Summary
Percutaneous left ventricular assist devices provide temporary circulatory support as bridge to recovery or heart transplantation in case of severe left ventricular failure and during percutaneous high-risk revascularisation procedures. We describe the first Swiss case of a high risk percutaneous coronary intervention assisted by a new, percutaneously inserted left ventricular assist device: the Impella Recover® LP 2.5 pump.
Key words: PCI; left ventricular assist device
Introduction
Left ventricular assist devices (LVAD) are able to maintain partial or total circulatory support in case of severe left ventricular failure. While the intraaortic balloon pump (IABP) solely decreases preload and afterload, LVADs actively increase cardiac output and may even completely replace left ventricular function. As opposed to the traditional extracorporeal circulation pumps, LVADs aspirate freshly oxygenated blood from the left atrium or ventricle, thus circumventing the need of an oxygenator.
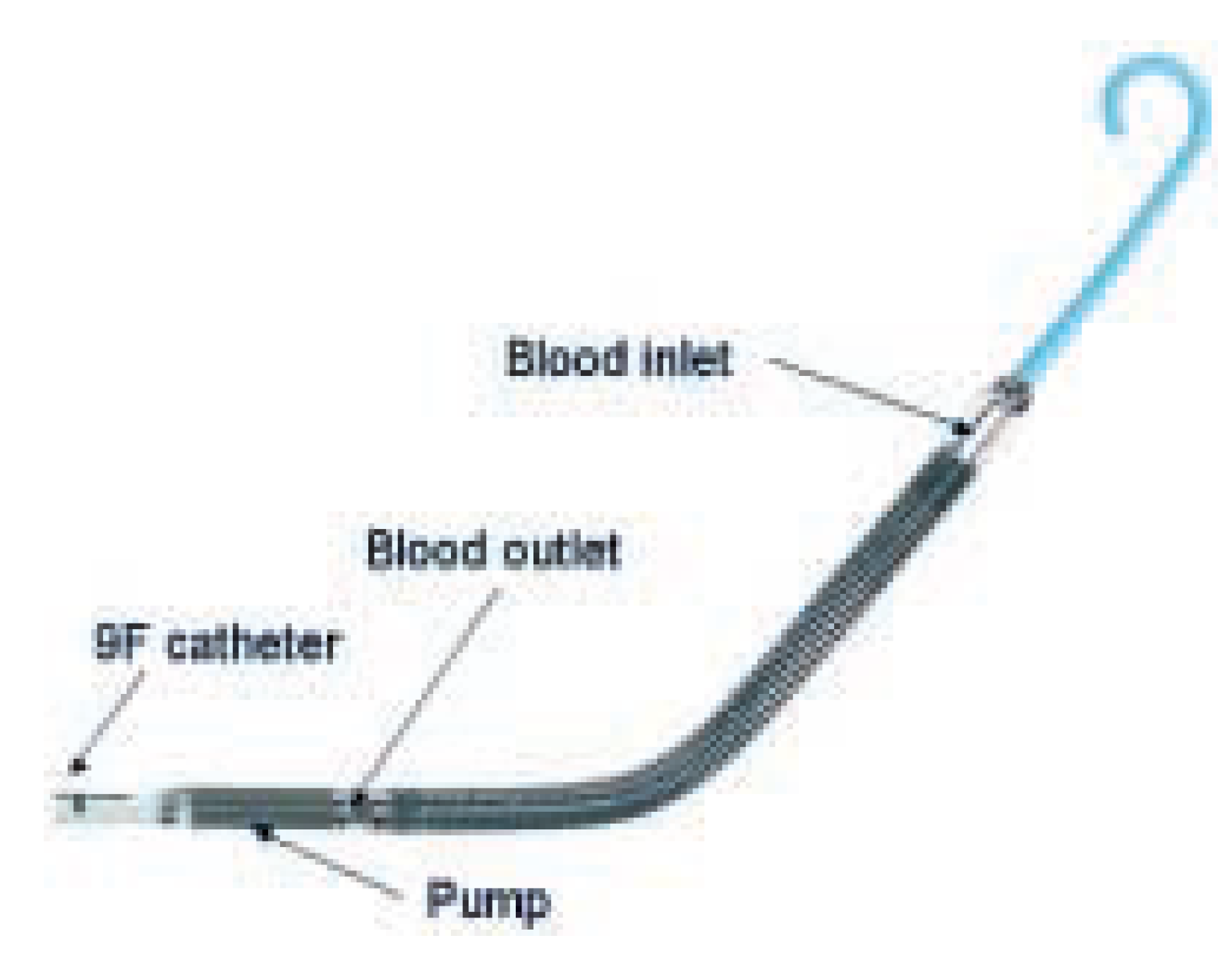
Figure 1.
