Rationale for FXI Inhibition as Novel Anticoagulant Principle
Anticoagulants are widely used as the mainstay treatment for many medical conditions e.g., the prevention and therapy of venous thrombosis (VT) and pulmonary embolism (PE), as well as the prevention of arterial thromboembolism, including stroke in atrial fibrillation (AF) [1, 2]. In very low doses and in combination with acetylsalicylic acid, they have shown effectiveness in preventing high risk atherothrombotic events [3]. The main goal of an anticoagulant is to optimally balance the prevention of thrombosis without major impairment of the normal hemostasis. In recent years, various effective and well-tolerated oral anticoagulant agents have been developed, with the most significant advancement being the introduction of the “direct oral anticoagulants” (DOACs) targeting factor XIIa and Xa. The DOACs have significantly, but not fully, replaced the standard vitamin K antagonist (VKA) and, in some cases, parenteral anticoagulants, such as low-molecular-weight heparin (LMWH). DOACs have become the preferred anticoagulant of choice for most patients due to a lower risk for major bleeding and in particular because of reduced intracranial hemorrhages compared to VKA as a class effect. Regarding prevention and therapy of thromboembolism, DOACs have been shown to achieve similar or better results. Nonetheless, the individual concerns of bleeding and the balance of thrombotic risk persist in any form of anticoagulation, including DOACs. Significant comorbidities like renal dysfunction, cirrhosis, thrombotic antiphospholipid syndrome (APS) or significant mitral valve disease (stenosis), as well as carriage of prosthetic valves or devices and the problems with artificial surfaces (hearts) remain critical [4,5,6].
The Unmet Needs
Patients with unmet needs for an improved anticoagulation strategy can be divided into two distinct groups. The first group comprises of patients who have an elevated risk of bleeding, including individuals with renal insufficiency, a history of prior bleeding events, cancer patients, and the elderly in general. As assessing the bleeding risk is challenging and no single approach is generally applicable, the decision to initiate, continue or stop anticoagulation therapy is left to the discretion of the physician. In cases where the risk of bleeding outweighs the risk of thrombosis, anticoagulants with an improved safety profile could serve as a valuable, long-awaited treatment option.
The second group that could benefit from a new treatment option consists of patients for whom the efficacy of DOACs has been tested and found to be inferior to VKA, or for whom there is insufficient evidence to support the use of DOACs. This includes patients with cardiovascular devices and/or artificial surfaces exposed to their circulatory system. For instance, in patients with mechanical heart valves, dabigatran and rivaroxaban were found to be less effective than VKA, and there is a lack of randomized trials comparing other DOACs to VKA in this population [7]. In dialysis patients, who have a high risk of bleeding, the benefit-to-risk ratio of oral anticoagulants is still a subject of debate. Observational studies have reported higher rates of bleeding with both VKA and, although somewhat less, with some DOACs, including apixaban and rivaroxaban [8]. Patients with left ventricular assist devices or under extracorporeal membrane oxygenation (ECMO) have not been included in randomized controlled trials assessing DOACs [9].
These limitations justified the search for novel anticoagulants. A search that resulted in the discovery of a target of the intrinsic coagulation pathway that plays a significant role in promoting and/or maintaining thrombosis but appears to be less critical for hemostasis: Factor XI (FXI).
Lessons Learned from Patients with Inherited Factor XI Deficiency
FXI deficiency, also known as Rosenthal syndrome or hemophilia C, was initially identified in the 1950s by Rosenthal and his colleagues [10]. They observed bleeding tendencies over four generations of a family following surgical and dental procedures. The rare autosomal bleeding disorder affects both sexes equally with a global incidence of 1 in 1,000,000. However, individuals of Ashkenazi Jewish heritage have a much higher prevalence of approximately 1 in 450. The clinical presentation of FXI deficiency varies considerably among patients with many individuals being asymptomatic and experiencing minimal or no increase in bleeding, especially in the absence of typical triggers such as trauma, surgery, or childbirth [11]. However, some patients have reported more severe bleeding after trauma or surgery, particularly in areas prone to fibrinolysis, like the nasopharynx, mouth or urinary tract [12].
