Telomere Biology in Cardiovasular Disease
Abstract
Zusammenfassung
Ageing and atherosclerosis
Telomeres: the mitotic clock

Vascular cell senescence in vascular ageing and atherosclerosis


Telomerase
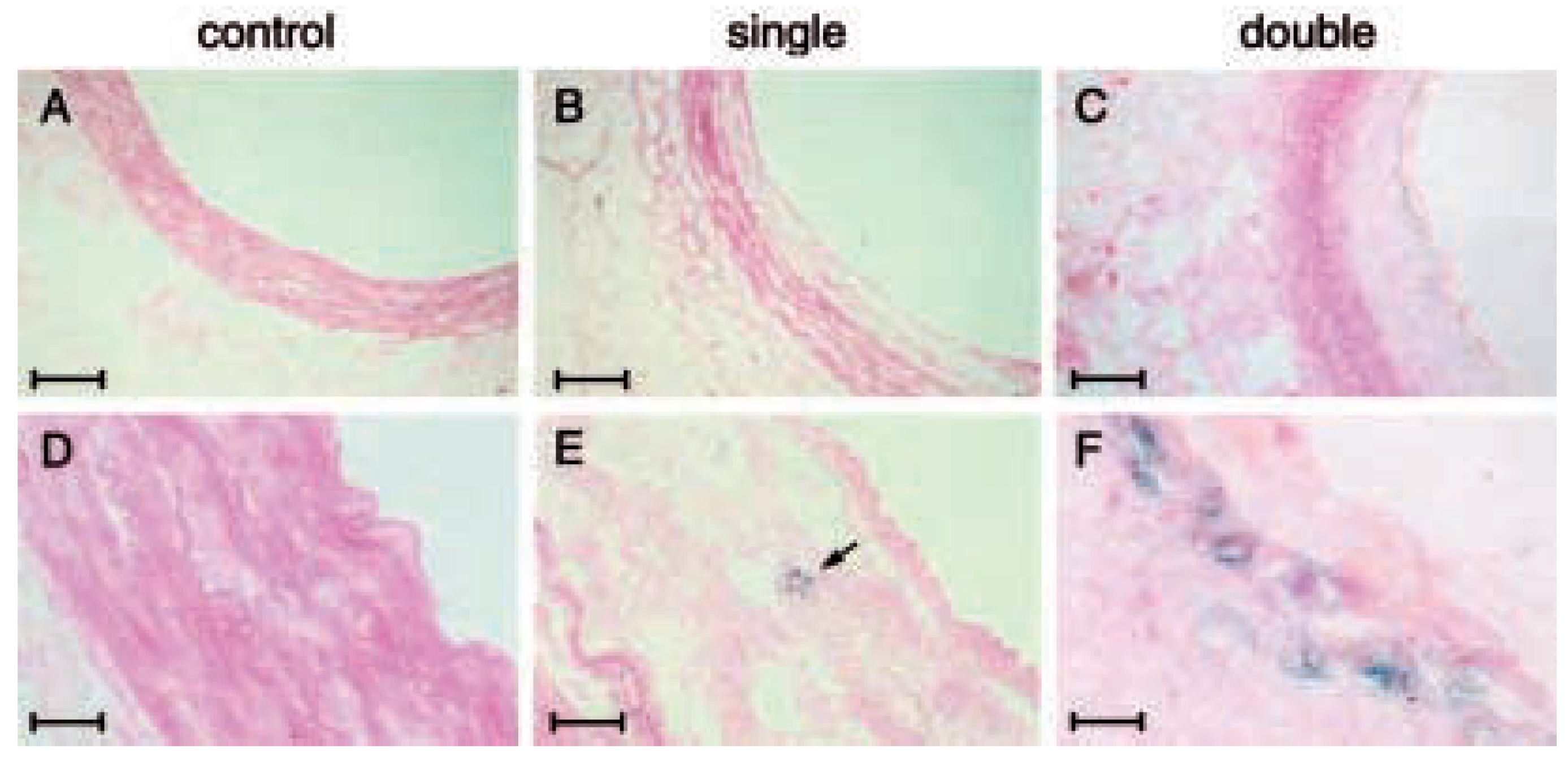
Telomerase activity in the vasculature
Do ageing mechanisms act in synergy?
