LDL-Cholesterol and Prevention of Cardiovascular Events
The causal relationship between increased low-density lipoprotein-cholesterol (LDL-C) and cardiovascular disease is one of the best documented facts in medicine [
1].
Prospective studies in patients with genetic hypercholesterolemia as well as Mendelian randomization studies underlined that an increased LDL-C from birth is associated with an increased risk of atherosclerotic cardiovascular disease (ASCVD) and that individuals with low LDL-C from birth, have a much lower cardiovascular risk than the general population.
The gold standard in LDL-lowering therapy are statins. These drugs reduce the cholesterol production in the liver, which accounts for approximately 85% of the total pool of cholesterol in the body, by inhibiting the HMG-coenzyme A reductase thereby leading to an upregulation of hepatic LDL receptors with a subsequent lowering of the LDL serum concentration [
2].
All studies evaluating the effects of statins uniformly show that a reduction in LDL-C is dose-dependently associated with a reduction of myocardial infarction, cerebrovascular accident stroke and premature death by about 25% per year. The absolute risk reduction of an LDL-C reduction depends on the level of individual risk, the cholesterol level and the extent and duration of the of LDL-C reduction [
3].
Accordingly, statins have been increasingly used since the end of the 1980s for all cardiac patients, especially those with coronary artery disease, but also in healthy individuals (with familial hypercholesterolemia) and patients with hypercholesterolemia associated with other cardiovascular risk factors, such as hypertension, smoking or diabetes mellitus.
In addition to statins, a combination with ezetimibe, an inhibitor of the Niemann-Pick C1-Like 1 (NPC1L1) protein in the intestine, is commonly used as recommended by the actual guidelines. Ezetimibe reduces the uptake of cholesterol from the diet resulting in a reduction of LDL-C of about 20–25% [
4]. The Improve-IT study showed that the combination of simvastatin and ezetimibe reduced cardiovascular events more than simvastatin alone [
5]. Unfortunately, many primary and secondary prevention patients do not reach the target LDL-C level under this therapy [
4]. Side effects, non-adherence or a step-down of the dose could partly explain the unsatisfying results.
In the last years, new drugs, such as bempedoic acid and PCSK9 inhibitors have become available for lowering LDL-C. This review will focus on the available efficacy, safety and outcome data obtained for bempedoic acid.
Bempedoic Acid
Bempedoic acid (ETC-1002) is a fairly small molecule, a prodrug that is activated in the liver by the enzyme very long-chain acyl-CoA synthetase 1 (ACSVL1) to form the active compound bempedoyl-CoA [
6].
The active metabolite of bempedoic acid inhibits the enzyme ATP citrate lyase, which is involved in the biosynthesis of cholesterol via the HMG-coenzyme A reductase, which is inhibited by statins (
Figure 1).
A particularly interesting property of bempedoic acid is the fact that the metabolically inactive prodrug is only converted into the active compound by the enzyme ACSVL1. ACSVL1 is mainly found in the liver and to some extent in the kidneys, but not in the skeletal muscle cells [
6] and is therefore particularly suitable for people with muscle complaints under statins.
Effect of Bempedoic Acid in Monotherapy: CLEAR Harmony, Tranquility and Serenity
The effect of bempedoic acid on the lipid metabolism has been documented in various studies. The most important one, the CLEAR (Cholesterol Lowering via Bempedoic Acid, an ACL-Inhibiting Regimen) Harmony trial [
7], included patients with clinically manifest atherosclerosis and/or heterozygous familial hypercholesterolemia and an LDL-C of at least 1.8 mmol/l on maximal tolerated statin therapy and randomized them 2:1 to treatment with bempedoic acid or placebo. In the CLEAR Harmony study, a reduction of LDL-C by 12.6–16.5% was observed under bempedoic acid compared to placebo. Similar to statins but in contrast to ezetimibe or PCSK9 inhibitors, there was also a reduction in the high-sensitive C-reactive protein (hsCRP) [
7].
