Association of Hypertension and Organ-Specific Cancer: A Meta-Analysis
Abstract
:1. Introduction
2. Methods
Literature Review and Search Criteria
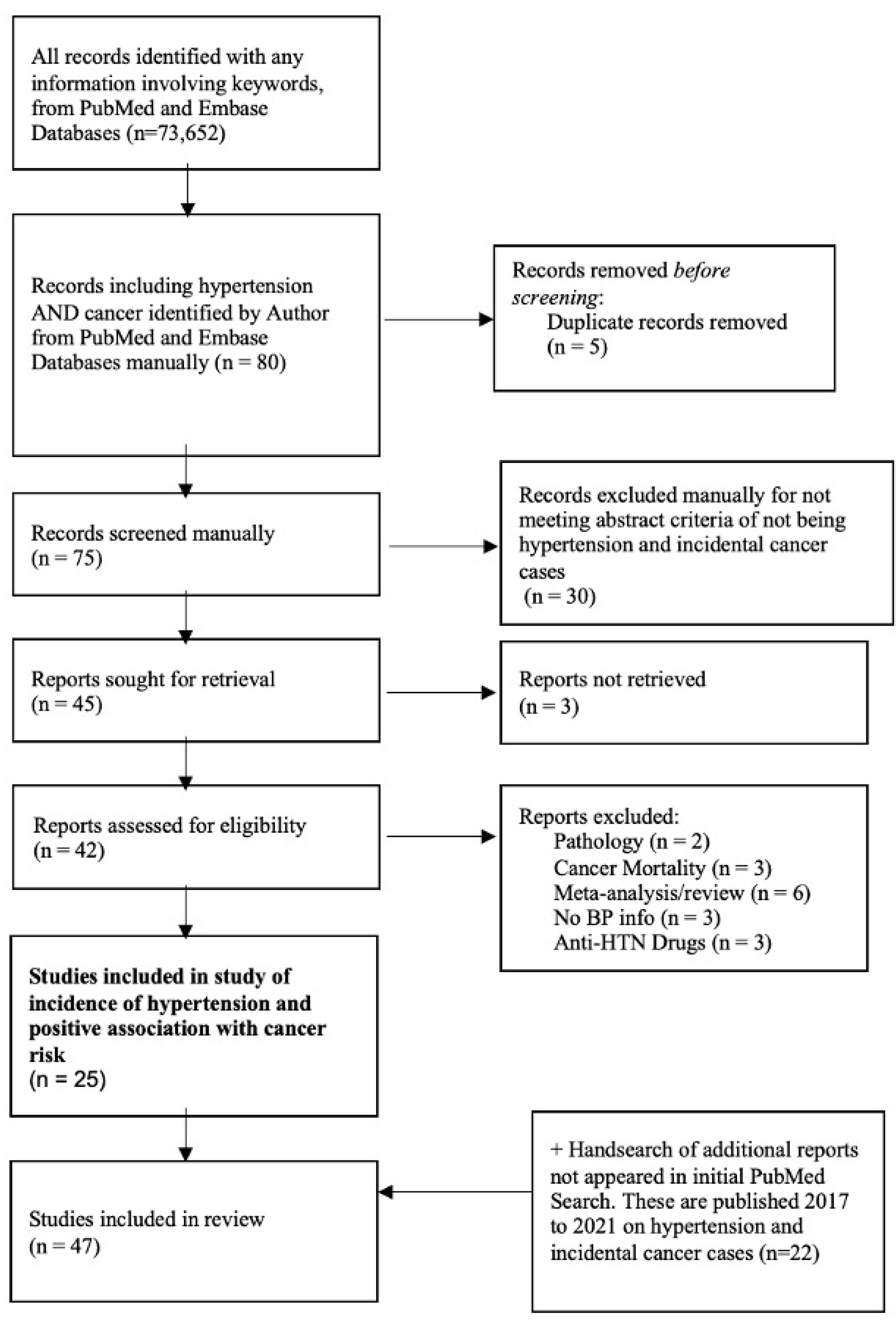
3. Data Extraction
4. Statistical Analysis
5. Results
5.1. Characteristics
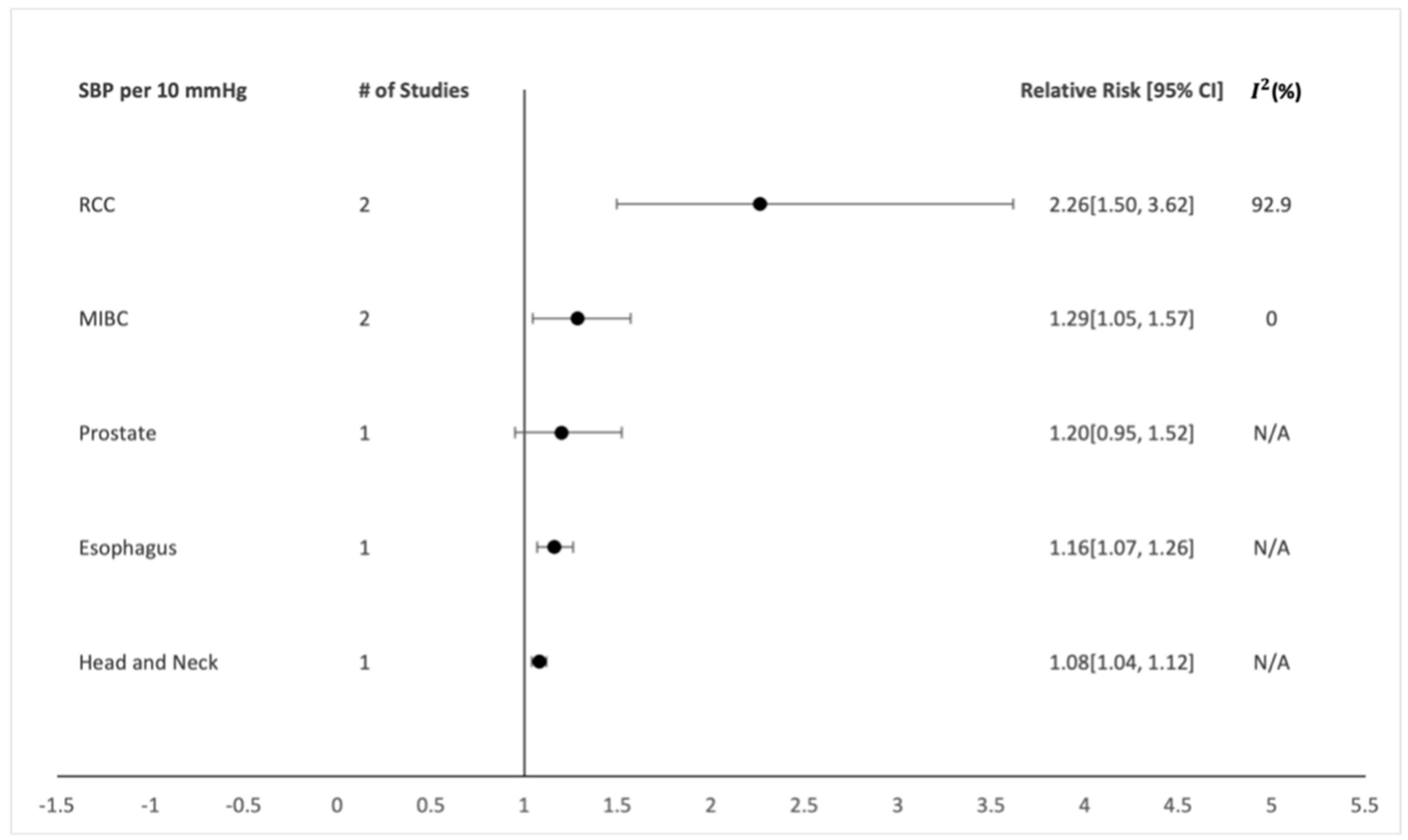
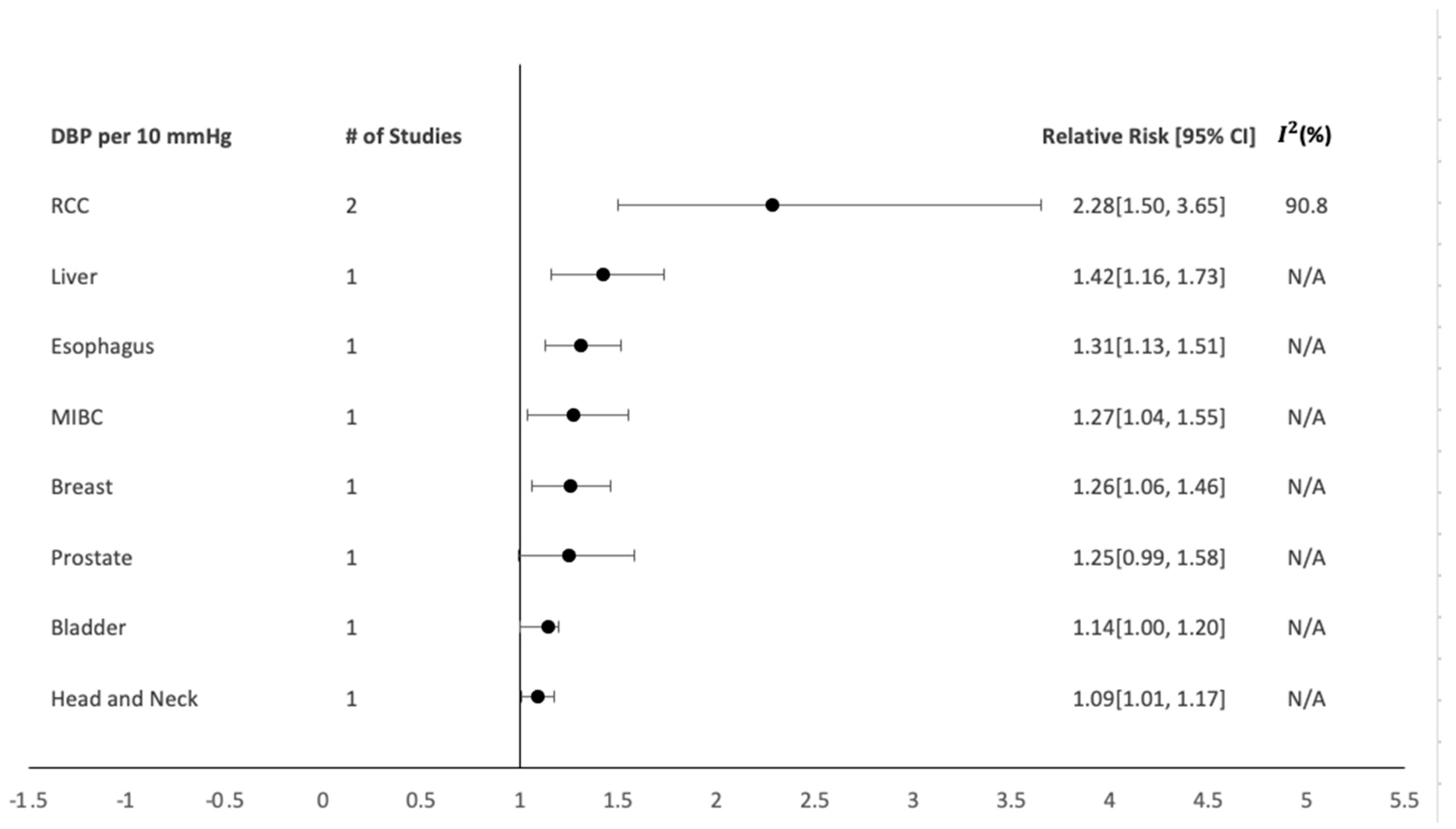
5.2. Overall Analysis
5.3. Evidence
6. Discussion
7. Remodeling of Extracellular Matrix
8. Vascular Endothelial Growth Factor Regulation
9. Reactive Oxygen Species and Renin–Angiotensin–Aldosterone System Regulation
10. Matrix Metalloproteinases Regulation
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Centers for Disease Control and Prevention. Facts about Hypertension. 2021. Available online: http://www.cdc.gov/bloodpressure/facts.htm (accessed on 1 November 2021).