Evidence from human genetic mutations
 |
Cellular senescence and organismal ageing
Concluding remarks
Acknowledgments
References
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a «set up» for vascular disease. Circulation 2003, 107, 139–46. [Google Scholar] [CrossRef]
- Zeiher, A.M.; Drexler, H.; Saurbier, B.; Just, H. Endothelium-mediated coronary blood flow modulation in humans. Effects of age, atherosclerosis, hypercholesterolemia, and hypertension. J Clin Invest 1993, 92, 652–62. [Google Scholar]
- van der Loo, B.; Labugger, R.; Skepper, J.N.; Bachschmid, M.; Kilo, J.; Powell, J.M. , et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med 2000, 192, 1731–44. [Google Scholar] [CrossRef]
- Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, et al. Age-dependent impairment of angiogenesis. Circulation 1999, 99, 111–20.
- Schneiderman, J.; Sawdey, M.S.; Keeton, M.R.; Bordin, G.M.; Bernstein, E.F.; Dilley, R.B.; et al. Increased type 1 plasminogen activator inhibitor gene expression in atherosclerotic human arteries. Proc Natl Acad Sci U S A 1992, 89, 6998–7002. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–47. [Google Scholar] [CrossRef]
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 1965, 37, 614–36. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, H.; McKeehan, W.L. Isolation, growth requirements, cloning, prostacyclin production and life-span of human adult endothelial cells in low serum culture medium. In Vitro Cell Dev Biol 1986, 22, 51–6. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; Kim, S.H.; Lim, C.S.; Rubio, M. Cellular senescence, cancer and aging: the telomere connection. Exp Gerontol 2001, 36, 1619–37. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.D.; Comeau, L.; Rosenfield, S.; Stansel, R.M.; Bianchi, A.; Moss, H.; et al. Mammalian telomeres end in a large duplex loop. Cell 1999, 97, 503–14. [Google Scholar] [CrossRef]
- de Lange, T. Protection of mammalian telomeres. Oncogene 2002, 21, 532–40. [Google Scholar] [CrossRef]
- Blackburn, E.H. Switching and signaling at the telomere. Cell 2001, 106, 661–73. [Google Scholar] [CrossRef]
- Levy, M.Z.; Allsopp, R.C.; Futcher, A.B.; Greider, C.W.; Harley, C.B. Telomere end-replication problem and cell aging. J Mol Biol 1992, 225, 951–60. [Google Scholar] [CrossRef]
- Schwartz, S.M.; Gajdusek, C.M.; Reidy, M.A.; Selden SC3rd Haudenschild, C.C. Maintenance of integrity in aortic endothelium. Fed Proc 1980, 39, 2618–25. [Google Scholar]
- Wright, H.P. Endothelial mitosis around aortic branches in normal guinea pigs. Nature 1968, 220, 78–9. [Google Scholar] [CrossRef] [PubMed]
- Zarins, C.K.; Glagov, S.; Giddens, D.P. What do we find in human atherosclerosis that provides insight into the hemodynamic factors in atherogenesis? In: Glagov S, Newman WP, Schafer SA, editors. Pathobiology of the Human Atherosclerotic Plaque. New York: Springer-Verlag;
- Rosen, E.M.; Mueller, S.N.; Noveral, J.P.; Levine, E.M. Proliferative characteristics of clonal endothelial cell strains. J Cell Physiol 1981, 107, 123–37. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Wight, T.N.; Strandness, E.; Thiele, B. Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol 1984, 114, 79–93. [Google Scholar]
- Maier, J.A.; Voulalas, P.; Roeder, D.; Maciag, T. Extension of the life-span of human endothelial cells by an interleukin-1 alpha antisense oligomer. Science 1990, 249, 1570–4. 20 Moyer CF, Sajuthi D, Tulli H, Williams JK. Synthesis of IL-1 alpha and IL-1 beta by arterial cells in atherosclerosis. Am J Pathol 1991, 138, 951–60. [Google Scholar]
- Comi, P.; Chiaramonte, R.; Maier, J.A. Senescence-dependent regulation of type 1 plasminogen activator inhibitor in human vascular endothelial cells. Exp Cell Res 1995, 219, 304–8. [Google Scholar] [CrossRef]
- Maier, J.A.; Statuto, M.; Ragnotti, G. Senescence stimulates U937–endothelial cell interactions. Exp Cell Res 1993, 208, 270–4. [Google Scholar]