The effect of bempedoic acid in patients with statin intolerance was examined in the CLEAR Serenity study including 345 patients with hypercholesterolemia and an intolerance to at least two statins [
8]. The patients were randomized 2:1 to bempedoic acid (180 mg) or placebo once a day for 24 weeks. Bempedoic acid reduced the placebo-corrected LDL-C by 21% and the apolipoprotein B-100 by 15%. Additionally, the hsCRP was reduced by 24%. Bempedoic acid was safe and well-tolerated in this patient group. Myalgias occurred less frequently (5%) than with placebo (7%). Similar results were shown in the CLEAR Tranquility trial [
8]. Since it is well documented that most patients who report statin-associated muscle complaints also report muscular problems with placebo in up to 90% (nocebo effect) [
9], muscular symptoms have also been reported with bempedoic acid.
Effect of Bempedoic Acid in Combination with Statins and Ezetimibe
The CLEAR Tranquility study investigated the efficacy and safety of bempedoic acid (180 mg) and ezetimibe (10 mg) in a fixed combination in a double-blind approach in 301 patients with hypercholesterolemia and a high risk for or existing atherosclerotic cardiovascular disease or heterozygous familial hypercholesterolemia [
10].
After twelve weeks, the fixed combination reduced placebo-corrected LDL-C by 38%. Ezetimibe alone reduced LDL-C by 23% and bempedoic acid by 17% (placebo-controlled 25% and 19%, respectively) [
10].
In the absence of statins, the LDL-C reduction gained from the combination was 45%. Thus, with the fixed combination of bempedoic acid and ezetimibe alone or in addition to a statin, a further significant LDL-C reduction and a better attainment of LDL-C target levels can be achieved.
Effect of Bempedoic Acid on Inflammation
Similar to statins, bempedoic acid reduced hsCRP by about 20–30%, making it a potential therapeutic option for patients with residual inflammatory or cholesterol risks (
Figure 2). This effect was not observed with ezetimibe or PCSK9 inhibitors.
A secondary analysis of the CLEAR Harmony trial specifically analyzed the effect of bempedoic acid on CRP, interleukin 6 (IL-6), fibrinogen and lipoprotein(a) in patients with residual inflammatory risk [
11]. In this subanalysis, biomarker analysis was performed in 817 patients (542 under bempedoic acid, 275 under placebo) with known atherosclerotic disease and/or heterozygous familial hypercholesterolemia who were under maximal tolerated statin therapy and had a residual inflammatory risk defined as a baseline hsCRP ≥2 mg/l. Bempedoic acid significantly lowered LDL-C as well as hsCRP but had no significant effect on fibrinogen or IL-6. These findings are comparable to prior statin studies [
11].
As such, the data suggests that bempedoic acid mimics the ability of statins in reducing LDL-C and hsCRP and may be potentially useful in patients with residual inflammatory or cholesterol risk.
Nevertheless, a difference must be acknowledged: under the combination of ezetimibe and statin no further reduction of hsCRP was observed confirming that ezetimibe per se, does not have an anti-inflammatory effect [
12]. On the other hand, the combination of ezetimibe and bempedoic acid was found to be associated with a further reduction in hsCRP compared to statins alone [
11].
Safety Profile
In terms of adverse drug reactions, the CLEAR Harmony trial found an increased incidence of gout (1.2 vs 0.3%) under the treatment with bempedoic acid compared to placebo due to an increase in uric acid levels. This is a pharmacological effect essentially caused by the inhibition of the organic anion transporter 2 in the renal tubule. This effect, which is also known for diuretics, leads to a further small increase in serum creatinine (0.8% vs 0.4% with placebo in the CLEAR Harmony study) [
7]. The increase in uric acid is completely reversible after discontinuation.
A drop in hemoglobin of more than 2 g/dl below the lower normal range occurred in 4.9% patients under bempedoic acid and 2.0% with placebo. A drop of more than 3 g/dl occurred in 1.5% and 1.1%, respectively.
Bempedoic acid inhibits the organic anion transporting polypeptides OATP1B1 and OATP1B3. The concomitant use of drugs that are substrates of OATP1B1 and/or OATP1B3 (e.g., high-dose statins) can lead to an increased plasma concentrations of the drugs. Accordingly, in combination with bempedoic acid, dosages of simvastatin above 20 mg should only be administered with caution.
CLEAR Outcomes
The effects of bempedoic acid on cardiovascular morbidity and mortality has been investigated in the CLEAR Outcomes trial [
13]. It is a randomized, double-blind, placebo-controlled clinical trial evaluating patients with 1) established atherosclerotic cardiovascular disease, 2) a high risk of developing it; 3) a documented intolerance to statins and 4) an LDL-C ≥2.6 mmol/l despite maximal tolerated lipid-lowering therapy.