- Soenarta, A.A.; Buranakitjaroen, P.; Chia, Y.-C.; Chen, C.-H.; Nailes, J.; Hoshide, S.; Minh, H.V.; Park, S.; Shin, J.; Siddique, S.; et al. An overview of hypertension and cardiac involvement in Asia: Focus on heart failure. J. Clin. Hypertens. 2020, 22, 423–430. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Hypertension. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 1 July 2021).
- World Health Organization. Cancer. 2021. Available online: www.who.int/news-room/fact-sheets/detail/cancer (accessed on 1 July 2021).
- Seretis, A.; Cividini, S.; Markozannes, G.; Tseretopoulou, X.; Lopez, D.S.; Ntzani, E.E.; Tsilidis, K.K. Association between Blood Pressure and Risk of Cancer Development: A Systematic Review and Meta-Analysis of Observational Studies. Sci. Rep. 2019, 9, 8565. Available online: https://www.nature.com/articles/s41598-019-45014-4 (accessed on 1 January 2021). [CrossRef] [PubMed] [Green Version]
- Stocks, T.; Van Hemelrijck, M.; Manjer, J.; Bjørge, T.; Ulmer, H.; Hallmans, G.; Lindkvist, B.; Selmer, R.; Nagel, G.; Tretli, S.; et al. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension 2012, 59, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, A.M.; Nissinen, A.M.; Tuomilehto, J.O.; Pukkala, E. Cancer pattern among hypertensive patients in North Karelia, Finland. J. Hum. Hypertens. 2005, 19, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Behrens, I.; Basit, S.; Jensen, A.; Lykke, J.A.; Nielsen, L.P.; Wohlfahrt, J.; Kjaer, S.K.; Melbye, M.; Boyd, H. Hypertensive disorders of pregnancy and subsequent risk of solid cancer—A nationwide cohort study: Hypertensive disorders of pregnancy and solid cancer. Int. J. Cancer 2016, 139, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Lofterød, T.; Frydenberg, H.; Flote, V.; Eggen, A.E.; McTiernan, A.; Mortensen, E.S.; Akslen, L.A.; Reitan, J.B.; Wilsgaard, T.; Thune, I. Exploring the effects of lifestyle on breast cancer risk, age at diagnosis, and survival: The EBBA-Life study. Breast Cancer Res. Treat. 2020, 182, 215–227. [Google Scholar] [CrossRef]
- Sun, L.-M.; Kuo, H.-T.; Jeng, L.-B.; Lin, C.-L.; Liang, J.-A.; Kao, C.-H. Hypertension and subsequent genitourinary and gynecologic cancers risk: A population-based cohort study. Medicine 2015, 94, e753. [Google Scholar] [CrossRef]
- Yassin, S.; Younis, M.; Abuzerr, S.; Darwish, M.; Mustafa, A.A. Extrinsic risk factors for women breast cancer in Gaza strip, Palestine: Associations and interactions in a case-control study. Adv. Breast Cancer Res. 2019, 08, 11–30. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.; Garmendia, M.L.; Alvarado, M.E.; Albala, C. Hypertension and the risk of breast cancer in Chilean women: A case-control study. Asian Pac. J. Cancer Prev. 2012, 13, 5829–5834. [Google Scholar] [CrossRef] [Green Version]
- Chuang, S.-C.; Wu, G.-J.; Lu, Y.-S.; Lin, C.-H.; Hsiung, C.A. Associations between medical conditions and breast cancer risk in Asians: A nationwide population-based study in Taiwan. PLoS ONE 2015, 10, e0143410. [Google Scholar] [CrossRef]
- Beji, N.K.; Reis, N. Risk factors for breast cancer in Turkish women: A hospital-based case-control study. Eur. J. Cancer Care 2007, 16, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.J.; Song, M.; Choi, J.-Y.; Song, N.; Park, S.K.; Yoo, K.-Y.; Kang, D. Association of selected medical conditions with breast cancer risk in Korea. J. Prev. Med. Public Health 2013, 46, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, H.A.; Lee, S.S.; Kim, S.-E.; Shim, K.-N.; Jung, H.-K.; Jung, S.-A.; Chang, J.H.; Kwon, K.; Pyun, W.B.; et al. Clinical impact of pre-hypertension on the risk of cancer in male and female subjects. Sci. Rep. 2020, 10, 9974. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Yoon, S.-J.; Kim, D.; Kim, A.-R.; Kim, E.-J.; Seo, H.-Y. Metabolic risk profile and cancer in Korean men and women. J. Prev. Med. Public Health 2016, 49, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirai, H.W.; Ching, J.Y.L.; Wu, J.C.Y.; Sung, J.J.Y.; Chan, F.K.L.; Ng, S.C. Risk factors for advanced colorectal neoplasms in the proximal colon in 6218 subjects undergoing complete colonoscopy: Risk factor of proximal colonic lesions. J. Gastroenterol. Hepatol. 2019, 34, 113–119. [Google Scholar] [CrossRef]
- Samarakoon, Y.M.; Gunawardena, N.S.; Pathirana, A. Behavioral, familial and comorbid illness risk factors of colorectal cancer: A case control study. Ceylon Med. J. 2018, 63, 113–118. [Google Scholar] [CrossRef]
- Dickerman, B.A.; Torfadottir, J.E.; Valdimarsdottir, U.A.; Wilson, K.M.; Steingrimsdottir, L.; Aspelund, T.; Batista, J.L.; Fall, K.; Giovannucci, E.; Sigurdardottir, L.G.; et al. Midlife metabolic factors and prostate cancer risk in later life. Int. J. Cancer 2018, 142, 1166–1173. [Google Scholar] [CrossRef] [Green Version]