In terms of thrombotic events, individuals with genetic FXI deficiency exhibit significantly lower rates of venous thromboembolism (VTE), stroke and, possibly, myocardial infarction (MI) compared to the general population. Interestingly, these patients do not experience an elevated risk of spontaneous bleeding [13]. A study conducted in Israel with a large cohort of 10,193 patients diagnosed with moderate-to-severe FXI deficiency (classified as ≤30% FXI activity) revealed that these individuals had roughly half the risk of cardiovascular events (stroke, transient ischemic events or MI) compared to the control group (classified as ≥50% FXI activity) [14]. Additionally, their risk of VTE was approximately one-quarter of that observed in patients with normal FXI levels. Although the patients with FXI deficiency reported higher rates of prior gastrointestinal bleeding, they did not exhibit higher rates of major bleeding, including intracerebral hemorrhage. On the other hand, women with FXI deficiency bleed heavily during childbirth in approximately 20% [43]. The preclinical ferric chloride-induced arterial injury mouse model has also shown that FXI-deficient mice experience lower thrombosis rates without a concurrent increase in bleeding [15].
Conversely, elevated levels of FXI may indicate an increased risk of thrombosis. In the Longitudinal Investigation of Thromboembolism Etiology (LITE), individuals with FXI levels in the highest quintile showed a two-fold increased VTE risk [16]. A retrospective case-control study reported up to a five-fold risk of VTE and a four-fold risk of stroke or transient ischemic attack (TIA) in patients with FXI levels above the 95th percentile [17].
Given the data obtained from both FXI deficiency and elevations of FXI, targeting FXI pharmacologically has emerged as a potential therapeutic approach with the hope of minimal bleeding.
Development of Agents Targeting FXI
FXI is an inactive form (zymogen) of the blood coagulation protease called factor XIa (FXIa), which plays a crucial role in hemostasis by activating factor IX (FIX). FXI is composed of identical subunits forming a disulfide-linked dimer with a molecular weight of 160 kDa. Each subunit contains four apple domains (A1-A4) at the N-terminus, which interact with FIX and factor XIIa (FXIIa). The C-terminus of FXI consists of the trypsin-like catalytic domain [18].
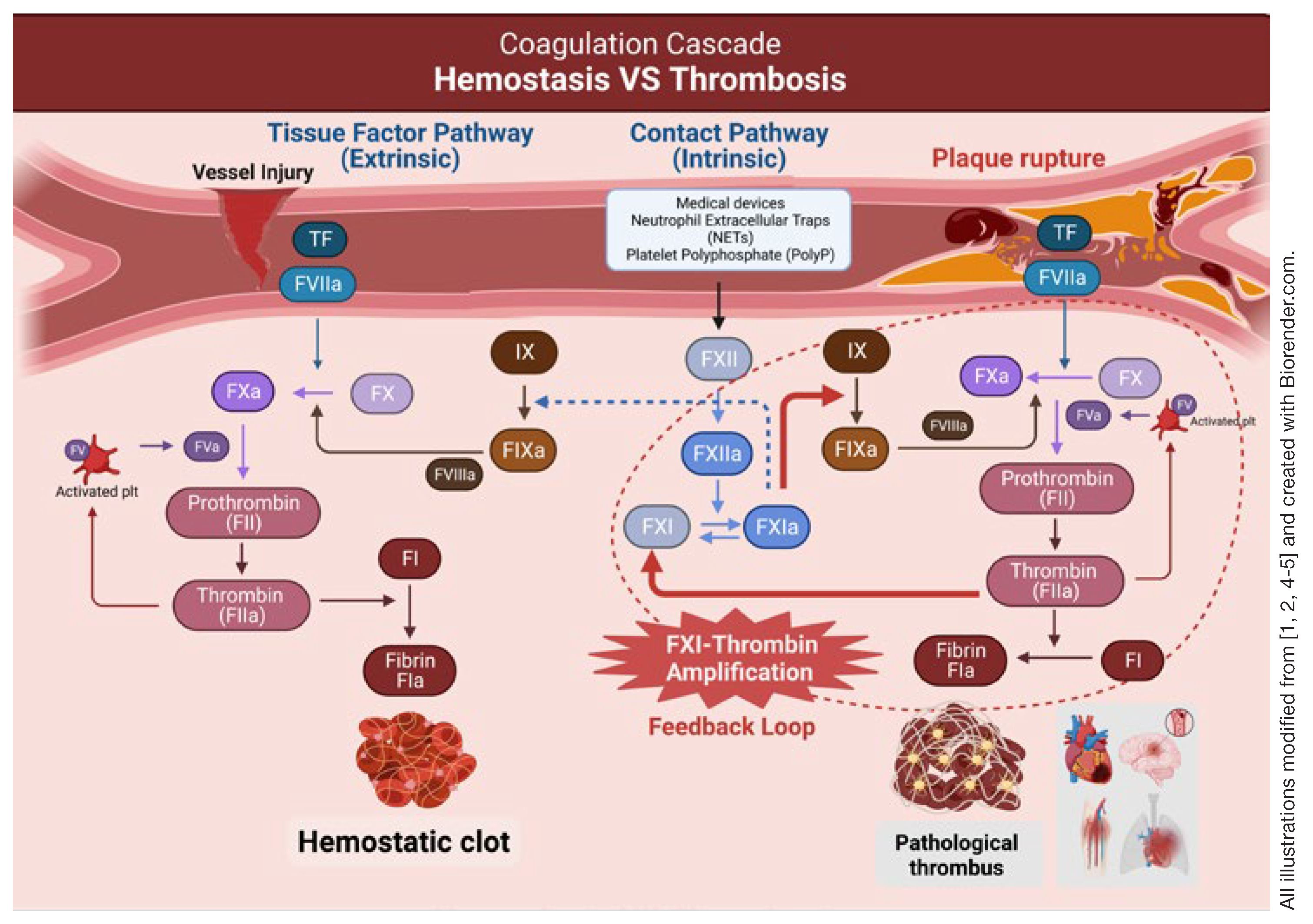
