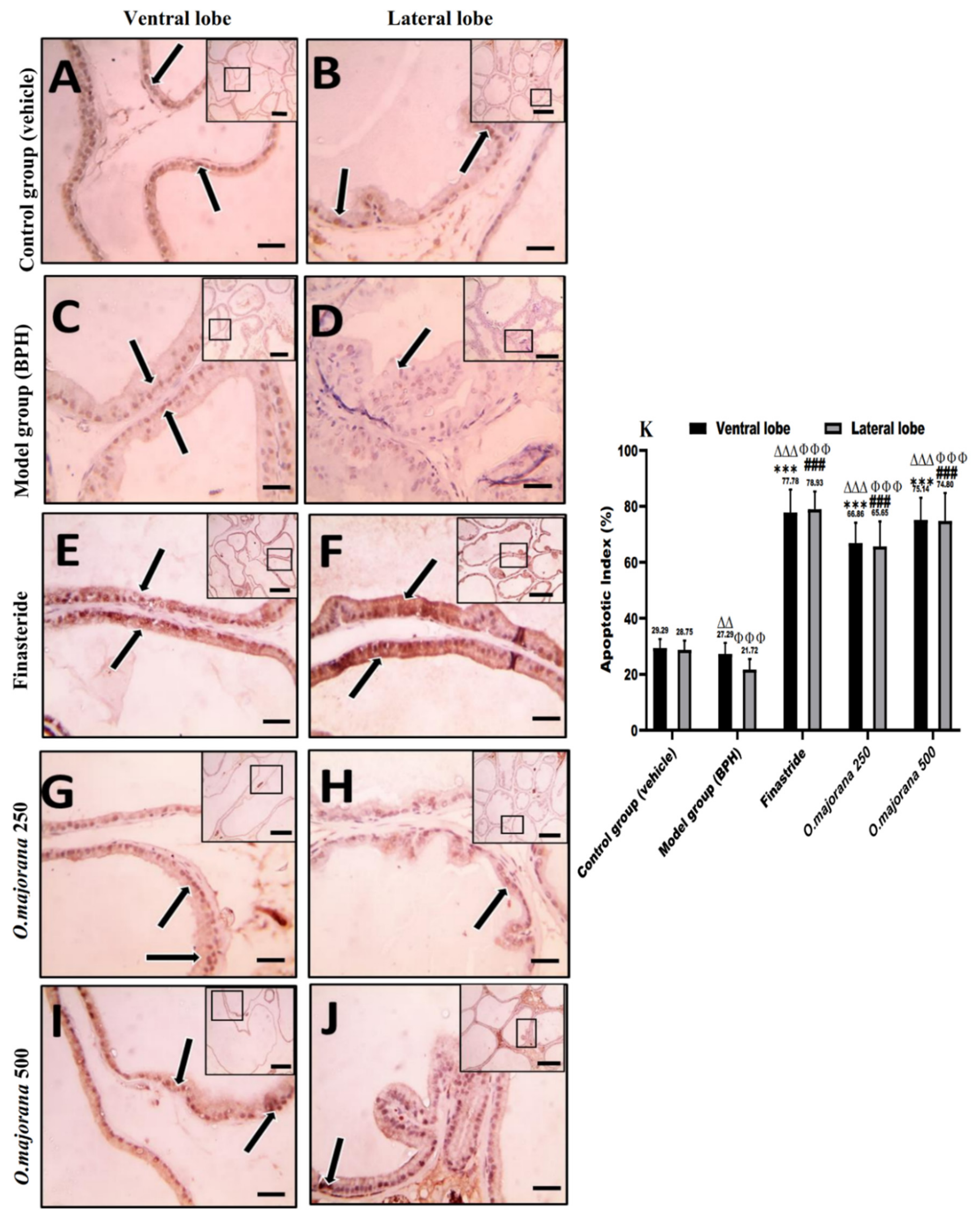
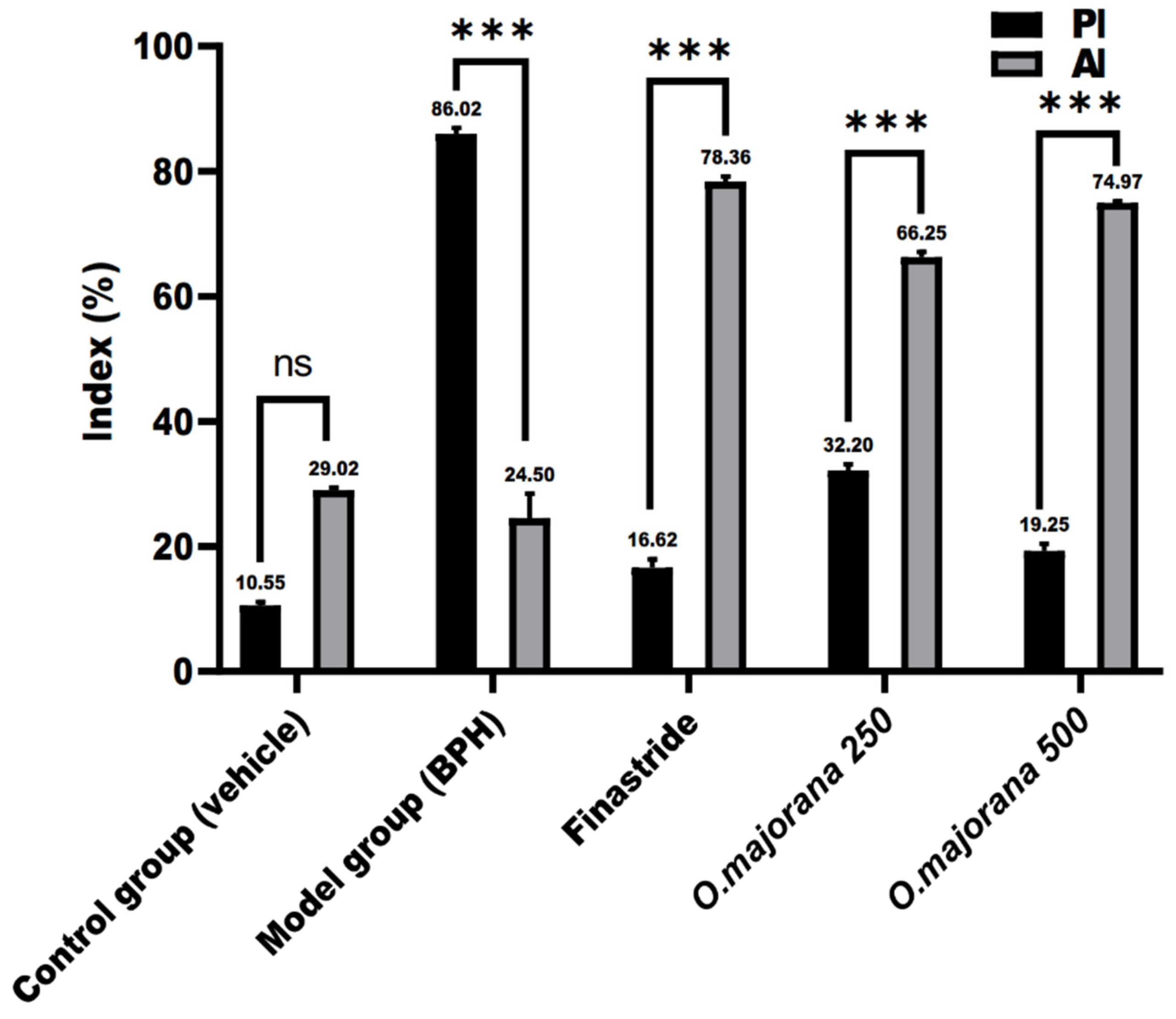
3.9. Apoptotic and Proliferative Indices
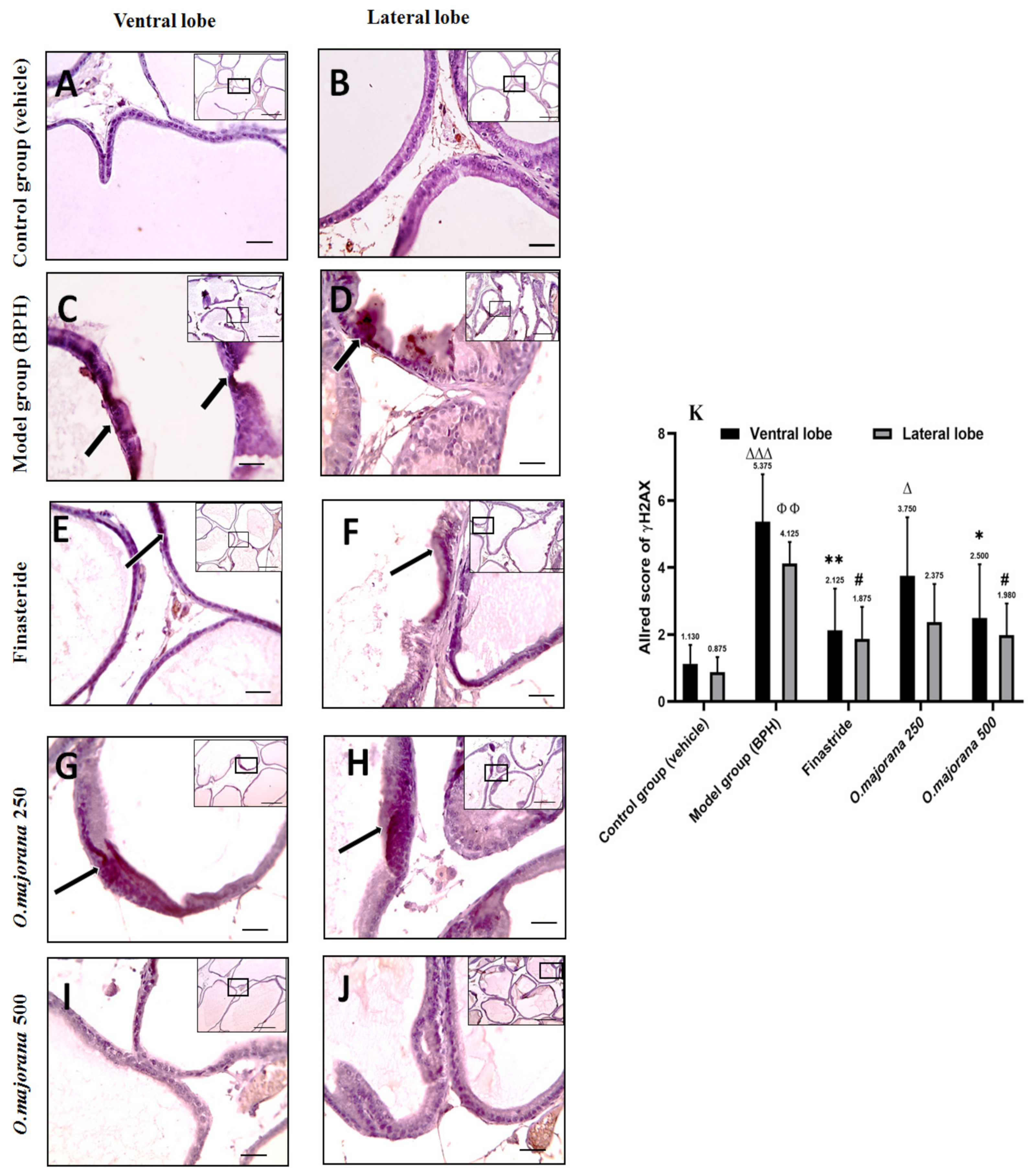
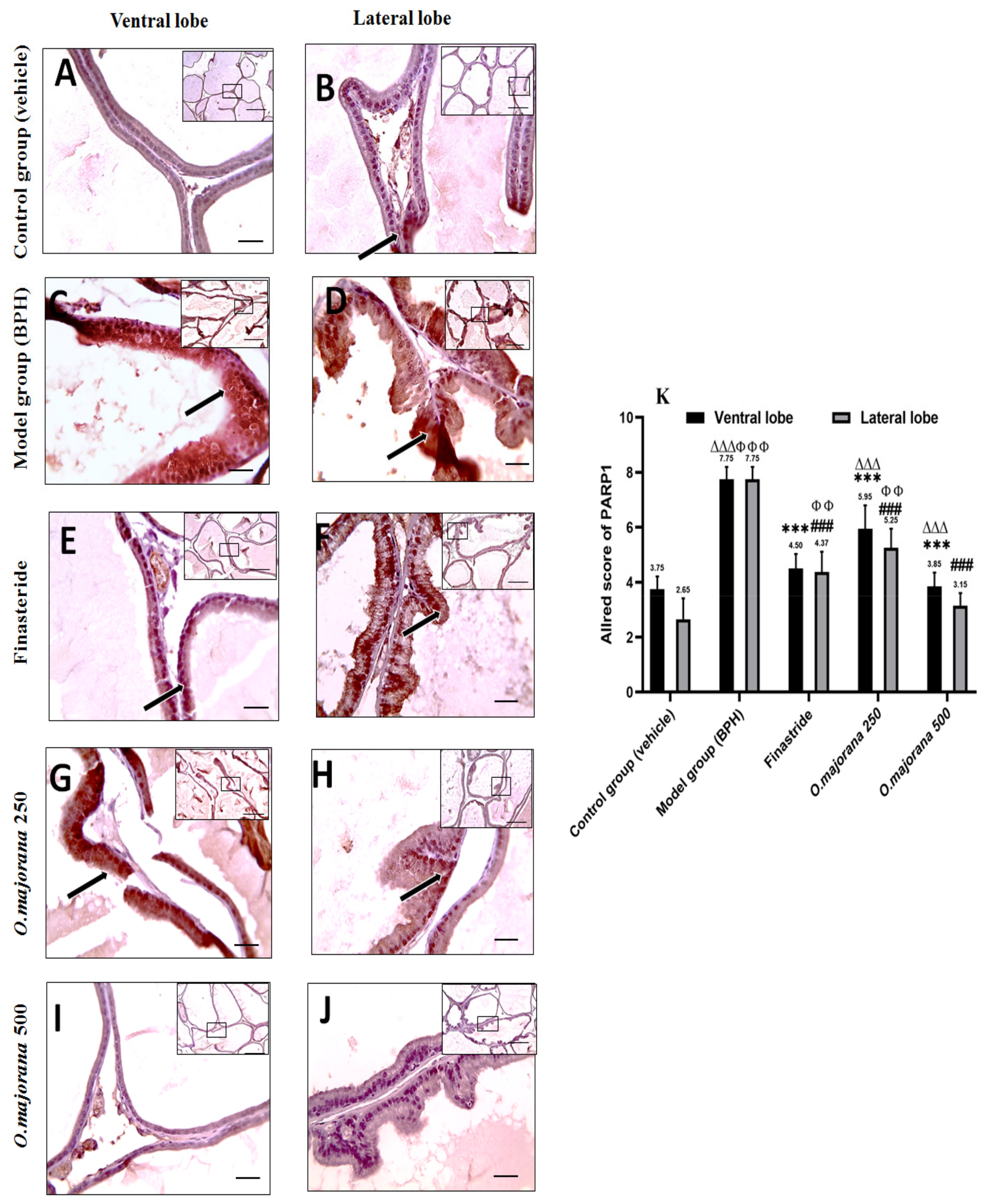
3.10. γ H2Ax and PARP1 Expression in Prostate Lobes
3.10.1. γ H2Ax Expression in Prostate Lobes
3.10.2. PARP1 Expression in Prostate Lobes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gacci, M.; Corona, G.; Salvi, M.; Vignozzi, L.; McVary, K.T.; Kaplan, S.A.; Roehrborn, C.G.; Serni, S.; Mirone, V.; Carini, M.; et al. A systematic review and meta-analysis on the use of phosphodiesterase 5 inhibitors alone or in combination with α-blockers for lower urinary tract symptoms due to benign prostatic hyperplasia. Eur. Urol. 2012, 61, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, M.; El-Shafey, M.; El-Din El-Agawy, M.S.; Mohamed, A.S.; Eisa, N.H.; Elsherbiny, N.M. Diacerein ameliorates testosterone-induced benign prostatic hyperplasia in rats: Effect on oxidative stress, inflammation and apoptosis. Int. Immunopharmacol. 2021, 100, 108082. [Google Scholar] [CrossRef] [PubMed]
- Atawia, R.T.; Tadros, M.G.; Khalifa, A.E.; Mosli, H.A.; Abdel-Naim, A.B. Role of the phytoestrogenic, pro-apoptotic and anti-oxidative properties of silymarin in inhibiting experimental benign prostatic hyperplasia in rats. Toxicol. Lett. 2013, 219, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-H.; Wang, L.-H.; Mei, X.-T.; Li, B.-J.; Lv, J.-L.; Xu, S.-B. Protective effects of seahorse extracts in a rat castration and testosterone-induced benign prostatic hyperplasia model and mouse oligospermatism model. Environ. Toxicol. Pharmacol. 2014, 37, 679–688. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, G.; Yan, Z.; Shang, X. The effect of Xialiqi capsule on testosterone-induced benign prostatic hyperplasia in rats. Evid. Based Complement. Alternat. Med. 2018, 2018, 5367814. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, Y.; Yang, Z.; Qin, X.; Yang, K.; Mao, C. The efficacy and safety of alpha-1 blockers for benign prostatic hyperplasia: An overview of 15 systematic reviews. Curr. Med. Res. Opin. 2013, 29, 279–287. [Google Scholar] [CrossRef]
- Gormley, G.J.; Stoner, E.; Bruskewitz, R.C.; Imperato-McGinley, J.; Walsh, P.C.; McConnell, J.D.; Andriole, G.L.; Geller, J.; Bracken, B.R.; Tenover, J.S.; et al. The effect of finasteride in men with benign prostatic hyperplasia. N. Engl. J. Med. 1992, 327, 1185–1191. [Google Scholar] [CrossRef]
- Lowe, F.C.; McConnell, J.D.; Hudson, P.B.; Romas, N.A.; Boake, R.; Lieber, M.; Elhilali, M.; Geller, J.; Imperto-McGinely, J.; Andriole, G.L.; et al. Long-term 6-year experience with finasteride in patients with benign prostatic hyperplasia. Urology 2003, 61, 791–796. [Google Scholar] [CrossRef]
- Lecce, L.; Lam, Y.T.; Lindsay, L.A.; Yuen, S.C.; Simpson, P.J.; Handelsman, D.J.; Ng, M.K. Aging impairs VEGF-mediated, androgen-dependent regulation of angiogenesis. Mol. Endocrinol. 2014, 28, 1487–1501. [Google Scholar] [CrossRef]
- Uygur, M.; Gür, E.; Arik, A.; Altuǧ, U.; Erol, D. Erectile dysfunction following treatments of benign prostatic hyperplasia: A prospective study. Andrologia 1998, 30, 5–10. [Google Scholar] [CrossRef]