- van der Wal, A.C.; Das, P.K.; Tigges, A.J.; Becker, AE. Adhesion molecules on the endothelium and mononuclear cells in human atherosclerotic lesions. Am J Pathol 1992, 141, 1427–33. [Google Scholar]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 1995, 92, 9363–7. [Google Scholar]
- Kurz, D.J.; Decary, S.; Hong, Y.; Erusalimsky, J.D. Senescenceassociated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci 2000, 113 Pt 20, 3613–22. [Google Scholar] [CrossRef]
- van der Loo, B.; Fenton, M.J.; Erusalimsky, JD. Cytochemical detection of a senescence-associated beta-galactosidase in endothelial and smooth muscle cells from human and rabbit blood vessels. Exp Cell Res 1998, 241, 309–15. [Google Scholar] [CrossRef]
- Fenton, M.; Barker, S.; Kurz, D.J.; Erusalimsky, J.D. Cellular senescence after single and repeated balloon catheter denudations of rabbit carotid arteries. Arterioscler Thromb Vasc Biol 2001, 21, 220–6. [Google Scholar] [CrossRef] [PubMed]
- Vasile, E.; Tomita, Y.; Brown, L.F.; Kocher, O.; Dvorak, H.F. Differential expression of thymosin beta-10 by early passage and senescent vascular endothelium is modulated by VPF/ VEGF: evidence for senescent endothelial cells in vivo at sites of atherosclerosis. Faseb J 2001, 15, 458–66. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Miyauchi, H.; Yoshida, T.; Ishida, Y.; Yoshida, H.; Komuro, I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 2002, 105, 1541–4. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993, 362, 801–9. [Google Scholar] [CrossRef]
- Chang, E.; Harley, C.B. Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci U S A 1995, 92, 11190–4. [Google Scholar] [CrossRef]
- Okuda, K.; Khan, M.Y.; Skurnick, J.; Kimura, M.; Aviv, H.; Aviv, A. Telomere attrition of the human abdominal aorta: relationships with age and atherosclerosis. Atherosclerosis 2000, 152, 391–8. [Google Scholar] [CrossRef]
- Aviv, H.; Khan, M.Y.; Skurnick, J.; Okuda, K.; Kimura, M.; Gardner, J.; et al. Age dependent aneuploidy and telomere length of the human vascular endothelium. Atherosclerosis 2001, 159, 281–7. [Google Scholar] [CrossRef]
- Ogami, M.; Ikura, Y.; Ohsawa, M.; Matsuo, T.; Kayo, S.; Yoshimi, N.; et al. Telomere shortening in human coronary artery diseases. Arterioscler Thromb Vasc Biol 2004, 24, 546–50. [Google Scholar] [CrossRef]
- Blasco, MA. Telomerase beyond telomeres. Nat Rev Cancer 2002, 2, 627–33. [Google Scholar] [CrossRef]
- Wright, W.E.; Piatyszek, M.A.; Rainey, W.E.; Byrd, W.; Shay, J.W. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet 1996, 18, 173–9. [Google Scholar] [CrossRef]
- Forsyth, N.R.; Wright, W.E.; Shay, J.W. Telomerase and differentiation in multicellular organisms: turn it off, turn it on, and turn it off again. Differentiation 2002, 69, 188–97. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.; et al. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–5. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.D.; Neumann, A.A.; Yeager, T.R.; Reddel, R.R. Alternative lengthening of telomeres in mammalian cells. Oncogene 2002, 21, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Mitsialis, S.A.; Kourembanas, S. Hypoxia extends the life span of vascular smooth muscle cells through telomerase activation. Mol Cell Biol 2001, 21, 3336–42. [Google Scholar] [CrossRef]
- Hsiao, R.; Sharma, H.W.; Ramakrishnan, S.; Keith, E.; Narayanan, R. Telomerase activity in normal human endothelial cells. Anticancer Res 1997, 17, 827–32. [Google Scholar]
- Vasa, M.; Breitschopf, K.; Zeiher, A.M.; Dimmeler, S. Nitric oxide activates telomerase and delays endothelial cell senescence. Circ Res 2000, 87, 540–2. [Google Scholar] [CrossRef]
- Kurz, D.J.; Hong, Y.; Trivier, E.; Huang, H.L.; Decary, S.; Zang, G.H.; et al. Fibroblast growth factor-2, but not vascular endothelial growth factor, upregulates telomerase activity in human endothelial cells. Arterioscler Thromb Vasc Biol 2003, 23, 748–54. [Google Scholar] [CrossRef]