FXI is primarily synthesized in hepatocytes alongside most coagulation factors, such as prothrombin (factor II) and factor XII (FXII). It can be activated not only by FXIIa but also by FXIa itself and thrombin through a positive feedback loop. This loop leads to increased thrombin production and amplification of the coagulation cascade. In addition, thrombin can also activate the thrombin-activatable fibrinolysis inhibitor (TAFI), which then hampers the breakdown of fibrin clots and enhances clot stability.
Figure 1 shows the coagulation cascade in detail.
The contact activation (intrinsic) pathway initiates when blood comes into contact with artificial surfaces, resulting in FXII activation.
The FXIIa then triggers a FXI activation, subsequently leading to the activation of FIX, factor X and prothrombin. Therefore, FXII might be a natural target for inhibiting the intrinsic pathway. However, data from FXII studies are conflicting. Studies in patients with FXII deficiency didn’t indicate a reduced risk of thrombotic events. Observational studies also found no association of FXII with VTE, ischemic stroke or MI [16, 19]. In contrast, animal experimental studies have shown protection against thrombosis [20]. The discrepancy in FXII studies might stem from the differences between animal and human coagulation systems. Additionally, reducing thrombosis with low concentrations of FXII might be counteracted by a reduction in thrombus stability leading to more embolization.
On the other hand, patients with inherited FXI deficiency experience both a lower incidence and severity of bleeding episodes. Inhibition of FXI, similar to FXII inhibition in animal models, prevents thrombosis triggered by artificial surfaces. Notably, individuals with FXI deficiency produce lower levels of activated TAFI and display resistance to its effects [21]. As a result, they become more susceptible to bleeding from tissues with heightened local fibrinolytic activity. Furthermore, it is worth noting that a large thrombin burst originating from the contact activation pathway is adequate to initiate the formation of hemostatic plug, obviating the need for thrombin amplification. This reasoning means that FXI may become dispensable in normal hemostasis. These findings concur with the observation of relatively mild bleeding patterns observed in FXI deficient patients. Given the current evidence, it appears that FXI plays a more substantial role in pathological intravascular thrombosis than in normal hemostasis, potentially making it a better target for anticoagulants compared to FXII.