- Chiba, K.; Yamaguchi, K.; Li, F.; Ando, M.; Fujisawa, M. Finasteride-associated male infertility. Fertil. Steril. 2011, 95, 1786.e9–1786.e11. [Google Scholar] [CrossRef]
- Sharma, M.; Chadha, R.; Dhingra, N. Phytotherapeutic agents for benign prostatic hyperplasia: An overview. Mini Rev. Med. Chem. 2017, 17, 1346–1363. [Google Scholar] [CrossRef]
- Kim, S.-D.; Lee, B.-H.; Sohn, D.-W.; Cho, Y.-H.; Lee, S.-M.; Kim, J.-O.; Kim, S.W. The effect of herbal formulation KH-305 mainly consisted of Rubus coreanus on benign prostatic hyperplasia-induced rat. Korean J. Pharmacogn. 2008, 39, 80–85. [Google Scholar]
- Sun, H.; Li, T.-J.; Sun, L.-N.; Qiu, Y.; Huang, B.-B.; Yi, B.; Chen, W.S. Inhibitory effect of traditional Chinese medicine Zi-Shen pill on benign prostatic hyperplasia in rats. J. Ethnopharmacol. 2008, 115, 203–208. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ahn, Y.-M.; Ahn, S.-Y.; Kim, Y.-O.; Lee, B.-C. The antihyperplastic effect of oral Curcuma longa ingestion in a rat model of benign prostatic hyperplasia. Korean J. Intern. Med. 2009, 30, 355–364. [Google Scholar]
- Kim, J.-S.; Han, Y.-H.; Kim, Y.-S. The effects of Scutellaria baicalensis on experimental rat model of benign prostatic hyperplasia. Korean J. Intern. Med. 2009, 30, 327–337. [Google Scholar]
- Shin, I.S.; Lee, M.Y.; Ha, H.K.; Seo, C.S.; Shin, H.-K. Inhibitory effect of Yukmijihwang-tang, a traditional herbal formula against testosterone-induced benign prostatic hyperplasia in rats. BMC Complement. Altern. Med. 2012, 12, 48. [Google Scholar] [CrossRef]
- Kim, S.K.; Seok, H.; Park, H.J.; Jeon, H.S.; Kang, S.W.; Lee, B.-C.; Yi, J.; Song, S.Y.; Lee, S.H.; Kim, Y.O.; et al. Inhibitory effect of curcumin on testosterone induced benign prostatic hyperplasia rat model. BMC Complement. Altern. Med. 2015, 15, 380. [Google Scholar] [CrossRef]
- Choi, H.-M.; Jung, Y.; Park, J.; Kim, H.-L.; Youn, D.-H.; Kang, J.; Jeong, M.Y.; Lee, J.H.; Yang, W.M.; Lee, S.G.; et al. Cinnamomi cortex (Cinnamomum verum) suppresses testosterone-induced benign prostatic hyperplasia by regulating 5α-reductase. Sci. Rep. 2016, 6, 31906. [Google Scholar] [CrossRef]
- Busatta, C.; Vidal, R.; Popiolski, A.; Mossi, A.; Dariva, C.; Rodrigues, M.; Corazza, F.C.; Corazza, M.L.; Vladimir Oliveira, J.; Cansian, R.L. Application of Origanum majorana L. essential oil as an antimicrobial agent in sausage. Food Microbiol. 2008, 25, 207–211. [Google Scholar] [CrossRef]
- El-Meligy, R.M.; Awaad, A.S.; Soliman, G.A.; Kenawy, S.A.; Alqasoumi, S.I. Prophylactic and curative anti-ulcerogenic activity and the possible mechanisms of action of some desert plants. Saudi Pharm. J. 2017, 25, 387–396. [Google Scholar] [CrossRef]
- Soliman, M.M.; Nassan, M.A.; Ismail, T.A. Origanum majoranum extract modulates gene expression, hepatic and renal changes in a rat model of type 2 diabetes. Iran. J. Pharm. Res. IJPR 2016, 15, 45. [Google Scholar] [PubMed]
- Yazdanparast, R.; Shahriyary, L. Comparative effects of Artemisia dracunculus, Satureja hortensis and Origanum majorana on inhibition of blood platelet adhesion, aggregation and secretion. Vasc. Pharmacol. 2008, 48, 32–37. [Google Scholar] [CrossRef]