The trial was conducted at 1,250 sites in 32 countries. Statin intolerance was defined as being unable or unwilling to receive statins due to an adverse effect that had started or aggravated during statin therapy and has disappeared or improved after its discontinuation. The use of other lipid-lowering therapies was permitted. Patients on a very low average daily statin dose (rosuvastatin <5 mg, atorvastatin <10 mg, simvastatin <10 mg, lovastatin <20 mg, pravastatin <40 mg, fluvastatin <40 mg, pitavastatin <2 mg) without unacceptable adverse effects could be enrolled.
Between December 2016 and August 2019, a total of 13,970 patients underwent randomization; 6,992 were assigned to the bempedoic acid and 6,978 to the placebo group [
13]. Among all enrolled patients, the mean age was 65.5 years, 48.2% were female and 45.6% had diabetes mellitus. 69.9% had had previous cardiovascular events, 22.7% were on a statin and 11.5% were receiving ezetimibe. Lipid-lowering therapy was required for primary prevention in 30% of patients and for secondary prevention in 70% of patients. The mean LDL-C was 3.59 mmol/l in both groups.
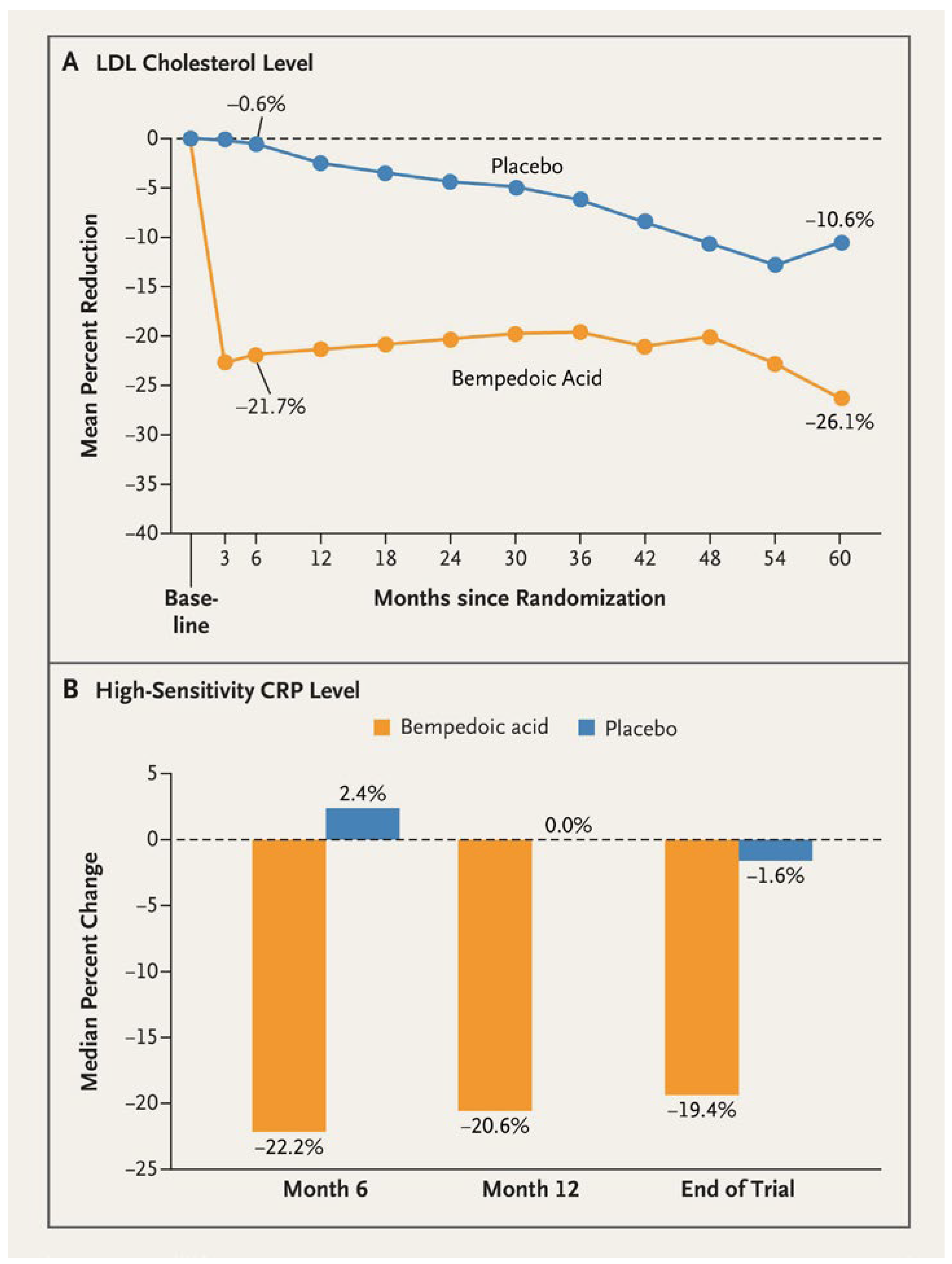
Additional lipid-lowering therapy was used in 9.4% and 15.6% of patients in the bempedoic acid and placebo group, respectively. The percental reduction of the LDL-C level was 21% greater with bempedoic acid than with placebo. The risk of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke or coronary revascularization (the primary composite endpoint) was 13% lower with bempedoic acid than with placebo over a median of 3.4 years (hazard ratio [HR] 0.87, 95% confidence interval [CI] 0.79–0.96, p=0.004) [
13]. There was no difference in the cardiovascular or all-cause mortality.
Bempedoic acid also reduced the risk of secondary end-point events including a three-component composite endpoint (death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke), fatal or nonfatal myocardial infarction and coronary revascularization [
13].
Death from cardiovascular causes, nonfatal stroke or nonfatal myocardial infarction occurred in 575 patients (8.2%) in the bempedoic acid group and in 663 patients (9.5%) in the placebo group (HR 0.85, 95% CI 0.76–0.96, p=0.006). Fatal or nonfatal myocardial infarction occurred in 261 patients (3.7%) in the bempedoic acid group and in 334 patients (4.8%) in the placebo group (HR 0.77, 95% CI 0.66–0.91, p=0.002). Coronary revascularization occurred in 435 patients (6.2%) in the bempedoic acid group and in 529 patients (7.6%) in the placebo group (HR 0.81, 95% CI 0.72–0.92; p=0.001) [
