Post-Operative Takotsubo Cardiomyopathy in Elective Mitral Valve Replacement
Abstract
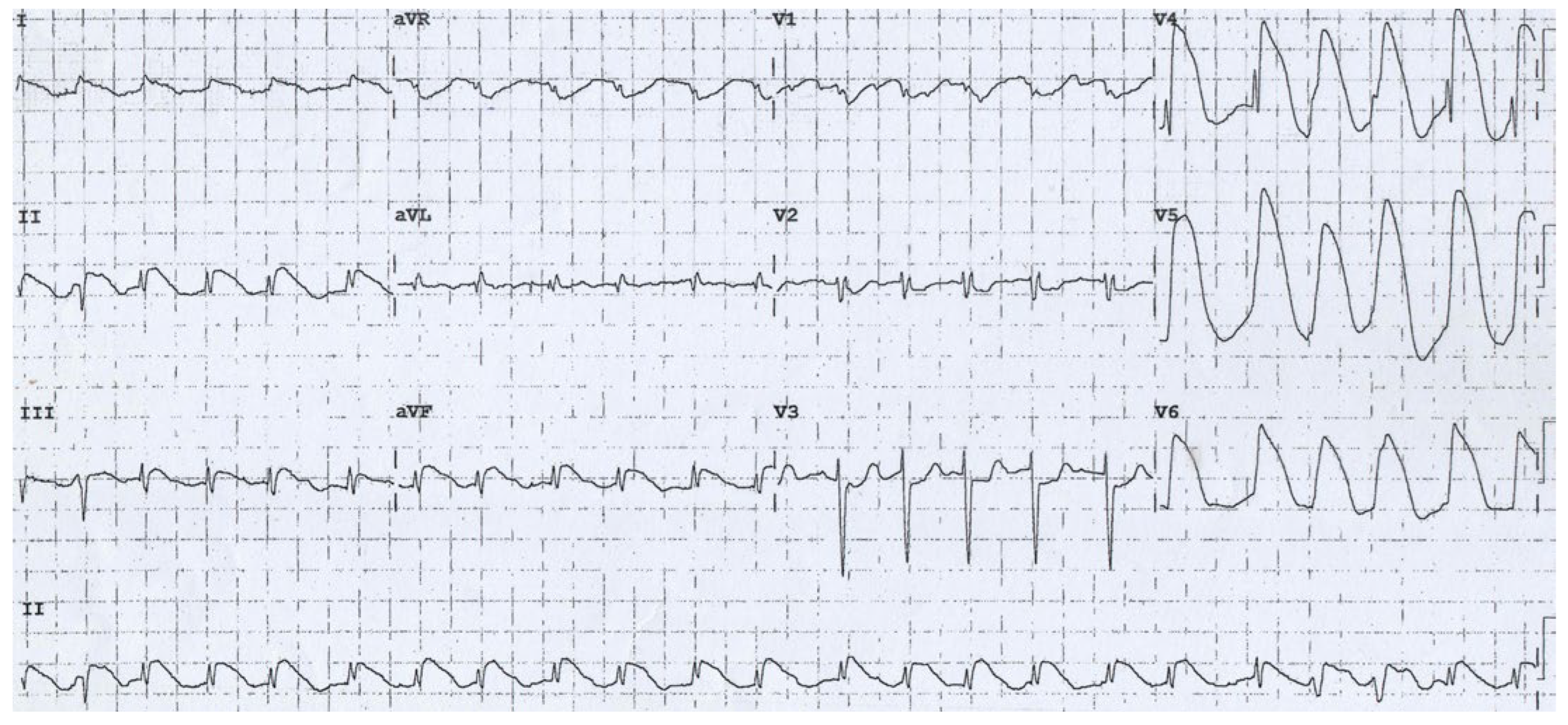
Case Presentation
Solution
Discussion
Author Contributions
Institutional Review Board Statement
Conflicts of Interest
References
- Bybee, K.A.; Kara, T.; Prasad, A.; Lerman, A.; Barsness, G.W.; Wright, R.S.; et al. Systematic review: Transient left ventricular apical ballooning: A syndrome that mimics ST-segment elevation myocardial infarction. Ann. Intern. Med. 2004, 141, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Sealove, B.A.; Tiyyagura, S.; Fuster, V. Takotsubo cardiomyopathy. J. Gen. Intern. Med. 2008, 23, 1904–1908. [Google Scholar] [CrossRef] [PubMed]
- Hussain, J.; Laufer, N.; Sorrof, S.; Pershad, A. Takotsubo cardiomyopathy after coronary intervention developed during hospitalization. Ann. Thorac. Surg. 2009, 88, e63–5. [Google Scholar] [CrossRef] [PubMed]
- Abehsira, G.; Bachelot, A.; Badilini, F.; Koehl, L.; Lebot, M.; Favet, C.; et al. Complex Influence of Gonadotropins and Sex Steroid Hormones on QT Interval Duration. J. Clin. Endocrinol. Metab. 2016, 101, 2776–2784. [Google Scholar] [CrossRef] [PubMed]
- Al-Wakeel, N.; Fernandes, J.F.; Amiri, A.; Siniawski, H.; Goubergrits, L.; Berger, F.; et al. Hemodynamic and energetic aspects of the left ventricle in patients with mitral regurgitation before and after mitral valve surgery. J. Magn. Reson. Imaging. 2015, 42, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Wehlin, L.; Vedin, J.; Vaage, J.; Lundahl, J. Peripheral blood monocyte activation during coronary artery bypass grafting with or without cardiopulmonary bypass. Scand. Cardiovasc. J. 2005, 39, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Halter, J.; Steinberg, J.; Fink, G.; Lutz, C.; Picone, A.; Maybury, R.; et al. Evidence of systemic cytokine release in patients undergoing cardiopulmonary bypass. J. Extra Corpor. Technol. 