- Abdel-Massih, R.M.; Fares, R.; Bazzi, S.; El-Chami, N.; Baydoun, E. The apoptotic and anti-proliferative activity of Origanum majorana extracts on human leukemic cell line. Leuk. Res. 2010, 34, 1052–1056. [Google Scholar] [CrossRef]
- El-Ashmawy, I.M.; El-Nahas, A.F.; Salama, O.M. Protective effect of volatile oil, alcoholic and aqueous extracts of Origanum majorana on lead acetate toxicity in mice. Basic Clin. Pharmacol. Toxicol. 2005, 97, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Seoudi, D.M.; Medhat, A.M.; Hewedi, I.H.; Osman, S.A.; Mohamed, M.K.; Arbid, M.S. Evaluation of the anti-inflammatory, analgesic, and anti-pyretic effects of Origanum majorana ethanolic extract in experimental animals. J. Radiat. Res. Appl. Sci. 2009, 2, 513–534. [Google Scholar]
- Refaie, A.A.E.-R.; Ramadan, A.; Mossa, A.-T.H. Oxidative damage and nephrotoxicity induced by prallethrin in rat and the protective effect of Origanum majorana essential oil. Asian Pac. J. Trop. Med. 2014, 7, S506–S513. [Google Scholar] [CrossRef]
- Ennaji, H.; Chahid, D.; Aitssi, S.; Badou, A.; Khlil, N.; Ibenmoussa, S. Phytochemicals screening, cytotoxicity and antioxidant activity of the Origanum majorana growing in Casablanca, Morocco. Open Biol. Sci. J. 2020, 5, 053–059. [Google Scholar]
- Jaganathan, S.K.; Supriyanto, E.; Mandal, M. Events associated with apoptotic effect of p-coumaric acid in HCT-15 colon cancer cells. World J. Gastroenterol. WJG 2013, 19, 7726. [Google Scholar] [CrossRef] [PubMed]
- Chaves, R.d.S.B.; Martins, R.L.; Rodrigues, A.B.L.; de Menezes Rabelo, É.; Farias, A.L.F.; Araújo, C.M.d.C.V.; Almeida, S.S. Larvicidal evaluation of the Origanum majorana L. essential oil against the larvae of the Aedes aegypti mosquito. bioRxiv 2019. [Google Scholar] [CrossRef]
- Nyati, K.K.; Prasad, K.N.; Rizwan, A.; Verma, A.; Paliwal, V.K.; Pradhan, S. Lymphocyte transformation test detects a response to Campylobacter jejuni antigens in patients with Guillain-Barré syndrome. Med. Microbiol. Immunol. 2010, 199, 109–116. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303. [Google Scholar] [CrossRef]
- Kim, K.S.; Yang, H.Y.; Chang, S.C.; Kim, Y.M.; Lee, K.Y.; Lee, B.M.; Kim, H.S. Potential repositioning of GV1001 as a therapeutic agent for testosterone-induced benign prostatic hyperplasia. Int. J. Mol. Med. 2018, 42, 2260–2268. [Google Scholar] [CrossRef]
- Choi, B.R.; Kim, H.K.; Soni, K.K.; Karna, K.K.; Lee, S.W.; So, I.; Park, J.K. Additive effect of oral LDD175 to tamsulosin and finasteride in a benign prostate hyperplasia rat model. Drug Des. Devel. Ther. 2018, 12, 1855. [Google Scholar] [CrossRef]
- Abdel-Naim, A.B.; Neamatallah, T.; Eid, B.G.; Esmat, A.; Alamoudi, A.J.; El-Aziz, A.; Ashour, O.M. 2-methoxyestradiol attenuates testosterone-induced benign prostate hyperplasia in rats through inhibition of HIF-1α/TGF-β/Smad2 axis. Oxid. Med. Cell. Longev. 2018, 2018, 4389484. [Google Scholar] [CrossRef]
- Soliman, A.M.; Desouky, S.; Marzouk, M.; Sayed, A.A. Origanum majorana attenuates nephrotoxicity of cisplatin anticancer drug through ameliorating oxidative stress. Nutrients 2016, 8, 264. [Google Scholar] [CrossRef]