13].
Results of a prespecified subgroup analysis of the primary endpoint were provided as supplementary data. Based on baseline cardiovascular disease risk category, patients in primary prevention had a better reduction of the primary endpoint (HR 0.68–0.53, 0.87, interaction P-value 0.03) than patients in secondary prevention (HR 0.91–0.82, 1.01) [
13].
Similar effects were seen in patients using concomitant ezetimibe and very-low-dose statins. Interestingly, a numerically greater effect of bempedoic acid on the primary endpoint was observed in the 30% of patients in the primary prevention cohort than for the 70% of patients in the secondary prevention cohort.
The overall rates of adverse events were similar in both groups. A similar proportion of patients reported myalgia (5.6% vs 6.8%), but incidences of gout, cholelithiasis and laboratory elevations in creatinine, uric acid and hepatic enzyme levels were higher with bempedoic acid than with placebo [
13].
Limitatio in Switzerland
In Switzerland, bempedoic acid is currently indicated for lowering elevated blood cholesterol levels in adults with elevated LDL-C levels that cannot be lowered sufficiently with drugs like statins. Bempedoic acid is used as an adjunct to dietary interventions and in combination with a statin or other drugs to lower a high cholesterol level.
According to the Limitatio defined by the BAG, it is covered by the health insurance according to the following rules:
- -
As adjunctive therapy to a combination therapy of a statin and ezetimibe for the treatment of hypercholesterolemia in the presence of a confirmed clinically manifest atherosclerotic cardiovascular disease with an LDL-C level ≥1.8 mmol/l or heterozygous familial hypercholesterolemia with an LDL-C level ≥2.6 mmol/l which could not be reduced below these values by diet and with maximum tolerable doses of statins and ezetimibe.
- -
Bempedoic acid is only reimbursed if the above-mentioned LDL-C values could not be reached for at least three months with the maximum tolerable dosage of an intensified LDL-C lowering therapy with a statin in combination with ezetimibe.
- -
The treatment may only be continued if the LDL-C value has been reduced by at least 10% during a check-up within one to six months after the start of treatment.
- -
Compensation in combination with the use of PCSK9 inhibitors is excluded.