2005, 37, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Sylvin, E.A.; Stern, D.R.; Goldstein, D.J. Mechanical support for postcardiotomy cardiogenic shock: Has progress been made? J Card Surg. 2010, 25, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Laghlam, D.; Touboul, O.; Herry, M.; Estagnasié, P.; Dib, J.C.; Baccouche, M.; et al. Takotsubo cardiomyopathy after cardiac surgery: A case-series and systematic review of literature. Front. Cardiovasc. Med. 2023, 9, 1067444. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef] [PubMed]
- Arcari, L.; Musumeci, M.B.; Cacciotti, L. The Swiss cheese model in takotsubo syndrome. Eur Heart J Case Rep. 2022, 6, ytac235. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Lerman, A.; Rihal, C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am. Heart J. 2008, 155, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, J.A.; González, J.M.; Dalmau, M.J.; López, J. Takotsubo cardiomyopathy after elective mitral valve replacement. Interact. Cardiovasc. Thorac. Surg. 2010, 11, 117–119. [Google Scholar] [CrossRef] [PubMed]


© 2024 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Potnis, A.; Potwar, S.; Jadhav, U. Post-Operative Takotsubo Cardiomyopathy in Elective Mitral Valve Replacement. Cardiovasc. Med. 2024, 27, 90. https://doi.org/10.4414/cvm.2024.1412187259
Potnis A, Potwar S, Jadhav U. Post-Operative Takotsubo Cardiomyopathy in Elective Mitral Valve Replacement. Cardiovascular Medicine. 2024; 27(3):90. https://doi.org/10.4414/cvm.2024.1412187259
Chicago/Turabian StylePotnis, Abhishek, Sushrut Potwar, and Uday Jadhav. 2024. "Post-Operative Takotsubo Cardiomyopathy in Elective Mitral Valve Replacement" Cardiovascular Medicine 27, no. 3: 90. https://doi.org/10.4414/cvm.2024.1412187259
APA StylePotnis, A., Potwar, S., & Jadhav, U. (2024). Post-Operative Takotsubo Cardiomyopathy in Elective Mitral Valve Replacement. Cardiovascular Medicine, 27(3), 90. https://doi.org/10.4414/cvm.2024.1412187259