- Abdelsalam, H.M.; Diab, A.A.; Zahra, M.H.; Eldehamy, S.; Hendawy, B.A. Therapeutic effects of Origanum majorana and propolis against nephrotoxicity induced by adenine. Biol. Eng. Med. 2019, 4, 1–9. [Google Scholar] [CrossRef]
- Desouky, S.; Marzouk, M.; Soliman, A.M.; Sayed, A.A. Modulatory effect of Origanum majorana extract against cisplatin-induced dyslipidemia in rats. Int. J. Curr. Res. Life Sci. 2015, 4, 228–234. [Google Scholar]
- Yang, X.; Yuan, L.; Xiong, C.; Yin, C.; Ruan, J. Abacopteris penangiana exerts testosterone-induced benign prostatic hyperplasia protective effect through regulating inflammatory responses, reducing oxidative stress and anti-proliferative. J. Ethnopharmacol. 2014, 157, 105–113. [Google Scholar] [CrossRef]
- Akbari, F.; Azadbakht, M.; Megha, K.; Dashti, A.; Vahedi, L.; Nejad, A.B.; Zahra, M.; Abdi Sarkami, S.; Sadati, M. Evaluation of Juniperus communis L. seed extract on benign prostatic hyperplasia induced in male Wistar rats. Afr. J. Urol. 2021, 27, 48. [Google Scholar] [CrossRef]
- An, Y.J.; Lee, J.Y.; Kim, Y.; Jun, W.; Lee, Y.-H. Cranberry powder attenuates benign prostatic hyperplasia in rats. J. Med. Food 2020, 23, 1296–1302. [Google Scholar] [CrossRef]
- Khoubnasabjafari, M.; Ansarin, K.; Jouyban, A. Critical review of malondialdehyde analysis in biological samples. Curr. Pharm. Anal. 2016, 12, 4–17. [Google Scholar] [CrossRef]
- Kosova, F.; Temeltaş, G.; Arı, Z.; Lekili, M. Possible relations between oxidative damage and apoptosis in benign prostate hyperplasia and prostate cancer patients. Tumor Biol. 2014, 35, 4295–4299. [Google Scholar] [CrossRef]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 1974; pp. 673–684. [Google Scholar]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Al-Trad, B.; Aljabali, A.; Al Zoubi, M.; Shehab, M.; Omari, S. Effect of gold nanoparticles treatment on the testosterone-induced benign prostatic hyperplasia in rats. Int. J. Nanomed. 2019, 14, 3145. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, Y.; Chen, Q.; Xu, H.; Wang, Y.; Yu, J.; Zhou, J.; Wang, X.; Xu, B. DNMT1 regulates IL-6-and TGF-β1-induced epithelial mesenchymal transition in prostate epithelial cells. Eur. J. Histochem. EJH 2017, 61, 2775. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 7th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Zhong, W.; Peng, J.; Wu, D.; Han, Z.; Bi, X.; Dai, Q. Ki-67 and PCNA expression in prostate cancer and benign prostatic hyperplasia. Clin. Investig. Med. 2008, 31, E8–E15. [Google Scholar] [CrossRef]
- Shariat, S.F.; Ashfaq, R.; Roehrborn, C.G.; Slawin, K.M.; Lotan, Y. Expression of survivin and apoptotic biomarkers in benign prostatic hyperplasia. J. Urol. 2005, 174, 2046–2050. [Google Scholar] [CrossRef]
- Zheng, C.; Luo, Y.; Chen, Y.; Chen, D.; Shao, C.; Huang, D.; Zhu, J.; Mao, X.; Li, L.; Sun, Z. Oral exposure of sulpiride promotes the proliferation of brown-Norway rat prostates. Exp. Ther. Med. 2020, 19, 2551–2562. [Google Scholar] [CrossRef]