- Kabat, G.C.; Kim, M.; Chlebowski, R.T.; Khandekar, J.; Ko, M.G.; McTiernan, A.; Neuhouser, M.L.; Parker, D.R.; Shikany, J.M.; Stefanick, M.L.; et al. A longitudinal study of the metabolic syndrome and risk of postmenopausal breast cancer. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 2046–2053. [Google Scholar] [CrossRef] [Green Version]
- Berger, S.M.; Gislason, G.; Moore, L.L.; Andersson, C.; Torp-Pedersen, C.; Denis, G.V.; Schmiegelow, M.D. Associations between metabolic disorders and risk of cancer in Danish men and women—A nationwide cohort study. BMC Cancer 2016, 16, 133. [Google Scholar] [CrossRef] [Green Version]
- Choi, I.; Chun, S.; Shin, D.; Han, K.; Jeon, K.; Yu, J.; Chae, B.; Suh, M.; Park, Y.-M. Changes in metabolic syndrome status and breast cancer risk: A nationwide cohort study. Cancers 2021, 13, 1177. [Google Scholar] [CrossRef]
- Trabert, B.; Wentzensen, N.; Felix, A.S.; Yang, H.P.; Sherman, M.E.; Brinton, L.A. Metabolic syndrome and risk of endometrial cancer in the United States: A study in the SEER–medicare linked database. Cancer Epidemiol. Biomarkers Prev. 2015, 24, 261–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christakoudi, S.; Kakourou, A.; Markozannes, G.; Tzoulaki, I.; Weiderpass, E.; Brennan, P.; Gunter, M.; Dahm, C.; Overvad, K.; Olsen, A.; et al. Blood pressure and risk of cancer in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2020, 146, 2680–2693. [Google Scholar] [CrossRef] [PubMed]
- Christakoudi, S.; Kakourou, A.; Markozannes, G.; Tzoulaki, I.; Weiderpass, E.; Brennan, P.; Gunter, M.; Dahm, C.; Overvad, K.; Olsen, A.; et al. Association of hypertension and obesity with renal cell carcinoma risk: A report from the Shanghai Men’s and Women’s Health Studies. Cancer Causes Control 2015, 26, 1173–1180. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.S.; Han, K.-D.; Choi, H.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. Association of hypertension and blood pressure with kidney cancer risk: A nationwide population-based cohort study: A nationwide population-based cohort study. Hypertension 2020, 75, 1439–1446. [Google Scholar] [CrossRef]
- Colt, J.S.; Schwartz, K.; Graubard, B.I.; Davis, F.; Ruterbusch, J.; DiGaetano, R.; Purdue, M.; Rothman, N.; Wacholder, S.; Chow, W.-H. Hypertension and risk of renal cell carcinoma among white and black Americans. Epidemiology 2011, 22, 797–804. [Google Scholar] [CrossRef] [Green Version]
- Kocher, N.J.; Rjepaj, C.; Robyak, H.; Lehman, E.; Raman, J.D. Hypertension is the primary component of metabolic syndrome associated with pathologic features of kidney cancer. World J. Urol. 2017, 35, 67–72. [Google Scholar] [CrossRef]
- Seo, J.-H.; Kim, Y.-D.; Park, C.-S.; Han, K.; Joo, Y.-H. Hypertension is associated with oral, laryngeal, and esophageal cancer: A nationwide population-based study. Sci. Rep. 2020, 10, 10291. [Google Scholar] [CrossRef]
- Teleka, S.; Häggström, C.; Nagel, G.; Bjørge, T.; Manjer, J.; Ulmer, H.; Liedberg, F.; Ghaderi, S.; Lang, A.H.; Jonsson, H.; et al. Risk of bladder cancer by disease severity in relation to metabolic factors and smoking: A prospective pooled cohort study of 800,000 men and women: Metabolic factors and bladder cancer risk. Int. J. Cancer 2018, 143, 3071–3082. [Google Scholar] [CrossRef] [Green Version]
- Teleka, S.; Jochems, S.H.J.; Häggström, C.; Wood, A.M.; Järvholm, B.; Orho-Melander, M.; Liedberg, F.; Stocks, T. Association between blood pressure and BMI with bladder cancer risk and mortality in 340,000 men in three Swedish cohorts. Cancer Med. 2021, 10, 1431–1438. [Google Scholar] [CrossRef]
- Peeters, P.H.; van Noord PAHoes, A.W.; Grobbee, D.E. Hypertension, antihypertensive drugs, and mortality from cancer among women. J. Hypertens. 1998, 16, 941–947. [Google Scholar] [CrossRef]
- Navin, S.; Ioffe, V. The association between hypertension and prostate cancer. Rev. Urol. 2017, 19, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Azzam, N.; AlRuthia, Y.; Alharbi, O.; Aljebreen, A.; Almadi, M.; Alarfaj, M.; Alsaleh, K.; Almasoud, A.; Alsharidah, M.; Alseneidi, S.; et al. Predictors of survival among colorectal cancer patients in a low incidence area. Cancer Manag. Res. 2020, 12, 451–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dibaba, D.T.; Ogunsina, K.; Braithwaite, D.; Akinyemiju, T. Metabolic syndrome and risk of breast cancer mortality by menopause, obesity, and subtype. Breast Cancer Res. Treat. 2019, 174, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Tini, G.; Sarocchi, M.; Tocci, G.; Arboscello, E.; Ghigliotti, G.; Novo, G.; Brunelli, C.; Lenihan, D.; Volpe, M.; Spallarossa, P. Arterial hypertension in cancer: The elephant in the room. Int. J. Cardiol. 2019, 281, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.L.; Sooriyakumaran, M.; Anstey, K.J.; Adams, R.; Balkau, B.; Brennan-Olsen, S.; Briffa, T.; Davis, T.M.; Davis, W.A.; Dobson, A.; et al. Hypertension, antihypertensive treatment and cancer incidence and mortality: A pooled collaborative analysis of 12 Australian and New Zealand cohorts. J. Hypertens. 2016, 34, 149–155. [Google Scholar] [CrossRef]