Components of the Impella Recover® LP 2.5 pump. Obtained with permission from Impella Cardiosystems, AG, Aachen, Germany.
Figure 1.
Components of the Impella Recover® LP 2.5 pump. Obtained with permission from Impella Cardiosystems, AG, Aachen, Germany.
The rationale for LVAD therapy is twofold [
1,
2]: (1.) To restore normal haemodynamics and endorgan perfusion in case of insufficient intrinsic left ventricular function. (2.) To ameliorate left ventricular remodeling by unloading of the left ventricle and improved microvascular perfusion.
Clinically, LVADs are used to provide temporary circulatory support as bridge to left ventricular recovery or heart transplantation, and during high-risk revascularisation procedures, ie, treatment of lesions subtending a large area at risk. Recently, percutaneous LVADs have been introduced into clinical practice to rapidly establish powerful circulatory support in the catheterisation laboratory without the need of surgical procedures or extracorporeal membrane oxygenators [
3].
The Impella Recover
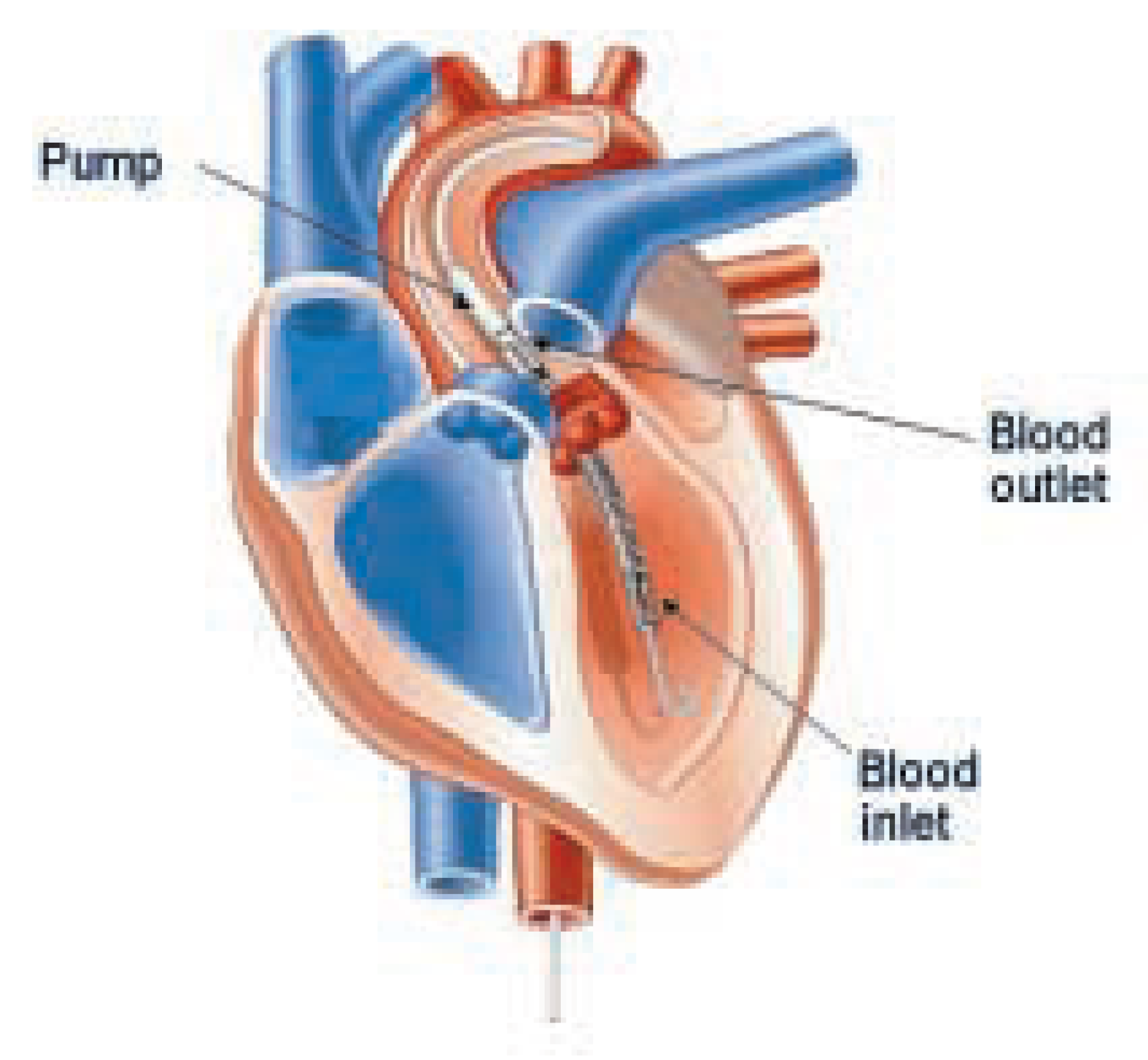
® LP 2.5 system (Impella Cardiosystems, AG, Aachen, Germany) is a new LVAD intended for percutaneous insertion in the catheterisation laboratory. The device features a pigtail at the catheter tip, a caged blood flow inlet, a nitinol ringed blood tube, and a 12 French (F) microaxial pump mounted on a 9F catheter, which is connected to a mobile driver console (fig. 1). The catheter is retrogradely inserted through a 13F peelaway sheath (outer diameter about 5 mm) via the femoral artery into the LV, which is facilitated by use of a 0.014 inch guidewire running through the pigtail catheter tip and the Impella housing (fig. 2). The microaxial pump rotates the impeller at high speed and thereby aspirates blood through the blood inlet positioned in the LV, from where it is directed through the hollow, nitinol tube and ejected past the impeller into the ascending aorta. Thus, the Impella Recover