- Ramos-Vara, J.; Miller, M. When tissue antigens and antibodies get along: Revisiting the technical aspects of immunohistochemistry—The red, brown, and blue technique. Vet. Pathol. 2014, 51, 42–87. [Google Scholar] [CrossRef]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Fedchenko, N.; Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—A review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef]
- Iqbal, B.M.; Buch, A. Hormone receptor (ER, PR, HER2/neu) status and proliferation index marker (Ki-67) in breast cancers: Their onco-pathological correlation, shortcomings and future trends. Med. J. Dr DY Patil Univ. 2016, 9, 674. [Google Scholar] [CrossRef]
- Wu, X.; Gu, Y.; Li, L. The anti-hyperplasia, anti-oxidative and anti-inflammatory properties of Qing Ye Dan and swertiamarin in testosterone-induced benign prostatic hyperplasia in rats. Toxicol. Lett. 2017, 265, 9–16. [Google Scholar] [CrossRef]
- Jung, Y.; Park, J.; Kim, H.-L.; Youn, D.-H.; Kang, J.; Lim, S.; Jeong, M.Y.; Sethi, G.; Park, S.J.; Ahn, K.S. Vanillic acid attenuates testosterone-induced benign prostatic hyperplasia in rats and inhibits proliferation of prostatic epithelial cells. Oncotarget 2017, 8, 87194. [Google Scholar] [CrossRef]
- La Torre, A.; Giupponi, G.; Duffy, D.; Conca, A.; Cai, T.; Scardigli, A. Sexual dysfunction related to drugs: A critical review. Part V: α-blocker and 5-ARI drugs. Pharmacopsychiatry 2016, 49, 3–13. [Google Scholar] [CrossRef]
- Garg, G.; Singh, D.; Saraf, S.; Saraf, S. Management of benign prostate hyperplasia: An overview of α-adrenergic antagonist. Biol. Pharm. Bull. 2006, 29, 1554–1558. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; Khedr, E.G.; El-Bahrawy, H.A.; Helmy, N.N. Modulatory effect of silymarin on apoptosis in testosterone-induced benign prostatic hyperplasia in rats. J. Clin. Oncol. 2020, 26, 1947–1956. [Google Scholar] [CrossRef]
- Gheitasi, I.; Motaghi, N.; Sadeghi, H.; Sadeghi, H.; Moslemi, Z.; Eftekhari, M.; Shakerinasab, N.; Doustimotlagh, A.H. Antioxidant and anti-inflammatory effects of Origanum majorana L. methanolic extract on bile duct ligation in male rats. Evid. Based Complement. Alternat. Med. 2021, 2021, 9927196. [Google Scholar] [CrossRef] [PubMed]
- Veličković, D.T.; Nikolova, M.T.; Ivancheva, S.V.; Stojanović, J.B.; Veljković, V.B. Extraction of flavonoids from garden (Salvia officinalis L.) and glutinous (Salvia glutinosa L.) sage by ultrasonic and classical maceration. J. Serb. Chem. Soc. 2007, 72, 73–80. [Google Scholar] [CrossRef]
- Ahmad, M.; Suhail, N.; Mansoor, T.; Banu, N.; Ahmad, S. Evaluation of oxidative stress and DNA damage in benign prostatic hyperplasia patients and comparison with controls. Indian J. Clin. Biochem. 2012, 27, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.N.; Elsherbiny, N.M.; Shaheen, M.A.; Elseweidy, M.M. Modulation of NADPH oxidase and Nrf2/HO-1 pathway by vanillin in cisplatin-induced nephrotoxicity in rats. J. Pharm. Pharmacol. 2020, 72, 1546–1555. [Google Scholar] [CrossRef]
- Eid, B.G.; Abdel-Naim, A.B. Piceatannol attenuates testosterone-induced benign prostatic hyperplasia in rats by modulation of Nrf2/HO-1/NFκB axis. Front. Pharmacol. 2020, 11, 2219. [Google Scholar] [CrossRef]