- Emaus, A.; Veierød, M.B.; Tretli, S.; Finstad, S.E.; Selmer, R.; Furberg, A.-S.; Bernstein, L.; Schlichting, E.; Thune, I. Metabolic profile, physical activity, and mortality in breast cancer patients. Breast Cancer Res. Treat. 2010, 121, 651–660. [Google Scholar] [CrossRef]
- Hu, D.; Jia, R.; Zhang, X.; Lin, X.; Zhang, H.; Xia, Y.; Lin, J.; Zheng, X.; Peng, F.; Niu, W. Identification of optimal baseline blood pressure predicting postoperative digestive tract cancer-specific mortality in the FIESTA cohort involving 6865 patients. J. Cancer 2019, 10, 1794–1799. [Google Scholar] [CrossRef]
- Yang, P.; Elhalawani, H.; Shi, Y.; Tang, Y.; Han, Y.; Zhao, Y.; Lou, F.; Jin, H. A large-scale retrospective study of the overall survival outcome in nasopharyngeal carcinoma with hypertension in Chinese population. Oncotarget 2017, 8, 75577–75586. [Google Scholar] [CrossRef] [Green Version]
- Grant, R.L. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ 2014, 348, f7450. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yu, K.F. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998, 280, 1690–1691. [Google Scholar] [CrossRef] [Green Version]
- Altman, D.G.; Bland, J.M. How to obtain the P value from a confidence interval. BMJ 2011, 343, d2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neyeloff, J.L.; Fuchs, S.C.; Moreira, L.B. Meta-analyses and Forest plots using a microsoft excel spreadsheet: Step-by-step guide focusing on descriptive data analysis. BMC Res. Notes 2012, 5, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindgren, A.; Pukkala, E.; Tuomilehto, J.; Nissinen, A. Incidence of breast cancer among postmenopausal, hypertensive women. Int. J. Cancer 2007, 121, 641–644. [Google Scholar] [CrossRef]
- Beebe-Dimmer, J.L.; Dunn, R.L.; Sarma, A.V.; Montie, J.E.; Cooney, K.A. Features of the metabolic syndrome and prostate cancer in African-American men. Cancer 2007, 109, 875–881. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, A.; Pukkala, E.; Nissinen, A.; Tuomilehto, J. Blood pressure, smoking, and the incidence of lung cancer in hypertensive men in North Karelia, Finland. Am. J. Epidemiol. 2003, 158, 442–447. [Google Scholar] [CrossRef] [Green Version]
- Batty, G.D.; Shipley, M.J.; Marmot, M.G.; Davey Smith, G. Blood pressure and site-specific cancer mortality: Evidence from the original Whitehall study. Br. J. Cancer 2003, 89, 1243–1247. [Google Scholar] [CrossRef] [Green Version]
- Wallner, L.P.; Morgenstern, H.; McGree, M.E.; Jacobson, D.J.; Sauver, J.L.S.; Jacobsen, S.J.; Sarma, A.V. The effects of metabolic conditions on prostate cancer incidence over 15 years of follow-up: Results from the Olmsted County Study. BJU Int. 2011, 107, 929–935. [Google Scholar] [CrossRef] [Green Version]
- Teleka, S.; Hindy, G.; Drake, I.; Poveda, A.; Melander, O.; Liedberg, F.; Orho-Melander, M.; Stocks, T. Blood pressure and bladder cancer risk in men by use of survival analysis and in interaction with NAT2 genotype, and by Mendelian randomization analysis. PLoS ONE 2020, 15, e0241711. [Google Scholar] [CrossRef]
- Drahos, J.; Ricker, W.; Pfeiffer, R.M.; Cook, M.B. Metabolic syndrome and risk of esophageal adenocarcinoma in elderly patients in the United States: An analysis of SEER-Medicare data: MetS and EA in Elderly Patients in the US. Cancer 2017, 123, 657–665. [Google Scholar] [CrossRef]
- Staples, J.N.; Peres, L.C.; Camacho, F.; Alberg, A.J.; Bandera, E.V.; Barnholtz-Sloan, J.; Bondy, M.L.; Cote, M.L.; Funkhouser, E.; Moorman, P.G.; et al. Cardiometabolic comorbidities and epithelial ovarian cancer risk among African-American women in the African-American Cancer Epidemiology Study (AACES). Gynecol. Oncol. 2020, 158, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Michels, K.A.; McNeel, T.S.; Trabert, B. Metabolic syndrome and risk of ovarian and fallopian tube cancer in the United States: An analysis of linked SEER-Medicare data. Gynecol. Oncol. 2019, 155, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Kok, V.C.; Zhang, H.-W.; Lin, C.-T.; Huang, S.-C.; Wu, M.-F. Positive association between hypertension and urinary bladder cancer: Epidemiologic evidence involving 79,236 propensity score-matched individuals. Ups J. Med. Sci. 2018, 123, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hektoen, H.H.; Robsahm, T.E.; Andreassen, B.K.; Stenehjem, J.S.; Axcrona, K.; Mondul, A.; Gislefoss, R.E. Lifestyle associated factors and risk of urinary bladder cancer: A prospective cohort study from Norway. Cancer Med. 2020, 9, 4420–4432. [Google Scholar] [CrossRef] [PubMed]