Various approaches are being investigated as potential therapeutic strategies to inhibit the generation and activity of FXI. These include antisense oligonucleotides (ASOs) targeting hepatocytes to reduce FXI synthesis, small molecules targeting the active or heparin-allosteric site on FXIa, monoclonal antibodies that block activation or inhibit FXIa activity, and DNA aptamers [22].
Figure 2 illustrates the various strategies of FXI inhibitors.
These interventions differ in their mechanisms of action and in their routes of administration (oral or parenteral), onset and duration of effect. ASOs, aptamers and monoclonal antibodies require parenteral administration while small molecules can be also administered orally. The varying onset and duration provide flexibility for different clinical scenarios; acute thrombotic events requiring fast-acting agents, and chronic prophylaxis and prevention where longer-acting options appear more suitable. Furthermore, conditions such as trauma or surgery which are associated with a high risk of bleeding complications may benefit from shorter-acting agents.
Considering all these factors, each strategy has its own strengths and weaknesses for clinical development.
Table 1 provides a summery outlining the main pharmacological characteristics of each type of drug.
Clinical Data on FXI Inhibitors
Currently, several phase II and III clinical trials investigate the use of FXI inhibitors in various clinical conditions. This includes VTE prophylaxis in specific scenarios, such as total knee arthroplasty (TKA), end-stage renal disease (ESRD) and cancer-associated thrombosis (CAT), as well as stroke prevention in patients with AF, after stroke or MI.
Figure 3A gives an overview of ongoing clinical trials in the beforementioned areas.
We conducted a systematical search across three electronic databases (PubMed, Cochrane Central Register of Controlled Trials [CENTRAL], Scopus) up until July 31, 2023. The evaluation of titles, abstracts, full texts (when applicable) for data extraction from relevant studies was carried out by the authors in an independent manner. Detailed summaries of each clinical trial can be found in table 2.
The results of recent phase II trials have provided valuable insights into the safety profile of FXI inhibitors, despite the limited number of participants involved. A recent meta-analysis, which included eight published phase II clinical trials of FXI inhibitors, revealed a 51% lower rate of bleeding of any type regardless of the dosage used [23]. Additionally, there was a 38% reduction in the trial-defined efficacy endpoint when compared to LMWH. Interestingly, when comparing FXI inhibitors to DOACs, no significant differences were observed in terms of major bleeding or efficacy endpoints. Moreover, when compared to placebo, FXI inhibitors were associated with a 25% increased risk of bleeding, but no difference was observed in the trial-defined efficacy endpoint.
Venous Thromboembolism Prophylaxis and Management
Several clinical trials have been conducted to evaluate the effectiveness of FXI inhibitors in preventing postoperative deep vein thrombosis (DVT) in patients undergoing elective TKA. In the AXIOMATIC-TKR trial [24], different doses of oral milvexian were tested in 1,028 patients. The results showed a dose-dependent reduction in the incidence of VTE, with no significant difference in major bleeding events compared to enoxaparin. Interestingly, patients randomized to a low dose of milvexian had similar rates of VTE as compared with enoxaparin. Monoclonal antibodies, such as osocimab [25] and abelacimab [26], also demonstrated a reduction in VTE incidence without an increase in bleeding events compared to enoxaparin with superiority achieved at higher doses. Of note, apixaban showed lower rates of bleeding events compared to osocimab. Similarly, in ASO trials, superior efficacy to enoxaparin was observed with a high dose of fesomersen (IONIS-FXI-LRx) with lower bleeding risks [27]. A recent meta-analysis of four randomized controlled trials confirmed that FXI inhibitors were associated with a significant reduction in VTE incidence and bleeding events of any type in patients undergoing TKA while there was no significant difference between the two groups in terms of adverse events or severe adverse events [28].