- Minamino, T.; Kourembanas, S. Mechanisms of telomerase induction during vascular smooth muscle cell proliferation. Circ Res 2001, 89, 237–43. [Google Scholar] [CrossRef]
- Breitschopf, K.; Zeiher, A.M.; Dimmeler, S. Pro-atherogenic factors induce telomerase inactivation in endothelial cells through an Akt-dependent mechanism. FEBS Lett 2001, 493, 21–5. [Google Scholar] [CrossRef]
- Haendeler, J.; Hoffmann, J.; Brandes, R.P.; Zeiher, A.M.; Dimmeler, S. Hydrogen peroxide triggers nuclear export of telomerase reverse transcriptase via Src kinase family-dependent phosphorylation of tyrosine 707. Mol Cell Biol 2003, 23, 4598–610. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; Kusdra, L.; Collins, K. Subnuclear shuttling of human telomerase induced by transformation and DNA damage. Nat Cell Biol 2002, 4, 731–6. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chang, E.; Cherry, A.M.; Bangs, C.D.; Oei, Y.; Bodnar, A.; et al. Human endothelial cell life extension by telomerase expression. J Biol Chem 1999, 274, 26141–8. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, H.; Benchimol, S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol 1998, 8, 279–82. [Google Scholar] [CrossRef]
- Bodnar, A.G.; Ouellette, M.; Frolkis, M.; Holt, S.E.; Chiu, C.P.; Morin, G.B.; et al. Extension of life-span by introduction of telomerase into normal human cells. Science 1998, 279, 349–52. [Google Scholar] [CrossRef]
- Yang, J.; Nagavarapu, U.; Relloma, K.; Sjaastad, M.D.; Moss, W.C.; Passaniti, A.; et al. Telomerized human microvasculature is functional in vivo. Nat Biotechnol 2001, 19, 219–24. [Google Scholar] [CrossRef]
- Trivier, E.; Kurz, D.J.; Hong, Y.; Huang, H.L.; Erusalimsky, J.D. Differential regulation of telomerase in endothelial cells by fibroblast growth factor-2 and vascular endothelial growth factor-a: association with replicative life span. Ann N Y Acad Sci 2004, 1019, 111–5. [Google Scholar] [PubMed]
- Masutomi, K.; Yu, E.Y.; Khurts, S.; Ben-Porath, I.; Currier, J.L.; Metz, G.B.; et al. Telomerase maintains telomere structure in normal human cells. Cell 2003, 114, 241–53. [Google Scholar] [CrossRef]
- Kurz, D.J.; Erusalimsky, J.D. Role of telomerase in human endothelial cell proliferation. Arterioscler Thromb Vasc Biol 2003, 23, e54. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Blasco, M.A. Putting the stress on senescence. Curr Opin Cell Biol 2001, 13, 748–53. [Google Scholar] [CrossRef]
- Toussaint, O.; Medrano, E.E.; von Zglinicki, T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol 2000, 35, 927–45. [Google Scholar] [CrossRef]
- von Zglinick, T. Oxidative stress shortens telomeres. Trends Biochem Sci 2002, 27, 339–44. [Google Scholar]
- Xu, D.; Neville, R.; Finkel, T. Homocysteine accelerates endothelial cell senescence. FEBS Lett 2000, 470, 20–4. [Google Scholar] [CrossRef] [PubMed]
- Kurz, D.J.; Decary, S.; Hong, Y.; Trivier, E.; Akhmedov, A.; Erusalimsky, J.D. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci 2004, 117, 2417–26. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Qi, B.; Park, Y.C.; Irani, K. Constitutive activation of rac1 results in mitochondrial oxidative stress and induces premature endothelial cell senescence. Arterioscler Thromb Vasc Biol 2003, 23, e1–6. [Google Scholar] [CrossRef]
- Unterluggauer, H.; Hampel, B.; Zwerschke, W.; Jansen-Durr, P. Senescence-associated cell death of human endothelial cells: the role of oxidative stress. Exp Gerontol 2003, 38, 1149–60. [Google Scholar] [CrossRef]
- Martens, U.M.; Zijlmans, J.M.; Poon, S.S.; Dragowska, W.; Yui, J.; Chavez, E.A.; et al. Short telomeres on human chromosome 17p. Nat Genet 1998, 18, 76–80. [Google Scholar] [CrossRef]
- Slagboom, P.E.; Droog, S.; Boomsma, D.I. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet, 1994; 55, 876–82. [Google Scholar]