- D’Amico, R.; Genovese, T.; Cordaro, M.; Siracusa, R.; Gugliandolo, E.; Peritore, A.F.; Interdonato, L.; Crupi, R.; Cuzzocrea, S.; Di Paola, R.; et al. Palmitoylethanolamide/baicalein regulates the androgen receptor signaling and NF-κB/Nrf2 pathways in benign prostatic hyperplasia. Antioxidants 2021, 10, 1014. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, H.; Liu, C.; Gu, M.; Chen, Q.; Zhan, M.; Wang, Z. Therapeutic effects of 25-hydroxyvitamin D on the pathological process of benign prostatic hyperplasia: An in vitro evidence. Dis. Markers 2021, 2021, 4029470. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Gasparutto, D.; Ravanat, J.-L. Oxidative damage to DNA: Formation, measurement and biochemical features. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2003, 531, 5–23. [Google Scholar] [CrossRef]
- Wang, J.; Yi, J. Cancer cell killing via ROS: To increase or decrease, that is the question. Cancer Biol. Ther. 2008, 7, 1875–1884. [Google Scholar] [CrossRef]
- Trachootham, D.; Zhou, Y.; Zhang, H.; Demizu, Y.; Chen, Z.; Pelicano, H.; Chiao, P.J.; Achanta, G.; Arlinghaus, R.B.; Liu, J.; et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethyl isothiocyanate. Cancer Cell. 2006, 10, 241–252. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Berlett, B.S. Reactive oxygen-mediated protein oxidation in aging and disease. Drug Metab. Rev. 1998, 30, 225–243. [Google Scholar] [CrossRef]
- Na, H.K.; Oliynyk, S. Effects of physical activity on cancer prevention. Ann. N. Y. Acad. Sci. 2011, 1229, 176–183. [Google Scholar] [CrossRef]
- Al Dhaheri, Y.; Eid, A.; AbuQamar, S.; Attoub, S.; Khasawneh, M.; Aiche, G.; Hisaindee, S.; Iratni, R. Mitotic arrest and apoptosis in breast cancer cells induced by Origanum majorana extract: Upregulation of TNF-α and downregulation of survivin and mutant p53. PLoS ONE 2013, 8, e56649. [Google Scholar]
- Rao, S.; Timsina, B.; Nadumane, V.K. Evaluation of the anticancer potentials of Origanum marjorana on fibrosarcoma (HT-1080) cell line. Asian Pac. J. Trop. Dis. 2014, 4, S389–S394. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Mighri, H.; Aouni, M.; Gharsallah, N.; Kadri, A. Chemical composition and in vitro evaluation of antioxidant, antimicrobial, cytotoxicity and anti-acetylcholinesterase properties of Tunisian Origanum majorana L. essential oil. Microb. Pathog. 2016, 95, 86–94. [Google Scholar] [CrossRef]
- García-Risco, M.R.; Mouhid, L.; Salas-Pérez, L.; López-Padilla, A.; Santoyo, S.; Jaime, L.; Ramírez de Molina, A.; Reglero, G.; Fornari, T. Biological activities of Asteraceae (Achillea millefolium and Calendula officinalis) and Lamiaceae (Melissa officinalis and Origanum majorana) plant extracts. Plant Foods Hum. Nutr. 2017, 72, 96–102. [Google Scholar] [CrossRef]
- Makrane, H.; El Messaoudi, M.; Melhaoui, A.; El Mzibri, M.; Benbacer, L.; Aziz, M. Cytotoxicity of the aqueous extract and organic fractions from Origanum majorana on human breast cell line MDA-MB-231 and human colon cell line HT-29. Adv. Pharmacol. Sci. 2018, 2018, 3297193. [Google Scholar] [CrossRef]