- van de Pol, J.A.A.; George, L.; van den Brandt, P.A.; Baldewijns, M.M.L.L.; Schouten, L.J. Etiologic heterogeneity of clear-cell and papillary renal cell carcinoma in the Netherlands Cohort Study. Int. J. Cancer 2021, 148, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Du, H.; Li, S.; Liu, J. The association between metabolic syndrome and gastric cancer in Chinese. Front. Oncol. 2018, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Al-Madani, W.; Ahmed, A.E.; Arabi, H.; Al Khodairy, S.; Al Mutairi, N.; Jazieh, A.R. Modelling risk assessment for cervical cancer in symptomatic Saudi women. Saudi Med. J. 2019, 40, 447–451. [Google Scholar] [CrossRef]
- Porto, L.A.M.; Lora, K.J.B.; Soares, J.C.M.; Costa, L.O.B.F. Metabolic syndrome is an independent risk factor for breast cancer. Arch. Gynecol. Obstet. 2011, 284, 1271–1276. [Google Scholar] [CrossRef]
- Jeon, K.H.; Shin, D.W.; Han, K.; Kim, D.; Yoo, J.E.; Jeong, S.-M.; Cho, J.H. Female reproductive factors and the risk of lung cancer in postmenopausal women: A nationwide cohort study. Br. J. Cancer 2020, 122, 1417–1424. [Google Scholar] [CrossRef]
- Bashamakha, G.; Bin Sumait, H.; Bashamakha, M.; Al Serouri, A.; Khader, Y. Risk factors of breast cancer in Hadramout Valley and desert, Yemen. Int. J. Prev. Med. 2019, 10, 161. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Fuchs, C.S.; Colditz, G.A.; Stampfer, M.J.; Speizer, F.E.; Willett, W.C.; Curhan, G.C. A prospective study of body mass index, hypertension, and smoking and the risk of renal cell carcinoma (United States). Cancer Causes Control 2005, 16, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Hã¤Ggstrã¶m, C.; Rapp, K.; Stocks, T.; Manjer, J.; Bjã¸rge, T.; Ulmer, H.; Engeland, A.; Almqvist, M.; Concin, H.; Selmer, R.; et al. Correction: Metabolic factors associated with risk of renal cell carcinoma. PLoS ONE 2013, 8, e57475. [Google Scholar] [CrossRef]
- Kasmari, A.J.; Welch, A.; Liu, G.; Leslie, D.; McGarrity, T.; Riley, T. Independent of cirrhosis, hepatocellular carcinoma risk is increased with diabetes and metabolic syndrome. Am. J. Med. 2017, 130, 746.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, R.M.; Vatten, L.; Gunnell, D.; Romundstad, P.; Nilsen, T.I.L. Components of the metabolic syndrome and risk of prostate cancer: The HUNT 2 cohort, Norway. Cancer Causes Control 2009, 20, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Corrao, G.; Scotti, L.; Bagnardi, V.; Sega, R. Hypertension, antihypertensive therapy and renal-cell cancer: A meta-analysis. Curr. Drug Saf. 2007, 2, 125–133. [Google Scholar] [CrossRef]
- Heath, C.W., Jr.; Lally, C.A.; Calle, E.E.; McLaughlin, J.K.; Thun, M.J. Hypertension, diuretics, and antihypertensive medications as possible risk factors for renal cell cancer. Am. J. Epidemiol. 1997, 145, 607–613. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, J.N.; Corley, D.A.; Zhao, W.K.; Colt, J.S.; Shuch, B.; Chow, W.-H.; Purdue, M.P. Chronic kidney disease and risk of renal cell carcinoma: Differences by race. Epidemiology 2015, 26, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Fraser, G.E.; Phillips, R.L.; Beeson, W.L. Hypertension, antihypertensive medication and risk of renal carcinoma in California Seventh-Day Adventists. Int. J. Epidemiol. 1990, 19, 832–838. [Google Scholar] [CrossRef]
- Setiawan, V.W.; Stram, D.O.; Nomura, A.M.Y.; Kolonel, L.N.; Henderson, B.E. Risk factors for renal cell cancer: The multiethnic cohort. Am. J. Epidemiol. 2007, 166, 932–940. [Google Scholar] [CrossRef]
- Schouten, L.J.; van Dijk, B.A.; Oosterwijk, E.; Kaa, C.A.H.-V.D.; Kiemeney, L.; Goldbohm, R.A.; Schalken, J.A.; Brandt, P.V.D. Hypertension, antihypertensives and mutations in the Von Hippel-Lindau gene in renal cell carcinoma: Results from the Netherlands Cohort Study. J. Hypertens. 2005, 23, 1997–2004. [Google Scholar] [CrossRef] [Green Version]
- Largent, J.A.; McEligot, A.J.; Ziogas, A.; Reid, C.; Hess, J.; Leighton, N.; Peel, D.; Anton-Culver, H. Hypertension, diuretics and breast cancer risk. J. Hum. Hypertens. 2006, 20, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Largent, J.A.; Bernstein, L.; Horn-Ross, P.L.; Marshall, S.F.; Neuhausen, S.; Reynolds, P.; Ursin, G.; Zell, J.; Ziogas, A.; Anton-Culver, H. Hypertension, antihypertensive medication use, and breast cancer risk in the California Teachers Study cohort. Cancer Causes Control 2010, 21, 1615–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peeters, P.H.; van Noord, P.A.; Hoes, A.W.; Fracheboud, J.; Gimbrère, C.H.; Grobbee, D.E. Hypertension and breast cancer risk in a 19-year follow-up study (the DOM cohort). Diagnostic investigation into mammarian cancer. J. Hypertens. 2000, 18, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Q.; Zhao, X.; Meng, H.; Yu, J. Effect of antihypertensive drugs on breast cancer risk in female hypertensive patients: Evidence from observational studies. Clin. Exp. Hypertens. 2018, 40, 22–27. [Google Scholar] [CrossRef]