- Nawrot, T.S.; Staessen, J.A.; Gardner, J.P.; Aviv, A. Telomere length and possible link to X chromosome. Lancet 2004, 363, 507–10. [Google Scholar] [CrossRef]
- Jeanclos, E.; Schork, N.J.; Kyvik, K.O.; Kimura, M.; Skurnick, J.H.; Aviv, A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension 2000, 36, 195–200. [Google Scholar] [CrossRef]
- Allsopp, R.C.; Vaziri, H.; Patterson, C.; Goldstein, S.; Younglai, E.V.; Futcher, A.B.; et al. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A 1992, 89, 10114–8. [Google Scholar] [CrossRef] [PubMed]
- Samani, N.J.; Boultby, R.; Butler, R.; Thompson, J.R.; Goodall, A.H. Telomere shortening in atherosclerosis. Lancet 2001, 358, 472–3. [Google Scholar] [CrossRef] [PubMed]
- Brouilette, S.; Singh, R.K.; Thompson, J.R.; Goodall, A.H.; Samani, N.J. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol 2003, 23, 842–6. [Google Scholar] [CrossRef] [PubMed]
- Benetos, A.; Gardner, J.P.; Zureik, M.; Labat, C.; Xiaobin, L.; Adamopoulos, C.; et al. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension 2004, 43, 182–5. [Google Scholar] [CrossRef]
- Kurz, D.J.; Akhmedov, A.; Kloeckener, B.; Buehler, I.; Berger, W.; Bertel, O.; et al. Calcific aortic valve stenosis is associated with shorter telomere length. (abstract). Eur Heart J 2004, 25, s590. [Google Scholar]
- von Zglinicki, T.; Serra, V.; Lorenz, M.; Saretzki, G.; LenzenGrossimlighaus, R.; Gessner, R.; et al. Short telomeres in patients with vascular dementia: an indicator of low antioxidative capacity and a possible risk factor? Lab Invest 2000, 80, 1739–47. [Google Scholar] [CrossRef]
- Cawthon, R.M.; Smith, K.R.; O’Brien, E.; Sivatchenko, A.; Kerber, R.A. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003, 361, 393–5. [Google Scholar] [CrossRef]
- Nowak, R.; Siwicki, J.K.; Chechlinska, M.; Markowicz, S. Telomere shortening and atherosclerosis. Lancet 2002, 359, 976. [Google Scholar] [CrossRef]
- Davis, T.; Faragher, R.G.; Jones, C.J.; Kipling, D. Investigation of the signaling pathways involved in the proliferative life span barriers in werner syndrome fibroblasts. Ann N Y Acad Sci 2004, 1019, 274–7. [Google Scholar] [CrossRef]
- Wallis, C.V.; Sheerin, A.N.; Green, M.H.; Jones, C.J.; Kipling, D.; Faragher, R.G. Fibroblast clones from patients with Hutchinson-Gilford progeria can senesce despite the presence of telomerase. Exp Gerontol 2004, 39, 461–7. [Google Scholar] [CrossRef]
- Marciniak, R.A.; Johnson, F.B.; Guarente, L. Dyskeratosis congenita, telomeres and human ageing. Trends Genet 2000, 16, 193–5. [Google Scholar] [CrossRef]
- Tchirkov, A.; Lansdorp, P.M. Role of oxidative stress in telomere shortening in cultured fibroblasts from normal individuals and patients with ataxia-telangiectasia. Hum Mol Genet 2003, 12, 227–32. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Cancer and ageing: rival demons? Nat Rev Cancer 2003, 3, 339–49. [Google Scholar] [CrossRef] [PubMed]
- Tyner, S.D.; Venkatachalam, S.; Choi, J.; Jones, S.; Ghebranious, N.; Igelmann, H.; et al. p53 mutant mice that display early ageing-associated phenotypes. Nature 2002, 415, 45–53. [Google Scholar] [CrossRef]
- Shay, J.W. At the end of the millennium, a view of the end. Nat Genet 1999, 23, 382–3. [Google Scholar] [CrossRef] [PubMed]
© 2004 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurz, D.J. Telomere Biology in Cardiovasular Disease. Cardiovasc. Med. 2004, 7, 433. https://doi.org/10.4414/cvm.2004.01065
Kurz DJ. Telomere Biology in Cardiovasular Disease. Cardiovascular Medicine. 2004; 7(12):433. https://doi.org/10.4414/cvm.2004.01065
Chicago/Turabian StyleKurz, David J. 2004. "Telomere Biology in Cardiovasular Disease" Cardiovascular Medicine 7, no. 12: 433. https://doi.org/10.4414/cvm.2004.01065
APA StyleKurz, D. J. (2004). Telomere Biology in Cardiovasular Disease. Cardiovascular Medicine, 7(12), 433. https://doi.org/10.4414/cvm.2004.01065