- Benhalilou, N.; Alsamri, H.; Alneyadi, A.; Athamneh, K.; Alrashedi, A.; Altamimi, N.; Al Dhaheri, Y.; Eid, A.H.; Iratni, R. Origanum majorana ethanolic extract promotes colorectal cancer cell death by triggering abortive autophagy and activation of the extrinsic apoptotic pathway. Front. Oncol. 2019, 9, 795. [Google Scholar] [CrossRef]
- Pakdemirli, A.; Karaca, C.; Sever, T.; Daşkin, E.; Leblebici, A.; Yiğitbaşi, T.; Başbinar, Y. Carvacrol alters soluble factors in HCT-116 and HT-29 cell lines. Turk. J. Med. Sci. 2020, 50, 271–276. [Google Scholar]
- Elbe, H.; Yigitturk, G.; Cavusoglu, T.; Baygar, T.; Ozgul Onal, M.; Ozturk, F. Comparison of ultrastructural changes and the anticarcinogenic effects of thymol and carvacrol on ovarian cancer cells: Which is more effective? Ultrastruct. Pathol. 2020, 44, 193–202. [Google Scholar] [CrossRef]
- Bouyahya, A.; Belmehdi, O.; Benjouad, A.; El Hassani, R.A.; Amzazi, S.; Dakka, N.; Bakri, Y. Pharmacological properties and mechanism insights of Moroccan anticancer medicinal plants: What are the next steps? Ind. Crops Prod. 2020, 147, 112198. [Google Scholar] [CrossRef]
- Marti, A.; Jaggi, R.; Vallan, C.; Ritter, P.M.; Baltzer, A.; Srinivasan, A.; Dharmarajan, A.M.; Friis, R.R. Physiological apoptosis in hormone-dependent tissues: Involvement of caspases. Cell Death Differ. 1999, 6, 1190–1200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Omezzine, A.; Mauduit, C.; Tabone, E.; Nabli, N.; Bouslama, A.; Benahmed, M. Caspase-3 and-6 expression and activation are targeted by hormone action in the rat ventral prostate during the apoptotic cell death process. Biol. Reprod. 2003, 69, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z.; Hobisch, A.; Cronauer, M.V.; Radmayr, C.; Hittmair, A.; Zhang, J.; Thurnher, M.; Bartsch, G.; Klocker, H. Regulation of prostatic growth and function by peptide growth factors. Prostate 1996, 28, 392–405. [Google Scholar] [CrossRef]
- Tuxhorn, J.A.; Ayala, G.E.; Smith, M.J.; Smith, V.C.; Dang, T.D.; Rowley, D.R. Reactive stroma in human prostate cancer: Induction of myofibroblast phenotype and extracellular matrix remodeling. Clin. Cancer Res. 2002, 8, 2912–2923. [Google Scholar]
- Rick, F.G.; Schally, A.V.; Block, N.L.; Halmos, G.; Perez, R.; Fernandez, J.B.; Vidaurre, I.; Szalontay, L. LHRH antagonist Cetrorelix reduces prostate size and gene expression of proinflammatory cytokines and growth factors in a rat model of benign prostatic hyperplasia. Prostate 2011, 71, 736–747. [Google Scholar] [CrossRef]
- Lai, K.-P.; Huang, C.-K.; Fang, L.-Y.; Izumi, K.; Lo, C.-W.; Wood, R.; Kindblom, J.; Yeh, S.; Chang, C. Targeting stromal androgen receptor suppresses prolactin-driven benign prostatic hyperplasia (BPH). Mol. Endocrinol. 2013, 27, 1617–1631. [Google Scholar] [CrossRef][Green Version]
- Said, E.; Zaitone, S.A.; Eldosoky, M.; Elsherbiny, N.M. Nifuroxazide, a STAT3 inhibitor, mitigates inflammatory burden and protects against diabetes-induced nephropathy in rats. Chem. Biol. Interact. 2018, 281, 111–120. [Google Scholar] [CrossRef]
- Cardenas, H.; Vieth, E.; Lee, J.; Segar, M.; Liu, Y.; Nephew, K.P.; Matei, D. TGF-β induces global changes in DNA methylation during the epithelial-to-mesenchymal transition in ovarian cancer cells. Epigenetics 2014, 9, 1461–1472. [Google Scholar] [CrossRef]