In ESRD patients, fesomersen and AB023 have been found to be well-tolerated with minimal risk of bleeding, as demonstrated in two small studies involving 43 and 24 patients respectively [29,30]. Larger phase II trials are currently underway investigating ASO (fesomersen) and monoclonal antibodies (osocimab, MK-2060) interventions with 200-700 participants. The main goal of these trials is to assess bleeding rates and adverse events compared to placebo.
![Cardiovascmed 27 00008 i001 Cardiovascmed 27 00008 i001]() |
![Cardiovascmed 27 00008 i002 Cardiovascmed 27 00008 i002]() |
| Table 2. Data from clinical trials with factor XI (FXI) inhibitors (Continuation). |
| Acute ischemic stroke or transient ischemic attack |
PACIF-
IC-STROKE [32] | II
(published 2022) | 18083 | Asundexian 10, 20, 50 mg PO OD. | Placebo | 6 months | Ischemic stroke/CBI:
18.9% Asundexian 10 mg
22% Asundexian 20 mg
20.1% Asundexian 50 mg
19.1% Placebo | Major bleeding/ CRNMB:
4% Asundexian 10 mg
3% Asundexian 20 mg
4% Asundexian 50 mg
2% Placebo |
| AXIOMAT-IC-SSP [33] | II
(completed, preliminary results) | 23664 | Milvexian 25 mg PO OD; 25, 50, 100,
200 mg BID. | Placebo | 3 months | New ischemic stroke/new CBI:
16.7% Milvexian 25 mg OD
16.6% Milvexian 25 mg BID
15.6% Milvexian 50 mg BID
15.4% Milvexian 100 mg BID
15.3% Milvexian 200 mg BID
16.8% Placebo | Major bleeding (BARC type 3 and 5):
0.6% Milvexian 25 mg OD
0.6% Milvexian 25 mg BID
1.5% Milvexian 50 mg BID
1.6% Milvexian
100 mg BID
1.5% Milvexian
200 mg BID
0.6% Placebo
Adverse events:
58.5% Milvexian
25 mg OD
59.4% Milvexian
25 mg BID
59.1% Milvexian
50 mg BID
63.1% Milvexian
100 mg BID
61.3% Milvexian
200 mg BID
58.5% Placebo |
OCEAN-
IC-STROKE (NCT05686070) | III
(recruiting) | 9300 | Asundexian | Placebo | 31 months | Time to first ischemic stroke.
Time to first major bleeding (ISTH criteria). Time to first CV death, MI or stroke. |
LIBREX-
IA-STROKE (NCT05702034) | III
(recruiting) | 15,0005 | Milvexian | Placebo | 41 months | Time to first ischemic stroke.
Time to first CV death, MI or stroke.
Time to first major adverse vascular event. |
| Acute myocardial infarction (MI) |
| PACIFIC-AMI [34] | II
(published 2022) | 16016 | Asundexian 10, 20, 50 mg
oral OD. | Placebo | 12 months | CV death, MI, stroke or stent thrombosis:
6.8% Asundexian 10 mg
5.9% Asundexian 20 mg
5.4% Asundexian 50 mg
5.5% Placebo | Major bleeding (BARC type 2, 3, 5):
7.5% Asundexian
10 mg
8% Asundexian 20 mg
10.4% Asundexian
50 mg
9% Placebo
Serious adverse events:
20% Asundexian
10 mg
21.2% Asundexian
20 mg
17.7% Asundexian
50 mg
21.3% Placebo |
FXI inhibitors have also shown potential in the prevention of CAT. Active phase III trials, namely ASTER [NCT05171049] and MAGNOLIA [NCT05171075], are currently ongoing with a large number of participants (1,655 and 1,020 respectively). These trials evaluate the effects of abelacimab on VTE recurrence and bleeding compared to apixaban (ASTER) and dalteparin (MAGNOLIA). These studies aim to provide further insights into the role of FXI inhibitors in patients with malignancy.