® LP 2.5 system is capable of providing LV unloading and at the same time up to 2.5 l/min blood flow against physiologic afterload. We describe the first clinical utilisation of this device in Switzerland on December 7, 2004, in a patient undergoing a high-risk percutaneous coronary intervention (PCI).
Figure 2.
Retrograde placement of the Impella Recover® LP 2.5 pump through the aortic valve into the left ventricle. Obtained with permission from Impella Cardiosystems, AG, Aachen, Germany.
Figure 2.
Retrograde placement of the Impella Recover® LP 2.5 pump through the aortic valve into the left ventricle. Obtained with permission from Impella Cardiosystems, AG, Aachen, Germany.
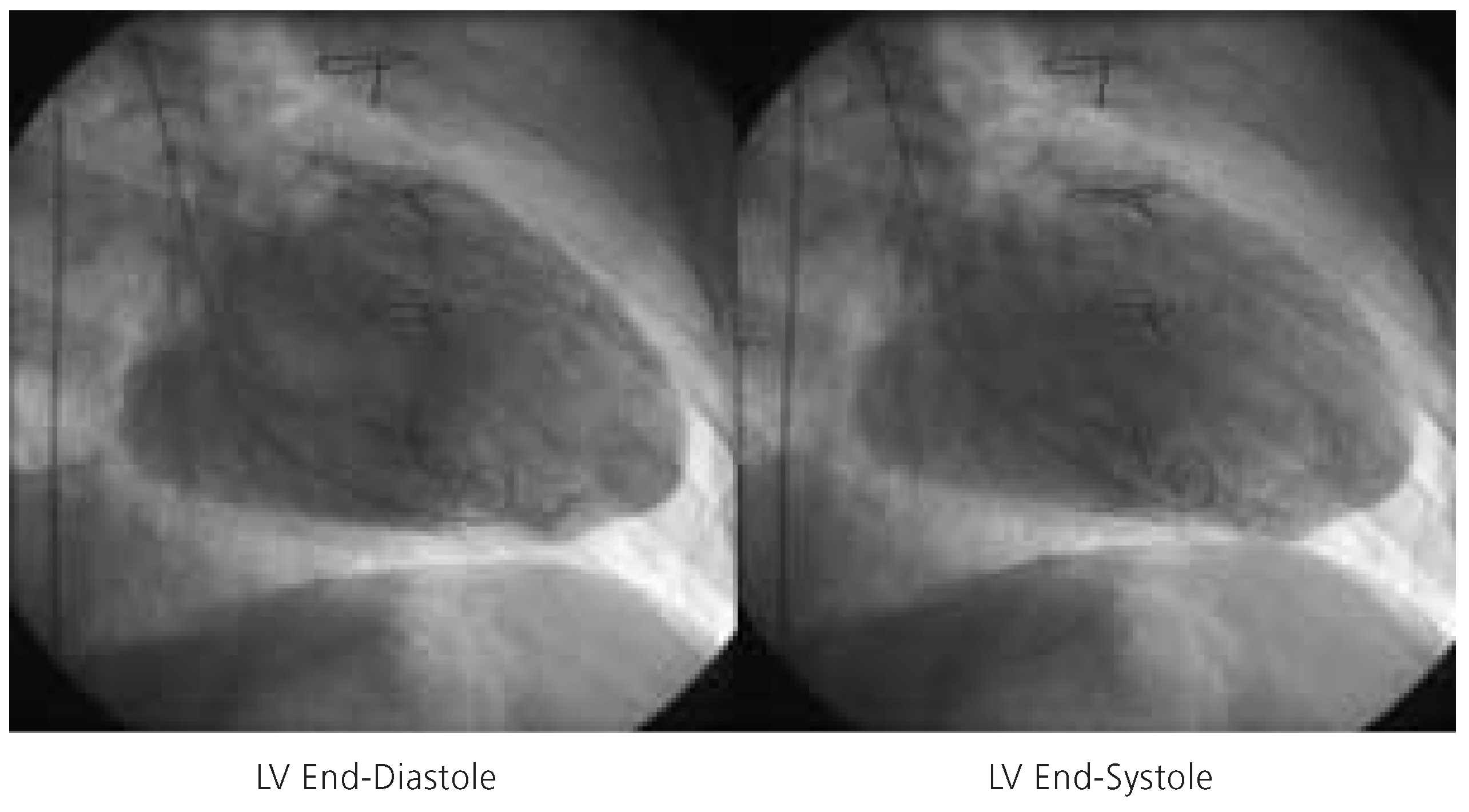
Figure 3.
Left ventricular end-diastolic frame (left) and left ventricular end-systolic frame (right) indicative of severely diminished left ventricular function (LVEF = 15%). LV = left ventricle.
Figure 3.
Left ventricular end-diastolic frame (left) and left ventricular end-systolic frame (right) indicative of severely diminished left ventricular function (LVEF = 15%). LV = left ventricle.