- Han, H.; Guo, W.; Shi, W.; Yu, Y.; Zhang, Y.; Ye, X.; He, J. Hypertension and breast cancer risk: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 44877. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123 Pt. 24, 4195–4200. [Google Scholar] [CrossRef] [Green Version]
- Intengan, H.D.; Schiffrin, E.L. Vascular remodeling in hypertension: Roles of apoptosis, inflammation, and fibrosis: Roles of apoptosis, inflammation, and fibrosis. Hypertension 2001, 38, 581–587. [Google Scholar] [CrossRef]
- Gkretsi, V.; Stylianopoulos, T. Cell adhesion and matrix stiffness: Coordinating cancer cell invasion and metastasis. Front. Oncol. 2018, 8, 145. [Google Scholar] [CrossRef]
- Deckers, I.A.; van den Brandt, P.A.; van Engeland, M.; van Schooten, F.-J.; Godschalk, R.W.; Keszei, A.P.; Schouten, L.J. Polymorphisms in genes of the renin-angiotensin-aldosterone system and renal cell cancer risk: Interplay with hypertension and intakes of sodium, potassium and fluid: RAAS Polymorphisms and RCC Risk: Interplay with Hypertension and Diet. Int. J. Cancer 2015, 136, 1104–1116. [Google Scholar] [CrossRef]
- Sobczuk, P.; Szczylik, C.; Porta, C.; Czarnecka, A.M. Renin angiotensin system deregulation as renal cancer risk factor. Oncol. Lett. 2017, 14, 5059–5068. [Google Scholar] [CrossRef] [Green Version]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochnowlei, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handorf, A.M.; Zhou, Y.; Halanski, M.A.; Li, W.-J. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 2015, 11, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobin, E.; Bagwell, K.; Wagner, J.; Mysona, D.; Sandirasegarane, S.; Smith, N.; Bai, S.; Sharma, A.; Schleifer, R.; She, J.-X. A pan-cancer perspective of matrix metalloproteases (MMP) gene expression profile and their diagnostic/prognostic potential. BMC Cancer 2019, 19, 581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laronha, H.; Caldeira, J. Structure and function of human matrix metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Friese, R.S.; Rao, F.; Khandrika, S.; Thomas, B.; Ziegler, M.G.; Schmid-Schönbein, G.W.; O’Connor, D.T. Matrix metalloproteinases: Discrete elevations in essential hypertension and hypertensive end-stage renal disease. Clin. Exp. Hypertens. 2009, 31, 521–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhingra, R.; Pencina, M.J.; Schrader, P.; Wang, T.; Levy, D.; Pencina, K.; Siwik, D.A.; Colucci, W.; Benjamin, E.J.; Vasan, R.S. Relations of matrix remodeling biomarkers to blood pressure progression and incidence of hypertension in the community. Circulation 2009, 119, 1101–1107. [Google Scholar] [CrossRef] [Green Version]
- Belo, V.A.; Guimarães, D.A.; Castro, M.M. Matrix metalloproteinase 2 as a potential mediator of vascular smooth muscle cell migration and chronic vascular remodeling in hypertension. J. Vasc. Res. 2015, 52, 221–231. [Google Scholar] [CrossRef]
- Yabluchanskiy, A.; Ma, Y.; Iyer, R.P.; Hall, M.E.; Lindsey, M.L. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology 2013, 28, 391–403. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Takino, T.; Endo, Y.; Sato, H. Activation of MMP-9 by membrane type-1 MMP/MMP-2 axis stimulates tumor metastasis. Cancer Sci. 2017, 108, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Joseph, C.; Alsaleem, M.; Orah, N.; Narasimha, P.L.; Miligy, I.M.; Kurozumi, S.; Ellis, I.O.; Mongan, N.; Green, A.R.; Rakha, E.A. Elevated MMP9 expression in breast cancer is a predictor of shorter patient survival. Breast Cancer Res. Treat. 2020, 182, 267–282. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Z.; Li, F.; Wang, C. The relationship between MMP-2 and MMP-9 expression levels with breast cancer incidence and prognosis. Oncol. Lett. 2017, 14, 5865–5870. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Study | Study Design | Cancer Outcome |
|---|---|---|
| Christakoudi, 2020, Europe | Cohort | RCC, Esophagus SCC, Head and Neck |
| Stocks, 2012, Europe | Cohort | Kidney, Colon, Rectum, Bladder, Pancreas, Liver, Endometrial, Cervix |
| Lindgren, 2003, Finland | Cohort | Lung |
| Lindgren, 2007, Finland | Cohort | Breast Cancer |
| Lindgren, 2005, Finland | Cohort | Kidney |
| Beebe-Dimmer, 2007, USA | Case-Control | Prostate |
| Batty, 2003, Europe | Cohort | Pancreas |
| Behrens, 2016, Denmark | Cohort | Endometrial |
| Seo, 2020, Korea | Cohort | Esophagus, Oral, Laryngeal |