| Gene | Primer Sequences |
|---|---|
| GAPDH | For 5′- CCTCTGACTTCAACAGCGAC-3 Rev 5′-TCCTCTTGTG CTCTTGCTGG-3′ |
| Caspase-3 | For 5′-GGTATTGAGACAGACAGTGG-3′ Rev 5′-CATGGGATCTGTTTCTTTGC-3′ |
| TGF-β1 | For 5′-CAATTCCTGGCGATACCTCAG-3″ Rev 5′-GCACAACTCCGGTGACATCAA-3 |
| Primary Antibody | Function | Manufacturer (Cat. No.) | Host Species | Dilution |
|---|---|---|---|---|
| γH2AX | A marker for DNA double-strand breaks | phospho-S139, Abclonal, Wuhan, China, AP0099) | Rabbit polyclonal | 1:100 |
| PARP | Sensor of DNA damage | Abclonal, Wuhan, China, A0942 | Rabbit polyclonal | 1:100 |
| PCNA | Cell proliferation marker | Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA (Sc-56) | Mouse monoclonal | 1:100 |
| Caspase-3 | Proapoptotic marker | Abcam (ab184787) | Rabbit monoclonal | 1:100 |
| TGF-β1 | Apoptosis, marker for Fibrogenesis. extracellular matrix formation, and morphogenesis | Santa Cruz Biotechnology, Inc. (SC-130348) | Mouse monoclonal | 1:100 |
| α-SMA | Marker for smooth muscle fibers surrounding the acini | Biolegend (MMS-466S) | Rabbit polyclonal | 1:100 |
| Groups | Gain in Body Weight (g) | Prostate Weight (mg) | % Inhibition Prostate Weight | Prostate Index × 10−3 | % Inhibition Prostate Index |
|---|---|---|---|---|---|
| Control group(vehicle) | 38.25 ± 4.65 | 380.59 ± 13.58 a | - | 1.14 ± 0.02 a | - |
| Model group (BPH) | 21.00 ± 3.16 * | 912.50 ± 85.39 b | - | 2.85 ± 0.26 b | - |
| Finasteride | 12.25 ± 2.85 * | 600.02 ± 81.64 c | 58.75 | 1.95 ± 0.28 c | 52.29 |
| O. majorana 250 | 20.00 ± 1.63 * | 812.50 ± 49.30 b,d | 18.80 | 2.59 ± 0.44 b,d | 15.03 |
| O. majorana 500 | 15.75 ± 8.42 * | 637.50 ± 47.87 c,e | 51.70 | 2.04 ± 0.16 c,e | 47.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsherbini, D.M.A.; Almohaimeed, H.M.; El-Sherbiny, M.; Mohammedsaleh, Z.M.; Elsherbiny, N.M.; Gabr, S.A.; Ebrahim, H.A. Origanum majorana L. Extract Attenuated Benign Prostatic Hyperplasia in Rat Model: Effect on Oxidative Stress, Apoptosis, and Proliferation. Antioxidants 2022, 11, 1149. https://doi.org/10.3390/antiox11061149
Elsherbini DMA, Almohaimeed HM, El-Sherbiny M, Mohammedsaleh ZM, Elsherbiny NM, Gabr SA, Ebrahim HA. Origanum majorana L. Extract Attenuated Benign Prostatic Hyperplasia in Rat Model: Effect on Oxidative Stress, Apoptosis, and Proliferation. Antioxidants. 2022; 11(6):1149. https://doi.org/10.3390/antiox11061149
Chicago/Turabian StyleElsherbini, Dalia Mahmoud Abdelmonem, Hailah M. Almohaimeed, Mohamed El-Sherbiny, Zuhair M. Mohammedsaleh, Nehal M. Elsherbiny, Sami A. Gabr, and Hasnaa Ali Ebrahim. 2022. "Origanum majorana L. Extract Attenuated Benign Prostatic Hyperplasia in Rat Model: Effect on Oxidative Stress, Apoptosis, and Proliferation" Antioxidants 11, no. 6: 1149. https://doi.org/10.3390/antiox11061149
APA StyleElsherbini, D. M. A., Almohaimeed, H. M., El-Sherbiny, M., Mohammedsaleh, Z. M., Elsherbiny, N. M., Gabr, S. A., & Ebrahim, H. A. (2022). Origanum majorana L. Extract Attenuated Benign Prostatic Hyperplasia in Rat Model: Effect on Oxidative Stress, Apoptosis, and Proliferation. Antioxidants, 11(6), 1149. https://doi.org/10.3390/antiox11061149