Atrial Fibrillation
The PACIFIC-AF trial [31] aimed to assess the effectiveness of oral asundexian in 755 patients with established AF requiring anticoagulation therapy. After twelve months, the trial revealed that both the 20 mg and 50 mg daily doses of asundexian had lower bleeding rates compared to the standard dosing of apixaban, while both groups had similar adverse events rate (47% in the asundexian and 49% in the apixaban groups). These promising results led to the initiation of the OCEANIC-AF trial [NCT05643573], a phase III study that focuses on evaluating the incidence of stroke and systemic embolism as the primary efficacy end-point over a period of 34 months.
In addition to asundexian, ongoing research in AF includes the AZALEA-TIMI 71 trial [NCT04755283], a phase II study investigating the safety of subcutaneously administered abelacimab once per month compared to oral rivaroxaban. Furthermore, the phase III LILAC-TIMI 76 trial [NCT05712200] currently in progress aims to determine the incidence of stroke in patients receiving abelacimab compared to those receiving a placebo over a duration of 30 months (patients who cannot or will not receive standard anticoagulants).
Stroke and Myocardial Infarction
In terms of secondary prevention of stroke, two small molecule FXI inhibitors, milvexian and asundexian, were evaluated in patients with ischemic stroke or TIA concurrently receiving antiplatelet therapy. These inhibitors did not exhibit a reduction in the composite outcome of covert brain infarcts or ischemic stroke when compared to a placebo at 90 and 180 days. This lack of reduction is likely attributed to the trials being underpowered and initially focusing on determining the safety dose for stroke prevention. However, it is noteworthy that neither of these small molecules showed an increase in bleeding rates [32, 33].
To further investigate the efficacy of these drugs in secondary stroke prevention, two large phase III trials, namely OCEANIC-STROKE [NCT05686070] and LIBREXIA-STROKE [NCT05702034], are currently underway. The trials aim to provide more comprehensive data on the effectiveness of milvexian and asundexian in preventing recurrent strokes.
Regarding MI, asundexian has shown promising outcomes, especially at a high dose. Although the trials were underpowered to effectively assess efficacy outcomes, they managed to reduce a composite of cardiovascular death, recurrent MI, stroke, and stent thrombosis, even in the presence of exceedingly low event rates in each arm (approximately 20 events) [34]. It is worth highlighting that the high dose of asundexian resulted in more than 90% inhibition of FXIa activity levels without causing any significant increase in major bleeding.
Potential Future Directions
Based on the available clinical data, FXI inhibitors show promise in various clinical scenarios, particularly in terms of their safety profiles. However, there are still unanswered questions regarding the efficacy of FXI inhibitors in large patient populations and the need for dose adjustments under specific conditions. Demonstrating superiority or non-inferiority compared to current, already effective anticoagulants may present a major challenge. However, the ongoing phase III trials hold the potential to provide valuable answers to these questions.
In addition to their established role in VTE prophylaxis and prevention of stroke/MI, FXI inhibitors may offer specific benefits to certain patient groups. Patients who are at a higher risk of bleeding associated with anticoagulants, such as the elderly, critically ill or septic patients, and pregnant and breastfeeding patients (where DOACs are contraindicated) could potentially benefit from novel anticoagulants (particularly monoclonal antibodies) with improved safety profiles.
Likewise, patients with diseases with limited options for current anticoagulants, for example, thrombotic APS, an autoimmune disease with an increased risk of DVT, could also benefit from these new anticoagulants. The current guidelines recommend using VKAs for individuals with triple positivity in arterial APS and discourage the use of DOACs. However, DOACs could be considered for patients with single or double positivity, but only after a comprehensive discussion with the patients and when VKAs are contraindicated. Nevertheless, the outcomes were not consistently conclusive and demand additional studies [35,36,37]. Sickle cell disease is an inherited disorder with an 11% risk of stroke. However, periodic red cell transfusion (and experimental anti p-selectin antibody) is the only proved beneficial prevention of stroke regardless of the anticoagulants used. Thus, small clinical trials could be interesting for this specific group of patients.