| Wallner, 2010, USA | Cohort | Prostate |
| Martin, 2009, Norway | Cohort | Prostate |
| Teleka, 2020, Europe | Cohort | Bladder |
| Teleka, 2018, Europe | Cohort | Bladder |
| Teleka, 2021, Europe | Cohort | Bladder |
| Drahos, 2016, USA | Case-Control | Esophagus |
| Lofterød, 2020, Europe | Cohort | Breast |
| Sun, 2015, Taiwan | Cohort | Kidney, Endometrial |
| Shen, 2015, China | Nested Case-Control | RCC |
| Kim, 2020, Korea | Cohort | Kidney |
| Yassin, 2019, Palestine | Case-Control | Breast |
| Staples, 2020, USA | Case-Control | Ovarian |
| Michels, 2019, USA | Case-Control | Ovarian |
| Pereira, 2012, Chili | Case-Control | Breast |
| Chuang, 2015, Taiwan | Nested Case-Control | Breast |
| Beji, 2007, Turkey | Case-Control | Breast |
| Colt, 2011, USA | Case-Control | RCC |
| Jung, 2013, Korea | Case-Control | Breast |
| Kok, 2018, Taiwan | Cohort | Bladder |
| Hektoen, 2020, Norway | Cohort | Bladder |
| Pol, 2020, Netherlands | Cohort | RCC |
| Ko, 2016, Korea | Cohort | Colon |
| Li, 2018, China | Case-Control | Gastric |
| Lee, 2020, Korea | Cohort | Colon |
| Al-Madani, 2019, Saudi Arabia | Cohort | Cervix |
| Hirai, 2018, China | N/A | Colon |
| Samarakoon, 2018, Asia | Case-Control | Colon |
| Dickerman, 2017, Iceland | Cohort | Prostate |
| Porto, 2011, Brazil | Case-Control | Breast |
| Kabat, 2009, USA | Cohort | Breast |
| Berger, 2016, Denmark | Cohort | Kidney, Liver, Gall Bladder |
| Choi, 2021, Korea | Cohort | Breast |
| Jeon, 2020, Korea | Cohort | Lung |
| Trabert, 2015, USA | Case-Control | Endometrial |
| Bashamakha, 2019, Yemen | Case-Control | Breast |
| Flaherty, 2005, USA | Cohort | RCC |
| Haggstrom, 2013, Europe | Cohort | RCC |
| Kasmari, 2017, USA | Cohort | Liver |
| Study | Exposure Assessment | Definition of Hypertension |
|---|---|---|
| Christakoudi, 2020, Europe | Measured | BP ≥ 140/90 mmHg |
| Stocks, 2012, Europe | Measured | BP ≥ 140/90 mmHg |
| Lindgren, 2003, Finland | Measured | Quartiles |
| Lindgren, 2007, Finland | Measured | Quartiles |
| Lindgren, 2005, Finland | Measured | Quartiles |
| Beebe-Dimmer, 2007, USA | Questionnaire | BP ≥ 140/90 mmHg |
| Batty, 2003, Europe | Measured | Quartiles |
| Behrens, 2016, Denmark | Database | N/A |
| Seo, 2020, Korea | Measured | BP ≥ 140/90 mmHg |
| Wallner, 2010, USA | Questionnaire | Yes/No |
| Martin, 2009, Norway | Measured | BP ≥ 130/85 mmHg |
| Teleka, 2020, Europe | Measured | Quartiles |
| Teleka, 2018, Europe | Measured | BP ≥ 140/90 mmHg |
| Teleka, 2021, Europe | Measured | BP ≥ 140/90 mmHg |
| Drahos, 2016, USA | Database | BP ≥ 140/90 mmHg |
| Lofterød, 2020, Europe | Measured | BP ≥ 140/90 mmHg |
| Sun, 2015, Taiwan | Database | Yes/No |
| Shen, 2015, China | Questionnaire | Yes/No |
| Kim, 2020, Korea | Database | BP ≥ 130/80 mmHg |
| Yassin, 2019, Palestine | Questionnaire | N/A |
| Staples, 2020, USA | Questionnaire | N/A |
| Michels, 2019, USA | Database | N/A |
| Pereira, 2012, Chili | Measured | BP ≥ 140/90 mmHg |
| Chuang, 2015, Taiwan | Database | Yes/No |
| Beji, 2007, Turkey | Questionnaire | Yes/No |
| Colt, 2011, USA | Questionnaire | Category of severity |
| Jung, 2013, Korea | Questionnaire | Yes/No |
| Kok, 2018, Taiwan | Database | BP ≥ 140/90 mmHg |
| Hektoen, 2020, Norway | Measured | BP ≥ 140/90 mmHg |
| Pol, 2020, Netherlands | Questionnaire | Yes/No |
| Ko, 2016, Korea | Measured | BP ≥ 130/85 mmHg |
| Li, 2018, China | Measured | BP ≥ 130–140/85–90 mmHg |
| Lee, 2020, Korea | Measured | Quartiles |
| Al-Madani, 2019, Saudi Arabia | Questionnaire | Yes/no |
| Hirai, 2018, China | N/A | N/A |
| Samarakoon, 2018, Asia | N/A | N/A |
| Dickerman, 2017, Iceland | Measured | BP ≥ 130/85 mmHg |
| Porto, 2011, Brazil | Measured | BP ≥ 130/85 mmHg |
| Kabat, 2009, USA | Measured | BP ≥ 130/85 mmHg |
| Berger, 2016, Denmark | Questionnaire | Yes/No |
| Choi, 2021, Korea | Measured | BP ≥ 130/85 mmHg |
| Jeon, 2020, Korea | Questionnaire | Yes/No |
| Trabert, 2015, USA | Measured | BP ≥ 130/85 mmHg |
| Bashamakha, 2019, Yemen | Questionnaire | Yes/No |
| Flaherty, 2005, USA | Questionnaire | Yes/No |
| Haggstrom, 2013, Europe | Database | BP ≥ 140/90 mmHg |
| Kasmari, 2017, USA | Database | Yes/No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Connaughton, M.; Dabagh, M. Association of Hypertension and Organ-Specific Cancer: A Meta-Analysis. Healthcare 2022, 10, 1074. https://doi.org/10.3390/healthcare10061074
Connaughton M, Dabagh M. Association of Hypertension and Organ-Specific Cancer: A Meta-Analysis. Healthcare. 2022; 10(6):1074. https://doi.org/10.3390/healthcare10061074
Chicago/Turabian StyleConnaughton, Morgan, and Mahsa Dabagh. 2022. "Association of Hypertension and Organ-Specific Cancer: A Meta-Analysis" Healthcare 10, no. 6: 1074. https://doi.org/10.3390/healthcare10061074