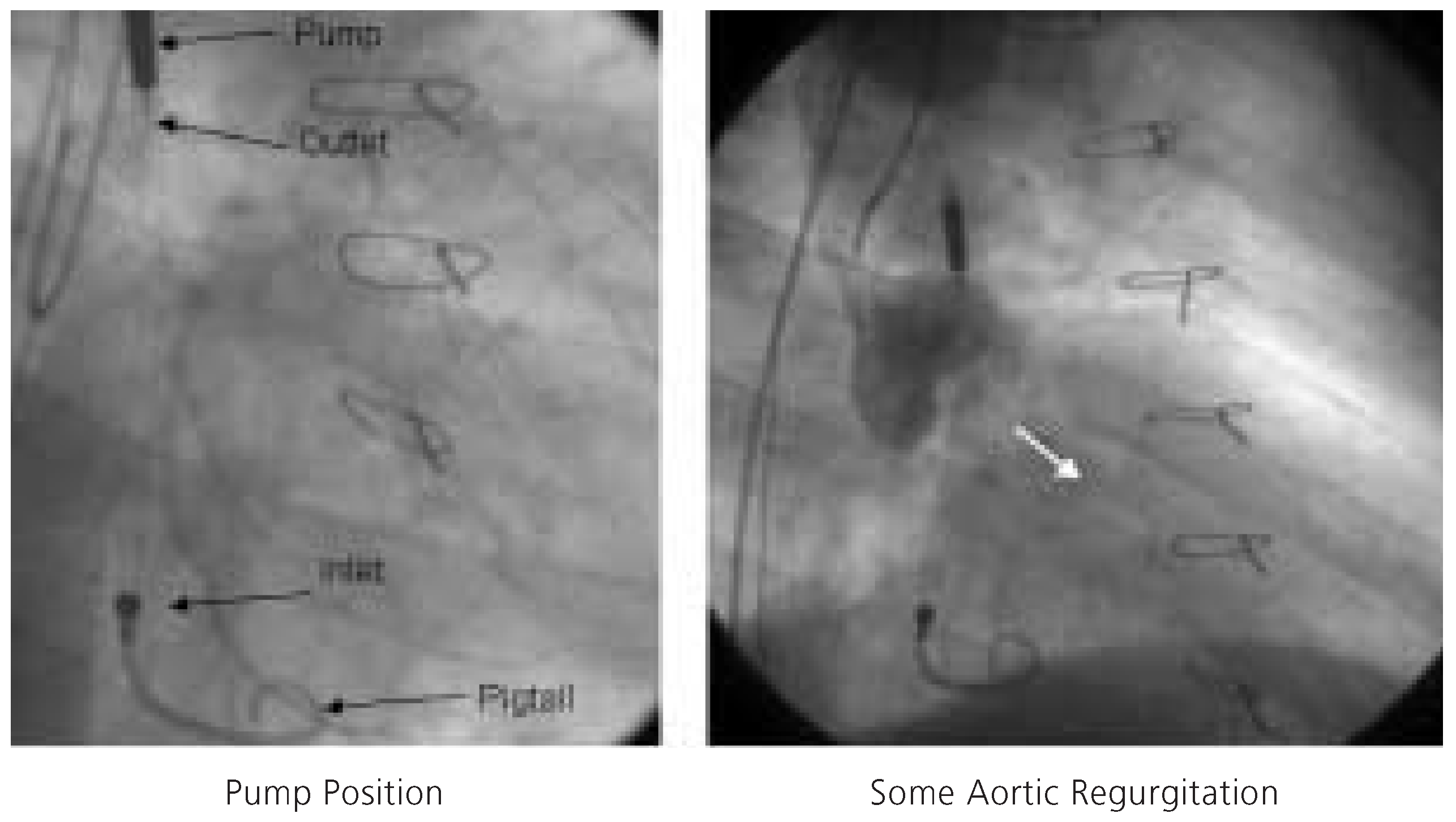
Figure 4.
Fluoroscopic appearance of the Impella Recover® LP 2.5 pump (left). Angiographic evidence of aortic regurgitation during aortography engendered by the 12F catheter traversing the aortic valve (right).
Figure 4.
Fluoroscopic appearance of the Impella Recover® LP 2.5 pump (left). Angiographic evidence of aortic regurgitation during aortography engendered by the 12F catheter traversing the aortic valve (right).
Case Report
A 64-year-old man with a long history of coronary artery disease was admitted due to crescendo angina pectoris and dyspnea on exertion. He had suffered an anterior and inferior myocardial infarction in 1986 and 1990, respectively, and undergone coronary artery bypass grafting with a left internal mammary artery to the left anterior descending coronary artery, and two saphenous vein grafts to the right coronary artery and the second marginal branch of the circumflex coronary artery in 1990. Cardiac catheterisation during this admission revealed native three vessel coronary artery disease with occlusion of the left anterior descending artery in the mid portion, three complex and high grade stenoses of the left circumflex artery, and proximal occlusion of the right coronary artery. The left internal mammary artery graft to the distal left anterior descending artery was patent, whereas the saphenous vein graft to the marginal branch of the left circumflex artery was occluded and the saphenous vein graft to the right coronary artery had been stented earlier for a high grade stenosis at the distal anastomosis. Left ventriculography revealed a severely diminished left ventricular function with an ejection fraction of 15% with dyskinesia of the entire anterolateral wall, akinesia of the inferior wall, and severe hypokinesia of the posterior and lateral wall (fig. 3). The left ventricular enddiastolic pressure measured 38 mm Hg.