Perioperative Management, Urgent Surgery and Management of Bleeding Events
The findings in phase II trials highlight the potential of FXI inhibitors as effective and safe alternatives in the prevention of thrombotic events. However, with great power comes great responsibility, and the efficacy of FXI inhibitors also carries the risk of bleeding. It is crucial to address specific considerations regarding the development of reversal strategies, perioperative management, dose finding and proper management of bleeding in patients using FXI inhibitors.
In patients with inherited FXI deficiency various treatments have been utilized for perioperative and bleeding management. Successful approaches include replacement therapy with fresh frozen plasma (FFP) and FXI concentrates. However, complications such as volume overload in high cardiovascular risk patients with FFP and early thrombosis in patients at high thrombotic risk with FXI concentrate raise concerns [41,42]. Moreover, the availability of FXI concentrate may vary depending on the location and may be challenging to obtain in certain areas. For example, hemoleven, a human plasma-derived FXI concentrate, is only available in France. Antifibrinolytic agents (tranexamic acid [TXA]) have also shown success in perioperative management and postpartum hemorrhage in FXI deficient patients. Low doses of recombinant factor VIIa (rFVIIa), typically within the range of 10–20 µg/kg, are administered alongside TXA as a strategy to prevent excessive bleeding without introducing a higher risk of thrombosis. In this approach, rFVIIa serves to bolster the initial thrombin burst, while TXA is employed to inhibit fibrinolysis [43, 44].
In terms of antidote, a study involving a mouse model treated with FXI ASO (ISIS 404071) demonstrated the capacity of a sequence-specific sense oligonucleotide to counteract the inhibitory impact of ASO on the target FXI mRNA. Additionally, the use of a purified human FXI protein concentrate was explored [44]. A study conducted in a rabbit AV-shunt thrombosis model showed the reversal of milvexian using prothrombin complex concentrate (PCC), as well as rFVIIa [46].
There is only one randomized trial that investigates the reversal of milvexian using PCC and rFVIIa in humans (47 participants). The results are estimated to be available this year [NCT04543383].
Sailomon and Gailani have proposed strategies to prevent and address bleeding in patients using therapeutic FXI inhibitors [47]. For perioperative management, they suggest discontinuing antiplatelet agents whenever possible and addressing any coagulopathies unrelated to FXI deficiency. Antifibrinolytics and rFVIIa can be employed for patients using any of the FXI inhibitors. In cases of bleeding management, they recommend combining antifibrinolytic agents with rFVIIa for severe or life-threatening bleeding, including intracranial or gastrointestinal hemorrhage and dissecting aneurysm of a major vessel. In these situations, administration of FFP or PCC should be considered. Plasma exchange to remove an inhibitor targeting FXIa with a long half-life may be an option for patients with refractory bleeding.
Conclusions
Recent progress in anticoagulant development, notably DOACs, has brought substantial clinical benefits in terms of effectiveness and safety over the past few decades. Limitations persist within specific patient groups marked by distinct medical conditions or significant comorbidities. These individuals are not recommended for the application of current anticoagulants. Given the upcoming results from multiple phase II and III trials, the use and adoption of FXI inhibitors in daily clinical practice may become a reality, particularly in patients with a high bleeding risk, and in special patient populations including kidney failure and ACSAT. Further studies are required to effectively assess potential reversal strategies.
Key Points
FXI inhibition is likely an attractive approach for reducing thrombosis while minimizing bleeding risk.
FXI inhibitors demonstrated a positive trend in reducing bleeding risks in phase II trials.
Ongoing phase III trials will provide important insights into the efficacy of FXI inhibitors in various clinical scenarios.
Specific patient groups, including those with mechanical devices and blood exposed to artificial surfaces, may benefit from FXI inhibitors and require further clinical studies.
It will be relevant to discuss and develop effective reversal strategies for FXI inhibitors to ensure comprehensive patient management, particularly in agents with a very long half-life.