Since the patient refused cardiac transplantation and due to clinical evidence of ischaemia, percutaneous coronary intervention of the native left circumflex artery was planned. In light of the severely diminished LV function, the elevated filling pressure despite intensive heart failure and diuretic therapy, and documented viability in the area to be intervened, percutaneous coronary intervention was considered high risk. Following written informed consent, the decision to perform an Impella Recover® LP 2.5-assisted percutaneous coronary intervention was made on December 7th, 2004. Both groins were used for vascular access. Following local anesthesia, a 13F peel-away sheath was placed into the right femoral artery. Systemic anticoagulation was achieved with administration of unfractionated heparin (70 U/kg), and the patient was pretreated with acetylsalicylic acid 100 mg/d and clopidogrel 75 mg/d. First, a 5F pigtail catheter was positioned in the LV and then exchanged over a 0.014 inch guidewire for the 12F Impella Recover® LP 2.5 catheter. The correct position of the device was verified by cinefluoroscopy (fig. 4) and an electronic placement signal. Circulatory support was initiated at the highest pump speed (P9, approximately 2.5 l/min) and maintained throughout the intervention.
Following this, the left femoral artery was punctured and a 5F Amplatz guiding catheter (Cordis, Johnson and Johnson, Miami, USA) used to selectively intubate the left main coronary ostium. Percutaneous coronary intervention of the left circumflex artery was performed by means of balloon pre-dilation, followed by insertion of a Cypher™ (Cordis, Johnson and Johnson, Miami, USA) stent (fig. 5). During the intervention no significant haemodynamic changes were noted, and the patient tolerated the balloon inflations well. At the end of the percutaneous coronary intervention, the Impella Recover® LP 2.5 system was withdrawn and haemostasis was ensured by manual compression for 30 minutes. Blood loss was estimated of less than 100 ml during the intervention. The periprocedural measurement of cardiac biomarkers was unrevealing and there were no electrocardiographic changes. The patient was ambulated after 6 hours and there were no peripheral access complications. He was discharged home on the second day and will be readmitted for implantation of an implantable cardioverter defibrillator in one week.
Discussion
The initial case of a novel percutaneous LVAD proofed easy to implant and provided partial circulatory support during a high-risk percutaneous coronary intervention without complications. The principal advantage of the Impella Recover
® LP 2.5 device over the TandemHeart™ (Cardiac Assist Inc, USA) [
4] is its ease of implantation and the smaller catheter size (13F
vs 17F). Moreover, there is no need for a transseptal puncture, and the retrograde insertion of the device over a guidewire through the aortic valve into the LV is familiar to all cardiologists. There is no extracorporeal blood and the central portion of the microaxial pump avoid the friction problem of the drive shaft of the Hemopump, the predecessor of the Impella device [
5]. The motor of the Hemopump was outside the body and overheating of the device shaft had been one of its major drawbacks. The circulatory support provided by the Impella Recover
® LP 2.5 is limited to 2.5 l/min, which does not suffice in patients without at least some LV function. The TandemHeart™ may deliver up to 3.5 l/min and completely replace LV function. Some aortic regurgitation is likely engendered by the 12F catheter traversing the aortic valve (fig. 4).
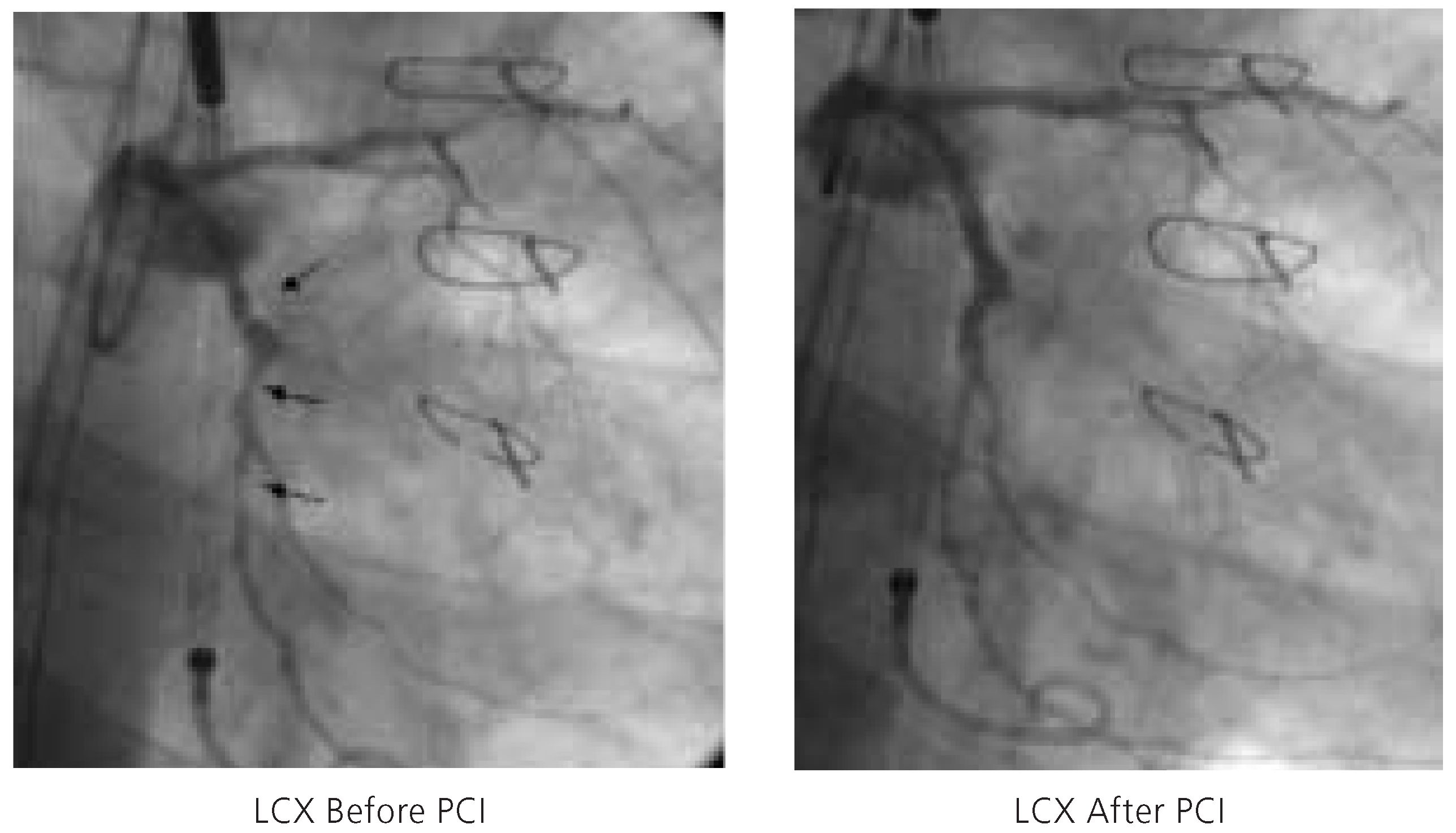
Figure 5.
Three complex, high-grade stenoses of the circumflex artery (LCX) before (left) and after (right) percutaneous coronary intervention. PCI = percutaneous coronary intervention.
Figure 5.
Three complex, high-grade stenoses of the circumflex artery (LCX) before (left) and after (right) percutaneous coronary intervention. PCI = percutaneous coronary intervention.
The introduction of easily manageable percutaneous LVADs constitutes an important advance in the practice of interventional cardiology [
6]. It provides a new armentarium in the management of patients with severe left ventricular dysfunction and cardiogenic shock and may serve as bridge to recovery or heart transplantation in carefully selected patients. In addition, it may provide a safety net during highrisk surgical or percutaneous coronary interventions in patients undergoing revascularisation of lesions subtending a large area at risk. Clinical trials are clearly needed to more precisely identify the risks and benefits associated with this new procedure before it can be embraced widely.